| Research Article | ||
Open Vet. J.. 2025; 15(7): 2972-2981 Open Veterinary Journal, (2025), Vol. 15(7): 2972-2981 Research Article Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus)José Goicochea-Vargas1,2*, Max Salvatierra-Alor2, Fidel Acosta-Pachorro3, Wilson Rondón-Jorge1, Cesar Caro-Magni4, Julissa Cajacuri-Aquino1, Edson Morales-Parra5, Eric Mialhe6, Mauricio Silva7 and Marcelo Ratto81Department of Surgery and Reproductive Biotechnology, Faculty of Veterinary Medicine and Zootechnics, University Nacional Hermilio Valdizán, Huánuco, Perú 2Molecular Biotechnology Laboratory, Central Laboratory Unit, Hermilio Valdizán National University, Huánuco, Peru 3National Institute of Health, Experimental Surgery for Children, San Borja, Lima, Perú 4Animal Pathology Laboratory, SENASA, Lima, Perú 5Catholic University Sedes Sapientiae, Faculty of Agricultural and Environmental Sciences, Lima, Perú 6INCABIOTEC SAC, Tumbes, Peru 7Department of Veterinary Medicine and Public Health, Faculty of Natural Resources, Catholic University of Temuco, Temuco, Chile 8Animal Science Institute, Faculty of Veterinary Sciences, Austral University of Chile, Valdivia, Chile *Corresponding Author: José F. Goicochea Vargas. Department of Surgery and Reproductive Biotechnology, Faculty of Veterinary Medicine and Zootechnics, Hermilio Valdizán National University, Huánuco, Peru. Email: jgoicochea [at] unheval.edu.pe Submitted: 08/04/2025 Revised: 18/06/2025 Accepted: 23/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
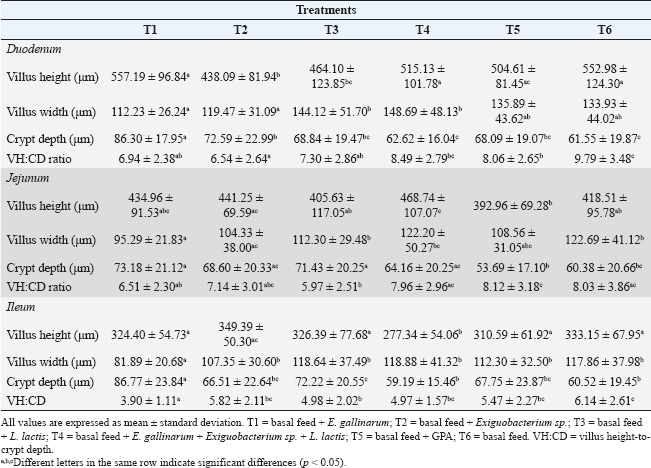
ABSTRACTBackground: Probiotics play an important role in improving host animal growth and influence intestinal morphology development. However, this effect varies depending on the type of probiotic used and the form of its administration. Aim: This study aimed to investigate the effect of probiotics based on native bacterial strains: Enterococcus gallinarum, Exiguobacterium sp., and Lactococcus lactis on the animal growth and intestinal histometry of guinea pigs (Cavia porcellus). Methods: Seventy-two weaned guinea pigs from a fattening line, with an initial average weight of 248.6 ± 42.2 g, were distributed into six pens. The subjects received oral administration of probiotics as follows: Treatment 1 (T1): E. gallinarum, Treatment 2 (T2): Exiguobacterium sp., Treatment 3 (T3): L. lactis, Treatment 4 (T4): a mixture of all three bacteria, Treatment 5 (T5): zinc bacitracin supplementation, and Treatment 6 (T6): control. After 63 days, weight gain and feed conversion ratio were determined. Additionally, the villus height and width, depth of crypt of Lieberkühn, and the villus height-to-crypt depth. (VH:CD) ratio in the duodenum, jejunum, and ileum were evaluated. Results: Probiotic application caused differences in weight gain (p=0.04), specifically between T1 and T3, but did not affect the weekly feed conversion ratio (p=0.72). On the other hand, intestinal histometry evaluations showed differences in villus height in each intestinal section (p < 0.05). In the jejunum, T4 had a greater villus height than the control (p < 0.05), whereas in the other treatments, probiotics did not increase height compared with the control. Differences were found in villus width in each intestinal section (p < 0.05) but were not greater than the width of the control. Treatments resulted in differences in crypt depth in all sections (p < 0.05), caused by an increase in depth compared with the control. Finally, VH:CD ratio also showed differences throughout the small intestine (p < 0.05), with values similar or lower in probiotics treatments compared with the control. Conclusions: The application of probiotics based on E. gallinarum, Exiguobacterium sp., and L. lactis did not affect the productive parameters of guinea pigs but had an impact on intestinal histomorphology. Keywords: Lieberkühn crypt, Duodenum, Jejunum, Ileum, Lactic acid