| Research Article | ||
Open Vet. J.. 2025; 15(7): 3035-3043 Open Veterinary Journal, (2025), Vol. 15(7): 3035-3043 Research Article Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trialMohammed Abdulabbas Hasan1,2*1Department of Pathological Analysis, College of Applied Medical Sciences, Al-Shatrah University, Al-Shatrah city, Iraq 2Department of Pharmacology and Microbiology, Faculty of Biomedical Science, Universiti Putra Malaysia, Seri Kembangan, Malaysia *Corresponding Author: Mohammed Abdulabbas Hasan. Department of Pathological Analysis, College of Applied Medical Sciences, Al-Shatrah University, Al-Shatrah city. Iraq; Department of Pharmacology and Microbiology, Faculty of Biomedical Science, Universiti Putra Malaysia, Seri Kembangan, Malaysia. Email: mohammedn10 [at] shu.edu.iq Submitted: 30/03/2025 Revised: 06/06/2025 Accepted: 21/07/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
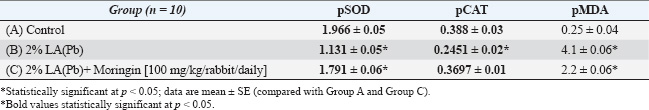
ABSTRACTBackground: Several researchers in the past have described lead acetate (LA), Pb, as a toxic heavy metal compound that can significantly affect the organs and the body systems, thereby influencing their functions. Aim: This article aimed to look at the negative effects of LA toxicity on rabbit liver samples, detect many histological abnormalities, and then examine the hepatoprotective properties of glucomoringin. Since ancient times, Moringa oleifera has been a rich source of antioxidants. Here, we orally gavaged Moringin extracted from M. oleifera seeds to the experimental animals to reduce toxicity. Methods: In this study, 30 rabbits were randomly categorized into three groups, with every group containing 10 rabbits (i.e., n=10). The animals in Group A (control) were administered distilled water, while those in Groups B and C were given a 2% oral LA solution. However, during the complete study duration, the animals in Group C were administered Moringin (100 mg/kg/rabbit). Results: The findings in this study indicated that oral LA administration caused significant pathological changes in the livers of the experimental animals which included degeneration, fibrosis, necrosis, inflammation, significant reduction in the plasma catalase and superoxide dismutase activities, and increased plasma malondialdehyde activities. It was hypothesized that the administration of Moringin would mitigate the histopathological changes in the liver tissues. The results indicated that the extract displayed a hepatoprotective effect and considerably reduced the harmful effects of LA on the functions and hepatic tissue. Conclusion: Finally, Moringin is highly efficacious in abating oxidative stress and cellular damage in rabbits. Keywords: Adverse effects, Antioxidant, Hepatopathy, Lead acetate, Moringin. IntroductionLead is widely recognized as a significant environmental contaminant due to its extensive application in various manufacturing sectors (Poli et al., 2022). Both industrialized nations and developing economies face substantial risks from environmental and occupational exposure to harmful pollutants (Medjedded et al., 2024). Even trace amounts of toxic heavy metals such as lead pose serious threats to human and animal well-being (Mesalam et al., 2023). Exposure to lead manifests through numerous health issues, including anaemia, weight loss (Kahalerras et al., 2022), kidney damage, infertility, liver toxicity, cardiovascular problems, reproductive dysfunction, and several other disorders (Gurer-Orhan et al., 2004). It exacerbates oxidative degradation in liver cells by promoting lipid peroxidation of membranes (Adeyomoye et al., 2022), a damaging sequence driven by oxygen-derived free radicals (Jomova et al., 2024). The toxic effects of lead are primarily linked to elevated oxidative stress levels in soft tissues and blood caused by lead exposure (Lakka et al., 2023). Cellular damage arises when the equilibrium between oxidants and antioxidants becomes disturbed (Abdelhamid et al., 2020). Research has shown that lead not only heightens lipid peroxide levels but also alters the antioxidant defence mechanisms in hepatic tissue (Rauf et al., 2024). Additionally, reactive oxygen species (ROS) have been identified as key contributors to the intensification of lead-induced toxicity in biological systems. In the past few years, many researchers have expressed their interest in determining the significance of oxidants and antioxidants in the field of medicine, biology, and nutrition research (Jomova et al., 2023). Studies have shown that the continuous production of pro-oxidants like the free oxygen radicals is essential for the sustenance of aerobic life. ROS signalling molecules, including nitric oxide, hydroxyl radical, and superoxide anion (O2.-), are highly reactive free radicals due to their unpaired electron. These radicals occur in the body as a consequence of various metabolic activities but are usually restricted within specific cellular compartments. Conversely, certain endogenous antioxidants—for instance, glutathione peroxidase (GPx), glutathione (GSH), vitamins C and E, and superoxide dismutase (pSOD)—are scavengers of radicals that are free, helping to neutralize excess ROS production (Abdollahi et al., 2014). A previous research revealed that free radicals caused by pathological mechanisms following lead exposure, suggesting that supplementing antioxidants might offer an effective alternative to conventional chelation therapies (Amadi et al., 2019). An uncommon glucosinolate extracted from Moringa oleifera seeds, known as the hydrolysis derivative of glucomoringin (GMG) or Moringin (GMG-ITC), comprises various bioactive compounds such as reducing sugars, alkaloids, tannins, sterols, terpenoids, anthraquinones, flavonoids, and saponins (Abd Karim et al., 2023). The seeds are particularly abundant in rhamnose sugars and unique compounds such as glucosinolates and isothiocyanates, with their beneficial effects largely attributed to phytochemicals such as flavonoids and isothiocyanates. Additional bioactive components include glucosinolates, phenolic acids, niazirin, terpenes, niazinin, quercetin, gossypetin, sitosterol, and ascorbic acid (Patil et al., 2022). Beyond M. oleifera, extracts from Garcinia hombroniana bark and essential oils obtained from Tarchonanthus camphoratus leaves and stems also displayed potent antioxidant properties (El Kamari et al., 2023). Research demonstrated that M. oleifera L extracts effectively suppressed lipid peroxidation by maintaining key antioxidant enzyme activities, including pSOD, catalase (pCAT), and GPx, in the plasma of treated animals. Another study assessed the influence of Juniperus phoenicea extracts on liver antioxidant enzymes in oxonate-administered rats (Gdoura et al., 2013; Vo et al., 2024). However, to our knowledge, no prior investigation has examined the efficacy of Moringin derived from M. oleifera seeds in alleviating lead-induced oxidative injury in hepatic tissue homogenates. Hence, in this study, we explored whether orally administering the extract to rabbits could mitigate liver damage, such as hepatocyte degeneration, necrosis, and fibrosis, triggered by lead acetate (LA) exposure. Research aimsInspect pathological mechanisms behind LA toxicity, utilizing rabbits as the experimental model. Gain deeper insights into the harmful impact of LA exposure across different toxic dosage levels. Lay the groundwork for future studies focused on LA toxicity and the adverse effects of trace elements in both humans and animals. Evaluate the protective potential of Moringin’s antioxidant activity against LA-induced hepatic injury. Materials and MethodsSetup of the experimentThirty rabbits of both sexes, aged between 6 and 12 months, with body weights ranging from 1,200 to 1,750 gm, were looked after in line with the World Health Organization’s (WHO) guidelines for breeding and caring for laboratory animals. More specifically, they were given a basic diet and plenty of fresh drinking water. They lived in stainless-steel cages maintained at a temperature of 21°C ± 2°C, a constant humidity level ranging from 40% to 70%, 12 hours of light, and 12 hours of darkness. GroupingThe 30 animals were separated randomly into three groups, with the control Group (A) receiving distilled water, the 2% LA Group (B) receiving 2% LA solution, and the LA+Moringin Group (C) receiving 2% LA+Moringin (100 mg/kg/rabbit) solution. Animal blood and tissue samplingAll rabbits enrolled in this experiment were euthanized 3 months after the final treatment session. Blood samples were drawn via cardiac puncture several hours before euthanasia to facilitate subsequent biochemical evaluations. These blood samples that were collected from each animal were separated into 2 parts, wherein one part of the sample was kept in a plain bottle, whereas the second part of the sample was added to a bottle containing ethyl di-amine tetra acetic acid. Also extracted the hepatic tissues from each animal and fixed the tissues in the 10% (v/v) saline formalin solution. The preserved liver tissues were sectioned, mounted onto glass slides, and subjected to haematoxylin and eosin (H&E) staining to carry out further histopathological examinations. Here, the procedures outlined in earlier research for sample preparation and plasma extraction are used to determine the activity of plasma enzymes. Such as pSOD, pCAT, and malondialdehyde (pMDA). Animal tissue preparation and microscopic examinationSpecimens of hepatic tissue were extracted from the animals immediately after sacrificing all the animals, 3 months after treatment. Thereafter, these tissues were sliced into sections and fixed with the help of a 10% (v/v) saline formalin solution. After fixing, these samples were processed and embedded in liquid paraffin. The paraffinized tissue blocks were sliced using a rotatory microtome into 5 µm-thick slices, deparaffinized, and stained using H&E stains. Finally, these samples were examined using an Olympus® light microscope to determine the LA treatment-induced histopathologic changes (Hayat and Giaquinta, 1970). Data collection method and statistical analysesAfter collecting the liver tissue samples from the animals in every group, the samples were homogenized, and the cell-free supernatant was used to determine the activities of enzymes such as pSOD, pCAT, and pMDA based on the processes described in the past (Ahmed et al., 2000). Here, the researchers carried out statistical analysis and presented the values as mean ± SE. The mean value differences were compared using the IBM® SPSS® 22.0 software and the one-way ANOVA test. The p < 0.05 was regarded as a statistically significant value. Table 1. Alterations in the antioxidant biomarkers and lipid peroxidation/enzymes (mean ± SE) of all the groups.