bacteria. IntroductionThe guinea pig (Cavia porcellus) is a rodent used as a model for medical research or as a food source in some regions because its meat is highly valued for its taste and high nutritional content (Enríquez, 2019; Herrera et al., 2022; Aphrodita et al., 2024). However, the breeding of these animals is hindered by infectious diseases caused by Salmonella sp., Escherichia coli, Streptococcus sp., Klebsiella sp., and Bordetella sp. (Obregón et al., 2018; Angulo-Tisoc et al., 2021; Mendoza-Rodriguez, 2022). Many of these pathogens are ingested with food, reaching the intestine where they are absorbed and can cause severe infections. Several studies have focused on the intestines of these animals, which are divided into small and large intestines (Imam et al., 2021). The first section is mainly responsible for nutrient absorption, whereas the large intestine absorbs water and ions, (Kiela and Ghishan, 2016). The small intestine comprises three regions without clear divisions: the duodenum, jejunum, and ileum, with the jejunum having the longest and largest surface area (Mitjans and Ferrer, 2004). The intestine has a high density of villi and microvilli, increasing its absorption area (Walton et al., 2016). Additionally, the intestine contains Lieberkühn crypts, structures associated with villus renewal (Rehman et al., 2007). Beneficial and pathogenic bacteria colonize and interact with epithelial cells to regulate various physiological processes (Hill et al., 2017; Yang et al., 2020). Among the strategies used to control infections, antibiotics are widely employed to reduce pathogen effects. However, their use alters the intestinal microbiota and can leave residues in guinea pig meat and organs (Ampuero-Riega and Morales-Cauti, 2021). An alternative has been the use of probiotics isolated from the intestines or feces of guinea pigs (Goicochea-Vargas et al., 2024), as they can regulate gastrointestinal microbial populations and provide beneficial effects for the host (FAO/WHO, 2001). However, their efficacy depends on the food type, probiotic preparation before administration, dosage, and inoculation rate. Once in the intestinal tract, probiotics enhance the intestinal barrier against pathogens and strengthen the immune system for rapid infection prevention (Raheem et al., 2021; Fijan, 2023; Mousa et al., 2023). Additionally, these microorganisms produce beneficial molecules for the host and improve digestive enzyme activity, promoting intestinal growth (Zuo et al., 2019; Raheem et al., 2021; Wang et al., 2021). Moreover, probiotics can modify the size of intestinal villi and crypts, facilitating nutrient absorption (Kalita et al., 2021; Obianwuna et al., 2023). The application of mixed probiotics increased villus length and the villus height-to-crypt depth (VH:CD) ratio in broiler chickens (Awad et al., 2009; Aliakbarpour et al., 2012). In mice, probiotics based on Lactobacillus increased villus height and the VH:CD ratio (Li et al., 2021), similar to treatments with Bacillus coagulans, which increased villus height, crypt depth, and VH:CD ratio (Zhao et al., 2023). The addition of Lactobacillus delbrueckii to piglet diets reduced crypt depth and increased the VH:CD ratio (Wang et al., 2021). In guinea pigs, probiotics also affect intestinal morphology. The application of probiotics containing Enterococcus hirae, Lactobacillus reuteri, Lactobacillus frumenti, Lactobacillus johnsonii Streptococcus thoraltensis, and Bacillus pumilus increased the VH:CD ratio and reduced Lieberkühn crypt depth in the duodenum and ileum (Carcelén, 2021). Furthermore, using this probiotic in Salmonella sp. infected animals increased villus height and widthin the ileum (López, 2018). Another study showed a positive linear effect on the VH:CD ratio (Puente et al., 2019). Although some reports have provided insights into probiotic effects on guinea pig