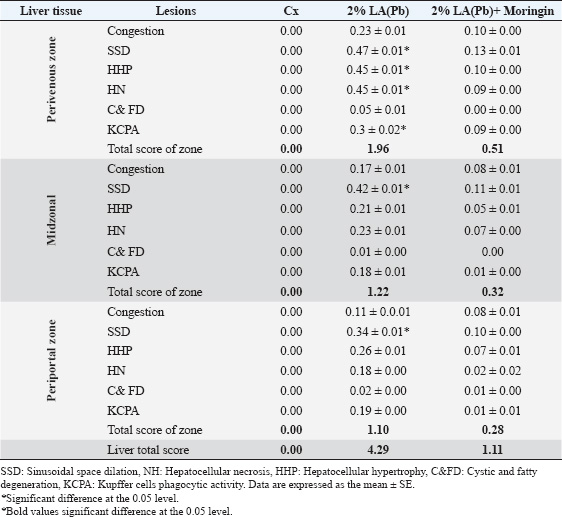
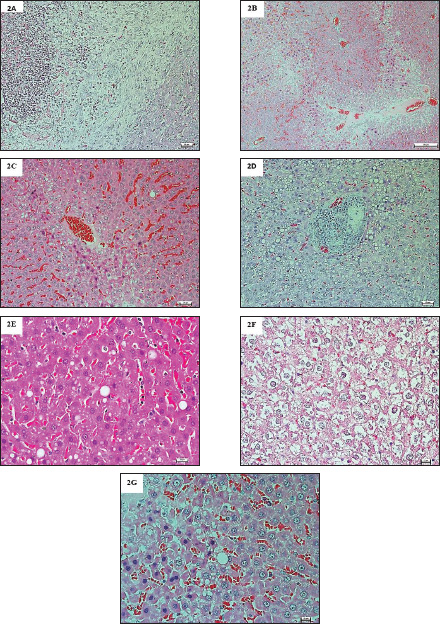
Fig. 1. Photomicrographs of H&E-stained livers in the sub-chronic trial at 10× magnification. The livers in Group A were normal, having sinusoidal capillaries and hepatocytes positioned regularly around the central vein. ResultsTherapeutic efficacy of Moringin and lead acetate (LA)-induced oxidative stress on liver enzymes and biomarkersThe findings in this study are described as mean ± SEM. Values showing p < 0.05* were deemed to be significant. After conducting the enzymatic analysis of the liver tissue samples, the animals in Group B showed a significant decrease (p < 0.05) in their pSOD and pCAT enzyme activities in comparison to Group A. However, the liver tissue extracts of the Group C animals did not present any significant variation (p > 0.05) in their pCAT and pSOD enzyme activities in comparison to the Group A animals. Additionally, when compared to Group A, the pMDA activity of the animals in Group B was seen to be significantly higher (p < 0.05), whereas the pMDA activity of the animals in Group C did not show any statistical difference (p > 0.05) (Table 1). Histopathological examinations and lesion scoring of animal Groups A and BIn this study, the histological lesions present in the liver tissue samples examined and the findings of all the experiments were compiled. The findings showed that the liver slices extracted from Group A animals displayed a normal level, wherein the tissues showed the presence of normally placed hepatocytes that surrounded the central vein, in addition to general sinusoidal capillaries (Figs. 1 and 4) (Table 2). However, the liver tissue slices extracted from the animals in Group B displayed significant treatment-induced histopathological changes, which included vacuolation, degeneration, and necrosis of hepatocytes with the accumulation of inflammatory cells, especially mononuclear cells; also noted clear swelling of hepatocytes, or became like balloons. Some samples displayed histological changes related to capsular and advanced septal fibrosis. Further histopathological analysis revealed that the rabbits had hepatitis since the infiltration of inflammatory cells was noted in addition to their congestion (Figs. 2A–G and 5A–C) (Table 2). On the other hand, the analysis of the liver sections extracted from the Group C animals did not display any structural variations (Figs. 3A and B, 6A and B) (Table 2). DiscussionIn this work, the enzymatic and histological investigations were employed to determine if the extended exposure of the experimental rabbits to Pb had caused liver damage. The findings showed that the Pb-administered rabbits (Group B) had higher levels of pMDA activity (Table 1). Comparable findings were previously reported by Hasan (2024), who observed a marked elevation in plasma enzyme activities, comprising alkaline phosphatase, alanine transaminase, and aspartate aminotransferase, along with increased hepatic pMDA levels. Elevated lipid peroxidation coupled with reduced GSH concentrations pointed to lead-induced oxidative stress within liver tissues (Hasan, 2024). The Pb-exposed animals absorb and retain the Pb ions within their soft tissues, particularly the liver (Hashim et al., 2024). The liver is the first organ in the body that gets exposed to the toxins and absorbed nutrients, which enter via the portal vein (Okediran et al., 2019; Mueed et al., 2023). As a result, we conducted a histological analysis of the liver tissue to investigate the resultant morphological and ultrastructural changes by using a light and transmission electron microscope, which indicated the toxic impact of Pb on hepatocytes. Our results showed that the hepatocytes displayed several significant changes, such as degeneration, vacuolation, and necrosis, with a higher fibrosis level, and hepatitis caused by the infiltration of inflammatory cells. A prior investigation (Mueed et al., 2023) documented comparable outcomes, noting that lead exposure through contaminated water triggered various pathological changes, including hepatic vein dilation, vascular congestion, and widespread hepatocellular degeneration and necrosis. Multiple studies (Shalan et al., 