intestinal histometry, research focused on new probiotics is needed to better understand their interaction with the intestinal tract and select the best probiotic strains. Previously, three strains with probiotic potential were isolated from guinea pig feces (Enterococcus gallinarum, Exiguobacterium sp., and Lactococcus lactis) (Goicochea-Vargas et al., 2024). The effects of these bacteria on different production parameters, including meat quality, have been studied. The objective of the present study was to investigate the effects of these strains on the intestinal histometry of guinea pigs and to determine their relationship with animal growth parameters. Materials and MethodsAnimals, feeding, and management systemA total of 72 guinea pigs from a fattening line in the weaning stage (14 ± 2 days post-birth) were housed in breeding pens at the Kotosh Livestock Experimental Center, Universidad Nacional Hermilio Valdizán (UNHEVAL). The compartments, floors, and walls were disinfected with diluted quaternary ammonium at the beginning of the rearing period and every 15 days thereafter. The pens were cleaned every 3 days, mainly to remove feces. The animals had an initial average weight of 248.6 ± 42.2 g and were fed daily with a basal diet consisting of a mixture of freshly harvested alfalfa (Medicago sativa) and corn stalks (Zea mays L.), representing 27% of the live weight of the guinea pigs. Additionally, balanced feed (BF) (Coricuy Crecimiento, Corina-Peru) was provided at a proportion of 6% of the live weight. The BF composition included 65% total digestible nutrients, 18% protein, 2.75 Mcal/kg of metabolizable energy, 11% fiber, 3% fat, 7% ash, 0.8% calcium, and 0.45% phosphorus. The diet was divided into two daily rations: BF was given at 09:00 AM and forage at 3:00 PM. The quantity provided was adjusted weekly based on weight gain (WG). Treatments and experimental designThe experimental units were distributed using a completely randomized design. Each pen contained 12 animals, which were divided by wooden partitions and wire mesh measuring 1.48 m in length, 1.42 m in width, and 0.5 m in height. The experimental assays lasted for 63 days. Six treatments were considered as follows: Treatment 1 (T1): basal feed + 3 ml Probiotic 01; Treatment 2 (T2): basal feed + 3 ml Probiotic 02; Treatment 3 (T3): basal feed + 3 ml Probiotic 03; Treatment 4 (T4): basal feed + 3 ml Probiotic 04; Treatment 5 (T5): basal feed + 300 ppm zinc bacitracin; and Treatment 6 (T6): basal feed (control). Preparation and administration of probiotics and antibioticsThe probiotics were prepared using the following bacterial strains: Enterococcus gallinarum (Probiotic 01), Exiguobacterium sp. (Probiotic 02), and Lactococcus lactis (Probiotic 03). These bacteria, with in vitro probiotic potential, were previously isolated from guinea pig feces (Goicochea-Vargas et al., 2024) and preserved in the Molecular Biotechnology Laboratory collection at UNHEVAL. Bacterial reactivation was performed in 5 ml of De Man Rogosa Sharpe (MRS) broth and incubated at 37°C for 48 hours. A portion of each culture was inoculated again in MRS broth until reaching a concentration between 109 and 1011 colony-forming units (CFU)/mL. After centrifugation at 4,000 g for 20 minutes, the bacterial pellet was resuspended in sterile distilled water containing 0.07% lactic acid at an approximate concentration of 107 (CFU)/mL (Carcelén et al., 2021). Each bacterial suspension was considered a probiotic. Probiotic 04 was prepared by mixing the three bacterial strains in equal proportions to reach a density of 107 (CFU)/mL. For T1–T4, probiotics were applied between days 1 and 10 after the start of the experiment (weaning stage) and from day 34 for 5 days (growth stage). The probiotics were administered orally using a 1 ml syringe at 3 ml/animal/day before feed intake. For T5, the growth-promoting antibiotic (GPA) zinc bacitracin 10% (Zinbax 10%, Pro-Premix Nutrición Sac, Peru) was mixed with a powdered concentrate feed at a concentration of 300 ppm and provided throughout the experiment (Carcelén et al., 2021). Animals in T5 and T6 received orally 3 ml/animal/day of a 0.07% lactic acid solution in distilled water using a 1 ml syringe, simultaneously with the administration of other treatments. Production parametersWG: Individual weights (g) were recorded at the beginning of the bioassay and weekly before feeding. Total WG was calculated as the difference between the weight at the 9th week of evaluation and the initial weight. Weekly feed conversion ratio (WFCR): The percentage of dry weight of feed (BF and forage) and residues was calculated based on the ratio of weight before and after drying 100 g in an oven at 70°C for 60 hours. The dry matter intake was then calculated by subtracting the dry weight of the residual feed from the weekly dry feed (Valdizán, 2018). Weekly feed conversion was calculated by establishing the ratio of weekly dry matter intake to weekly live WG. Intestinal morphometric parametersAt the end of the experiment, four animals from each group were randomly euthanized after a 12-hour fasting period. One-centimeter samples from the intestinal segments (duodenum, jejunum, and ileum) were collected and immediately fixed in 10% formalin for at least 48 hours. The samples were processed using a TP1020 automatic tissue processor (Leica, Germany), where they were fixed, dehydrated, cleared, and embedded in paraffin. The paraffin blocks were sectioned into 5 μm slices using a microtome and stained with hematoxylin and eosin (Cáceres et al., 2021). Each histological slide was prepared for each intestinal section and animal. The slides were observed under an Olympus BX-41 bright-field microscope (Olympus Corporation, Japan) equipped with an Infinity 3 digital camera (Lumenera, Canada) at 10× and 50× magnifications. The images were analyzed using LabSpec v. 6.0 software (Horiba, France) (Fig. 1). Twenty intact villi and crypts of Lieberkühn were evaluated per intestinal section. Villi height and width, as well as crypt depth, were measured using the measure tool of LabSpec v. 6.0 software. Additionally, the VH:CD ratio was calculated using the collected data. Statistical analysisData are presented in a table as mean ± SD using Microsoft Excel version 365 (Microsoft Corporation, Redmond, WA). Correlational statistical analyses were performed using SPSS Statistics for Windows, version 26.0 (IBM Corp, Armonk, NY). The normal distribution of data for each variable was assessed using the Shapiro–Wilk test, assuming normality when p > 0.05. Levene’s test was applied to determine variance homogeneity between groups when p < 0.05. These results influenced the choice between parametric and nonparametric tests for variable comparison. The effects of lactic acid bacteria and GPA on the production parameters and intestinal histometry were analyzed using ANOVA and Tukey’s post hoc test or the nonparametric Kruskal–Wallis test. Both tests were conducted at a 95% confidence level, with significant differences at p < 0.05. Ethical approvalAll animal procedures performed in this study were performed according to the protocol for handling animals for research approved by the Bioethics Committee of the Veterinary Medicine and Zootechnics Faculty of UNHEVAL, Peru (N°52-2021-UNHEVAL-FMVZ). ResultsProduction parametersWeight gainAmong all treatments, the highest average WG was observed in T3 (641.67 ± 37.45 g) and the lowest in T1 (550.09 ± 39.70 g). However, statistical differences were only observed between T3 and T1 (p=0.04) (Table 1). Weekly feed conversion ratioNo significant differences were observed when comparing the effect of the applied treatments on WFCR (p=0.72). However, the best average value was found in guinea pigs from T2 (5.71 ± 1.81) (Table 1).