2023) have also highlighted similar hepatotoxic impacts on liver tissues, revealing histological alterations such as enlarged blood vessels, dilated central veins, and sinusoidal hemorrhaging. Furthermore, many researchers claimed that Pb was very detrimental to liver tissue because it caused fibrosis and inflammation of hepatocytes. Lead (Pb) toxicity led to many pathological changes/abnormalities in the liver, which differed according to exposure duration, administration route, and animal model type (Shalan, 2024). The microscopy analysis of hepatic tissue samples in Group C animals revealed that many of the histopathological abnormalities observed in Group B had been significantly reduced. Group C animals (Figs. 3A and B, 6A and B) demonstrated a significant decrease in hepatic tissue cellular deteriorations in comparison to Group B (Figs. 2A and G and 5A and C), (Table 2). These findings indicated that Moringin displayed a protective effect against the different pathological events that occurred in the rabbits because of Pb-hepatotoxicity. Previous presentations of comparable findings have shown that Moringin successfully prevented hepatotoxicity (Sharida et al., 2012; Singh et al., 2014). Additionally, comparable outcomes were reported by Saalu et al., (2012),; Sharifudin et al. (2013)), where it was demonstrated that animals treated with M. oleifera leaf extract experienced notable protection against hepatic injury, showing no significant rise in lipid peroxidation levels even after exposure to multiple toxins and liver-damaging agents. They concluded that M. oleifera significantly decreased the animals’ oxidative stress caused by alcohol or paracetamol, or other ROS and RNS inducers. Several studies have postulated that ROS are key contributors to lead-induced toxicity (Gurer-Orhan et al., 2004). Previous research has extensively examined how different antioxidant enzymes and molecules influence Pb-triggered oxidative stress in both humans and animals. Diminished levels of GSH and glutathione disulfide, along with altered pSOD activity, were frequently cited as primary indicators of Pb-related hepatotoxicity in blood and tissue samples (Okediran et al., 2019). Some researchers (Borekar et al., 2024) suggested that antioxidant supplementation might serve as a viable alternative to chelation therapy, as Pb exposure typically generates free radicals during disease progression. Numerous studies have highlighted that ascorbic acid, commonly known as vitamin C, functions effectively as a chelating agent with antioxidant properties, offering cellular defence against oxidative stress (Borekar et al., 2024). Additionally, tomatoes and their derivatives are excellent sources of antioxidants due to their rich content of vitamins A, C, and lycopene (Abdollahi et al., 2014). The experimental results revealed no significant variation (p > 0.05) in the pSOD activities of Groups C and A. However, the animals in Group B showed significantly lower pSOD activities (p < 0.05) than Group A. Comparable observations were documented previously (Simatupang et al., 2024). Animals in Group B showed a marked decline (p < 0.05) in their plasma catalase pCAT activity compared to those in Group A. Conversely, there was no notable difference (p > 0.05) in pCAT activity levels between Groups A and C. Based on the above findings, we concluded that even though the animals in Group C were exposed to Pb toxicity, the dosage of Moringin that was administered to the animals could reduce their Pb-based oxidative stress. Additionally, no significant variation (p > 0.05) was found in both tissue and plasma MDA concentrations between Groups A and C. However, Group B revealed a significantly elevated (p < 0.05) pMDA concentration. These results suggest that Moringin extract, abundant in antioxidants, effectively countered the oxidative damage triggered by lead exposure in the animals receiving Moringin supplementation. In summary, since Moringin contains several types of antioxidants, it can effectively mitigate the hepatotoxicity resulting from the Pb-induced oxidative damage to the hepatic tissue. Table 2. Sub-chronic lesion scoring for liver tissue obtained from treated and untreated groups.