Fig. 1. Representative microphotograph (50×) demonstrating the intestinal morphology of Cavia porcellus guinea pigs. A transverse section of the jejunum stained with hematoxylin-eosin is shown, indicating intestinal villi HEIGHT (blue), considered from the base to the tip of the villus, villi WIDTH (red), measured in the in the middle area of each villus, and CRYPT of Lieberkühn DEPTH (green), located below the villi. These measurements were obtained in micrometers and were used for treatment comparison. Similar images were found in the rest of the evaluated fields of the other intestinal sections. Intestinal histometryVilla and crypt measurements are presented in Table 2. Intestinal villus heightSignificant differences were found in the height of each evaluated intestinal section (p < 0.05). In the duodenum, greater villus heights were detected at T1, T4, and T6, exceeding those at T2 and T3 (p < 0.05). Meanwhile, villi were taller in T5 than in T2 (p < 0.05). In the jejunum, villi in T4 were taller than those observed in T3, T5, and T6 (p < 0.05), and greater height was found in T2 than in T5 (p=0.02). Finally, in the ileum, villi in T4 were shorter than those in other treatments (p < 0.05), whereas in T2, the height was higher than in T5 (p < 0.05). Intestinal villus widthIn the experimental treatments, the measurement of villus width revealed differences in the duodenum (p < 0.05), jejunum (p < 0.05), and ileum (p < 0.05) compared with the control. Pairwise comparisons showed that the villi in the duodenum at T3 and T4 were wider than those at T1 (p < 0.05). Additionally, T4 had a greater width than T2 (p=0.01). Similarly, the intestinal villus width in the jejunum was greater in T3, T4, and the control group (T6) than in T1 (p < 0.05), and when comparing T6 and T2, the width was lower in the latter (p=0.03). Finally, in the ileum, the villus width in T1 was lower than in the other treatments (p < 0.05). Depth of Lieberkühn cryptsCrypt depth also showed significant differences in the three intestinal regions (p < 0.05). In the duodenum, T1 had the greatest depth compared with the other treatments (p < 0.05), and an elongation was found in T2 relative to T4 and T6 (p < 0.05). In the jejunum, probiotic application (T1–T4) resulted in greater crypt depth than in T5 (p < 0.05). Additionally, the control group showed less depth than T1 and T3 (p < 0.01). In the ileum, the crypts in T1 were the deepest (p < 0.05), while T4 and T6 had shallower depths than T3 (p < 0.05). Table 1. Effect of probiotic administration on WG and WFCR of guinea pig.
Villus height to crypt depth ratioThe VH:CD ratio evaluation revealed significant differences in the duodenum, jejunum, and ileum (p < 0.05). In the duodenum, VH:CD ratio had a higher value in T6 (p < 0.05) than at the other treatments, except at T4 (p=0.07). In addition, T2 had a lower ratio than T4 and T5 (p < 0.05). In the jejunum, a lower ratio was found in T3 compared with T4, T5, and T6 (p < 0.05), while T5 had a higher value than T1 (p=0.03). Finally, in the ileum, T1 showed the lowest VH:CD ratio compared with the other treatments (p < 0.05), and T3 had lower values than T6. DiscussionVarious studies have been conducted to evaluate the effect of probiotics on guinea pigs, although very few have achieved successful results (Barrera et al., 2022). Therefore, exploring new native bacteria with probiotic potential in farm animals will help develop more efficient probiotics that consistently improve production parameters. For this reason, the in vivo probiotic effect of lactic acid bacteria with probiotic potential, previously obtained from guinea pig feces (Goicochea-Vargas et al., 2024), has been evaluated, as there are no direct precedents for their application in guinea pigs. The community of bacteria essential for digestion resides primarily in the intestine; thus, understanding their impact on intestinal morphology and their relationship with host growth is of great interest. The consumption of probiotics can be highly beneficial because these microorganisms promote the digestion of complex molecules such as cellulose, amylose, and large proteins. This facilitates nutrient absorption (Amenyogbe et al., 2024), which enhances feed conversion. Additionally, along with their ability to inhibit pathogens, strengthen the immune system, and produce antimicrobial metabolites usable by the host (Raheem et al., 2021; Fijan, 2023; Evangelista et al., 2024), probiotics promote greater WG in animals. However, in this study, the addition of different probiotics did not show significant effects on WG. These results align with previous reports on guinea pigs after applying different doses of probiotics based on Lactobacillus and/or Saccharomyces (Ortiz, 2016; Canto et al., 2018; Andía and Ángeles, 2021) or a mixture of six native lactic acid bacteria species (Valdizán et al., 2019; Carcelén et al., 2021). Additionally, other studies agree with our findings, as they did not observe improvements in feed conversion ratio after applying different probiotic bacteria (Lactobacillus rhamnosus and Enterococcus faecium) (Guevara-Vasquez et al., 2021) or mixtures of different Lactobacillus species (Quijano et al., 2023). In the intestine, villi size is primarily related to the population of enterocytes, which are cells involved in enzymatic digestion, absorption, and nutrient transport (Awad et al., 2009; Chende et al., 2021). The proliferation of these cells can be increased by administering probiotics such as Lactobacillus (Preidis et al., 2012; Yan et al., 2017). This cell multiplication can be mediated by bacterial metabolites such as D8 and indole-3-aldehyde, which trigger a signaling pathway for STAT3 phosphorylation, accelerating the proliferation of intestinal epithelial cells (Gāliņa et al., 2020; Yan and Polk, 2020). In the present study, a positive effect on the height of intestinal villi in the jejunum was observed only when a mixture of three potential probiotic bacterial strains (T4) was applied. Regarding villi width, no probiotic strain could increase its value. On the contrary, some probiotic treatments reduced the villi height or width. The absence of a significant positive effect aligns with previous reports in which different probiotic strains added to guinea pig diets failed to increase villi height or width (Carcelén, 2021; Moreno, 2021; Sopla, 2024). However, the addition of multistrain probiotics in guinea pigs subjected to experimental infection, as well as the inclusion of prebiotics in the diet, has been reported to increase these dimensions (López, 2018; Carcelén, 2021). Table 2. Effect of probiotic supplementation on the height and width of intestinal villi, crypt depth of Lieberkühn, and villus height to crypt depth ratio of the small intestine of the guinea pig.
On the other hand, the crypts of Lieberkühn and their relationship with villi have been used as indicators to evaluate the effects of probiotics. Some probiotic strains can influence the stem cells located in these crypts, which are involved in the renewal of intestinal epithelial cells (Shaker and Rubin, 2010). This proliferation may be triggered by a high villus extrusion rate, immune factors, or inflammation caused by pathogenic microorganisms or toxins (Furlan et al., 2004; Rehman et al., 2007; Pearson and Brownlee, 2010). Our results indicate a trend toward crypt elongation or no differences compared with the control following the application of the probiotic strain. These findings are consistent with the absence of effects of administering the Biomodulator produced by Reinmark (E. hirae, L. reuteri, L. frumenti, L. johnsonii, S. thoraltensis, and