Fig. 2. (A). Photomicrographs of H&E-stained livers in the sub-chronic trial at 10× magnification. The livers in Group B were abnormal, appeared hepatocellular necrosis, with periductal fibrosis surrounding the bile ducts and severe dilation of sinusoidal spaces. (B). Photomicrographs of H&E-stained livers, at 10× magnification. The livers in Group B were abnormal, the central vein and sinusoidal spaces were severely dilated and congested, while appeared severe steatosis in the centrilobular zone. A huge number of inflammatory cells had infiltrated, especially in the periportal region. (C). Photomicrographs of H&E-stained livers at 20× magnification. The livers in Group B were abnormal, the central vein and sinusoidal spaces were severely dilated and congested, while severe steatosis was in the centrilobular zone. Hepatocellular necrosis was also observed, with periductal fibrosis surrounding the bile ducts. (D). Photomicrographs of H&E-stained livers at 20× magnification. The livers in Group B were abnormal, hepatocellular necrosis was observed, with periductal fibrosis surrounding the bile ducts. A highly infiltrated area of inflammatory cells especially in the periportal region, which formed non-small nodules. (E). Photomicrographs of H&E-stained livers at 40× magnification. The livers in Group B were abnormal, clear ballooned hepatocytes, with Mallory-Denk bodies, and a huge number of inflammatory cells had infiltrated, especially in the periportal region, as well as the Kupffer cells, hypertrophy and hyperplasia. (F). Photomicrographs of H&E-stained livers at 40× magnification. The livers in Group B were abnormal, clear ballooned hepatocytes, with Mallory-Denk bodies, and a huge number of inflammatory cells had infiltrated, especially in the periportal region, as well as periductal fibrosis surrounding the bile ducts. (G). Photomicrographs of H&E-stained livers at 40× magnification. The livers in Group B were abnormal, and hepatocellular necrosis was observed, with periductal fibrosis and clear ballooned hepatocytes, with Mallory-Denk bodies, as well as the Kupffer cells, hypertrophy and hyperplasia.
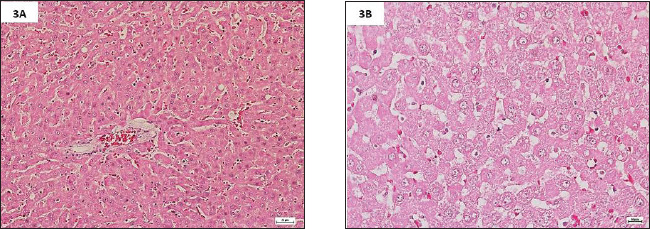
Fig. 3. (A). Photomicrographs of H&E-stained livers in the sub-chronic trial at 20× magnification. The livers in Group C had significantly smaller hepatotoxic lesions with minimal necrotic cells, no cystic or fatty degeneration, and no inflammatory cells infiltrations. (B). Photomicrographs of H&E-stained livers in the sub-chronic trial at 40× magnification. The livers in Group C, a fewer hypertrophic and hyperplastic periportal Kupffer cells and hepatocytes, withminimalwith minimal necrosis, no cystic or fatty degeneration, and no inflammation, despite necrosis and mild congestion. Moringin can therefore counteract the negative effects of Pb-induced liver damage and has a strong protective effect.
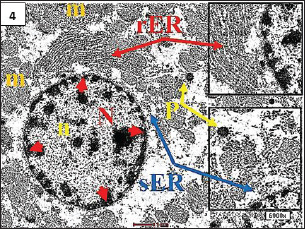
Fig. 4. TEM. from control group, showing normal appearance of hepatocyte with a euchromatic nucleus (N) as well as developed a nuclear membrane (red head arrow), and the cytoplasim contains normal mitochondria (m), an array of rough endoplasmic reticulum (rER) and smooth endoplasmic reticulum (sER), and normal peroxisome (p).
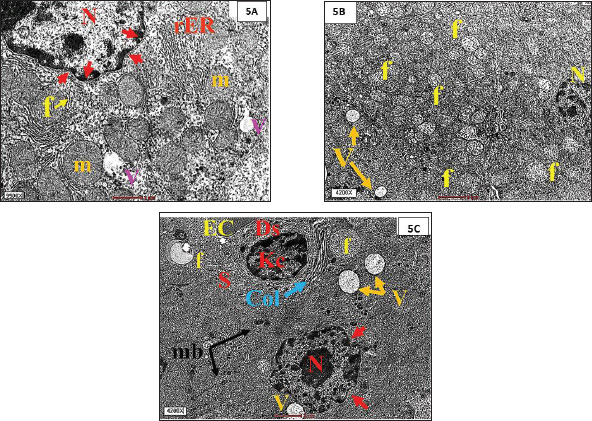
Fig. 5. (A). TEM from the LA-treated group, showing, hepatocytes with intracytoplasmic vacuolization (V), swollen mitochondria (m), electro-dense bodies Mallory bodies (mb)(f) and debris of necrotic nuclei, a lot of lysosomes (Ly). (B). TEM from the LA-treated group, showing, hepatocytes with intracytoplasmic vacuolization (V), swollen mitochondria (m), electro-dense bodies Mallory bodies (mb)(f) and debris of necrotic nuclei, a lot of lysosomes (Ly). (C). TEM from the LA-treated group, showing, dilatated blood sinusoids (S) with severe hypertrophic Kupffer cells (KC), severe dilated smooth endoplasmic reticulum (sER) and highly proliferation of peroxisome (P), and a higher magnification of cytosol immediately adjacent to the nucleus and collagen fibers adjacent to Kupffer cells.
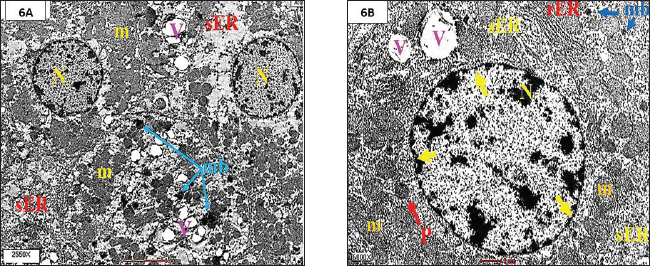
Fig. 6. A and B. TEM from the LA+Moringin group, illustrateing pronounced reduction in the hepatocellular degeneration and necrosis and Kupffer cells hypertrophy with significant diminution in the highly increased amount of sER and peroxisome, normal appearance of hepatocyte with an euchromatic nucleus (N) as well as developed a nuclear membrane (yellow head arrow). ConclusionIn summary, findings from this study demonstrated that Moringin, derived from M. oleifera seeds, exhibits powerful antioxidant, anti-proliferative, and liver-protective effects. Consistent oral dosing of Moringin at 100 mg/kg proved beneficial as both a preventive strategy and supportive therapy against the onset, progression, and persistence of lead-induced liver toxicity in rabbits. Moringin provides substantial hepatoprotective action, contributing to the prevention of hepatic lesions and minimizing their intensity over prolonged exposure periods. AcknowledgmentThank you toThe author would like to thank the Faculty of Medicine at Universiti Putra Malaysia (UPM) for granting access to all the facilities required to complete this study. Conflicts of interest and funding declarationThe manuscript was not funded, and there are no conflicts of interest. Ethical statementThe Animal Care and Use Committee (ACUC) of the Faculty of Bio-Medical Science at Universiti Putra Malaysia (UPM) approved this study (UPM/IACUC/AUP-R082/2015). Authors’ contributionThe author designed and implemented the research, analysed the results, and wrote the manuscript. ReferencesAbd Karim, N.A., Adam, A.H.B., Jaafaru, M.S., Rukayadi, Y. and Abdull Razis, A.F. 2023. Apoptotic potential of glucomoringin isothiocyanate (GMG-ITC) isolated from Moringa oleifera Lam seeds on human prostate cancer cells (PC-3). J. Mol. 28(7), 3214. Abdelhamid, F.M., Mahgoub, H.A. and Ateya, A.I. 2020. Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res. 27, 10950–10965. Abdollahi, M., Moridani, M.Y., Aruoma, O.I. and Mostafalou, S. 2014. Oxidative stress in aging. Oxid. Med. Cell Longev. 2014, 876834. Adeyomoye, O.I., Olaniyan, O.T., Adewumi, N. and Anyakudo, M.M. 2022. Honey supplemented with Vitamin C prevents dyslipidaemia and oxidative stress induced by exposure to lead acetate in Wistar rats. J. Physiol. Pharmacol. 65(4), 229–236. Ahmed, A.E., Aronson, J. and Jacob, S. 2000. Induction of oxidative stress and TNF-$α$ secretion by Dichloroacetonitrile, a water disinfectant by-product, as possible mediators of apoptosis or necrosis in a murine macrophage cell line (RAW). Toxicol. In Vitro 14(3), 199–210. Amadi, C.N., Offor, S. J., Frazzoli, C. and Orisakwe, O.E. 2019. Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res. 26, 18032–18052. Borekar, V.I., Somkuwar, A.P., Sawarkar, A.R., Limsay, R.P., Dubey, S.A. and Umap, S.A. 2024. Therapeutic effect of Zingiber officinale on serum biochemical parameters in lead intoxicated broiler chickens. Int. J. Vet. Sci. Anim. Husb. 9(6), 231–236. El Kamari, F., Chlouchi, A., El Hamzaoui, N., Harmouzi, A., Lhilali, I., ElMouhdi, K., El Omari H. and Abdellaoui, A. 2023. Chemical composition, antioxidant and antimicrobial activities of the essential oil of origanum majorana growing in middle Atlas of Morocco. Trop. J. Nat. Prod. Res. 7(10), 4232–4237. Gdoura, N., Murat, J.C., Abdelmouleh, A. and Elfeki, A. 2013. Effects of juniperus phoenicea extract on uricemia and activity of antioxidant enzymes in liver, erythrocyte and testis of hyperuricemic (oxonate-treated) rats. Afr. J. Pharm. Pharmacol. 7(8), 416–425. Gurer-Orhan, H., Sabır, H.U. and Özgüneş, H. 2004. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. J. Toxicol. 195(2–3), 147–154. Hasan, M.A. 2020. Ultrastructural changes in hepatocytes and chemopreventive effects of short-term administration of curcuma longa L. against oxidative stress-induced toxicity: improvement mechanisms of liver detoxification. eCAM. 2020:9535731; https://doi.org/10.1155/2020/9535731 Hasan, M.A. 2024. Efficacy of zingiber Officinale (ZO) in The Treatment of Lead Acetate-induced Hepatopathy in Rabbits: an Ultrastructural Assessment of Sub-acute Trial.: Efficacy of Zingiber Officinale (ZO) in the Treatment of Lead Acetate-Induced Hepatopathy in Rabbits. Iraq J Pharm Sci. 33(2), 112–120. Hashim, M., Al-Attar, A.M., Alomar, M.Y., Omar, A.M.S., Alkenani, N.A. and Zeid, I.M.A. 2024. Alleviation of carbendazim toxicity effect by Moringa oleifera oil and Linum usitatissimum L. oil on testes of male rats: physiological, histological and in silico study. Saudi J. Biol. Sci. 31(2), 103921. Hayat, M.A. and Giaquinta, R. 1970. Rapid fixation and embedding for electron microscopy. Tissue Cell 2(2), 191–195. Jomova, K., Alomar, S.Y., Alwasel, S.H., Nepovimova, E., Kuca, K. and Valko, M. 2024. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 98(5), 1323–1367. Jomova, K., Raptova, R., Alomar, S.Y., Alwasel, S.H., Nepovimova, E., Kuca, K. and Valko, M. 2023. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97(10), 2499–2574. Kahalerras, L., Otmani, I. and Abdennour, C. 2022. The allium triquetrum L. leaves mitigated hepatotoxicity and nephrotoxicity induced by lead acetate in wistar rats. Biol. Trace Elem. Res. 200, 1–11. Lakka, N., Pai, B., Mani, M.S. and Dsouza, H.S. 2023. Potential diagnostic biomarkers for lead-induced hepatotoxicity and the role of synthetic chelators and bioactive compounds. J. Toxicol. Res. 12(2), 178–188. Medjedded, H., Nemiche, S. and Nemmiche, S. 2024. Individual and combined effects of cadmium and lead exposure in rats. Int. J. Environ. Health Res. 34(7), 2649–2660. Mesalam, N.M., Ibrahim, M.A., Mousa, M.R. and Said, N.M. 2023. Selenium and vitamin E ameliorate lead acetate-induced hepatotoxicity in rats via suppression of oxidative stress, mRNA of heat shock proteins, and NF-kB production. J. Trace Elem. Med. Biol. 79, 127256. Mueed, A., Shibli, S., Jahangir, M., Jabbar, S. and Deng, Z. 2023. A comprehensive review of flaxseed (Linum usitatissimum L.): health-affecting compounds, mechanism of toxicity, detoxification, anticancer and potential risk. Crit. Rev. Food Sci. Nutr. 63(32), 11081–11104. Okediran, B.S., Suleiman, K.Y., Adah, A.S. and Sanusi, F. 2019. Mitigation of lead acetate induced toxicity by ginger (Zingiber officinale). Ann. Clin. Toxicol. 2(2), 1020. Patil, S.V, Mohite, B.V, Marathe, K.R., Salunkhe, N.S., Marathe, V. and Patil, V.S. 2022. Moringa tree, gift of nature: a review on nutritional and industrial potential. Curr. Pharmacol. Rep. 8(4), 262–280. Poli, V., Aparna, Y., Madduru, R. and Motireddy, S.R. 2022. Protective effect of Vitamin C and E on enzymatic and antioxidant system in liver and kidney toxicity of Cadmium in rats. J. Appl. Food Res. 2(1), 100098. Rauf, A., Khalil, A.A., Awadallah, S., Khan, S.A., Abu-Izneid, T., Kamran, M. and Wilairatana, P. 2024. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—a review. J. Food Sci. Nutr. 12(2), 675–693. Saalu, L.C., Ogunlade, B., Ajayi, G.O., Oyewopo, A.O., Akunna, G.G. and Ogunmodede, O.S. 2012. The hepato-protective potentials of Moringa oleifera leaf extract on alcohol-induced hepato-toxicity in wistar rat. Am. J. Biotechnol. Mol. Sci. 2(1), 6–14. Shalan, M.G, Al-Bakry, N.A. and Rashwan, H.M. 2023. The ameliorative effects of lactoferrin against lead acetate toxicity in female albino rat. Egypt. J. Zool. 80(80), 35–49. Shalan, M.G. 2024. Mitigating lead acetate-induced histopathologic and physiologic disorders in rats receiving vitamin C and glutathione supplement. Heliyon 11(1), e41256. Sharida, F., Syazana, A.S. and Palanisamy, A. 2012. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules 17, 8334–8350. Sharifudin, S.A., Fakurazi, S., Hidayat, M.T., Hairuszah, I., Aris Mohd Moklas, M. and Arulselvan, P. 2013. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. J. Pharm. Biol. 51(3), 279–288. Simatupang, M.M., Veronika, E., Irfandi, A. and Azteria, V. 2024. Potential impacts of lead on health: a review of environmental exposure, population at risk, and toxic effects. J. Environ. Health. 16(3), 277–288. Singh, D., Arya, P.V., Aggarwal, V.P. and Gupta, R.S. 2014. Evaluation of antioxidant and hepatoprotective activities of Moringa oleifera Lam. leaves in carbon tetrachloride-intoxicated rats. J. Antioxid. 3(3), 569–591. Vo, K.H., Nguyen, L.H., Nguyen, N.L., Phan, T.T.N., Zulfiqar, N., Hoang, T.V. and Ha, H.A. 2024. Screening of jellyfish venom inhibitors from beach morning glory (Ipomoea pes-caprae) against nemopilema nomurai. Matrix Sci. Pharma. 8(2), 24–30. | ||
| How to Cite this Article |
| Pubmed Style Mohammed Abdulabbas Hasan. Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Vet. J.. 2025; 15(7): 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 Web Style Mohammed Abdulabbas Hasan. Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. https://www.openveterinaryjournal.com/?mno=250122 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.14 AMA (American Medical Association) Style Mohammed Abdulabbas Hasan. Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Vet. J.. 2025; 15(7): 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 Vancouver/ICMJE Style Mohammed Abdulabbas Hasan. Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 Harvard Style Mohammed Abdulabbas Hasan (2025) Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Vet. J., 15 (7), 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 Turabian Style Mohammed Abdulabbas Hasan. 2025. Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Veterinary Journal, 15 (7), 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 Chicago Style Mohammed Abdulabbas Hasan. "Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial." Open Veterinary Journal 15 (2025), 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 MLA (The Modern Language Association) Style Mohammed Abdulabbas Hasan. "Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial." Open Veterinary Journal 15.7 (2025), 3035-3043. Print. doi:10.5455/OVJ.2025.v15.i7.14 APA (American Psychological Association) Style Mohammed Abdulabbas Hasan (2025) Histopathological and ultrastructural alterations reveal the hepatotoxicity of lead against Moringin: Sub-chronic-chemotherapeutic trial. Open Veterinary Journal, 15 (7), 3035-3043. doi:10.5455/OVJ.2025.v15.i7.14 |