B. pumilus) on crypt depth in guinea pigs (López, 2018). Additionally, reports not only from Moreno (2021) but also from Sopla (2024) indicate that adding a probiotic based on Bacillus amyloliquefaciens and Bacillus subtilis, respectively, increases crypt depth. However, other studies found a reduction in crypt depth after the application of the biomodulator commercialized by Reinmark (Carcelén, 2021). Furthermore, VH:CD ratio found in probiotic treatments was similar to or lower than that in the control treatment (T6). These findings align with López (2018), who found no differences between treatments, but differ from other studies showing a higher VH:CD ratio after applying the Biomodulator probiotic (Reinmark) in guinea pig diets (Puente et al., 2019; Carcelén et al., 2020). The impact of intestinal morphology on animal growth can be explained by the fact that a larger villi area implies a higher number of enterocytes and other epithelial cells. An increase in these cells can improve food digestibility and serve as an indicator of enhanced animal growth (Wang et al., 2019), as reported in fish (Hossain et al., 2022), poultry (Hashemitabar and Hosseinian, 2024), and pigs (Haupenthal et al., 2020). On the other hand, an increase in crypt depth is associated with cell proliferation for rapid villus renewal, but it can lead to high nutrient demand and lower growth efficiency (Hoste et al., 1988; Xu et al., 2003). However, if the crypt depth is high but the VH:CD ratio is also high, it would imply greater nutrient absorption (Xu et al., 2003). This result can be explained by the assumption of a high cell renewal rate combined with a reduced villus cell extrusion rate. In this study, the obtained results on production parameters align with intestinal villi and crypt measurements, as only a positive effect on jejunal villi height was found, which does not sufficiently contribute to improving guinea pig production parameters. Moreover, the use of probiotics in the diet promotes epithelial cell renewal without influencing villus growth, which may indicate animal stress or low levels of intestinal inflammation (Peuhkuri et al., 2010; Li et al., 2023). However, when GPA (T5) was used, similar findings were observed in villus growth due to minor or major changes in the microbiota caused by probiotics or antibiotics, which cannot be entirely attributed to stress or inflammation (Lobionda et al., 2019). The absence of the expected effects in this study after probiotic addition could be explained by the low colonization of these microorganisms in the intestinal tract. This may be due to the use of minimal probiotic concentrations in diet, as some studies report applying 108 (CFU)/mL or even higher doses to achieve an adequate response (Hadi et al., 2014; Nayel et al., 2019; Zhang et al., 2024). Additionally, since probiotics were administered orally, bacterial concentration may have been affected by low pH values in saliva and the stomach, making it difficult for a sufficient number of bacteria to reach the intestine. This issue can be overcome by adding complementary molecules to protect probiotic bacteria (Jumazhanova et al., 2023). Despite being native guinea pig bacteria, the intestinal microbiota of guinea pigs may present a challenging environment for the colonization of applied bacteria. Therefore, including prebiotics that support their growth could improve their effects, as reported in other studies (Carcelén et al., 2020). ConclusionIn conclusion, the application of probiotics based on E. gallinarum, Exiguobacterium sp., and L. lactis induced changes in the length and width of intestinal villi, as well as in the Lieberkühn crypts. However, these changes did not positively influence the improvement of productive parameters in guinea pigs. AcknowledgmentsThe authors would like to thank UNHEVAL for their support, along with the Kotosh Livestock Experimental Center, for the development of this project. They would also like to thank SENASA for collaborating with the preparation of the histological slides. Finally, they would like to thank UTC-Chile, UACH, and Incabiotec for their assistance in providing advice and analyzing the results. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research was supported by the Universidad Nacional Hermilio Valdizán, Perú, through the resolution N° 0003-2024-UNHEVAL-VRI. Authors’ contributionsAll authors participated in the drafting of the manuscript. JGV, MSA, FAP, and WRJ participated in the study design and conception. JGV, MSA, JCA, and CCM performed the tests and collected the required data. JGV, MSA FAC, MR, EM, and WRJ analyzed the collected data. All authors have revised and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAliakbarpour, H.R., Chamani, M., Rahimi., G., Sadeghi, A.A. and Qujeq, D. 2012. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. AJAS. 25(9), 1285. Amenyogbe, E., Droepenu, E.K., Ayisi, C.L., Boamah, G.A., Duker, R.Q., Abarike, E.D. and Huang, J.S. 2024. Impact of probiotics, prebiotics, and synbiotics on digestive enzymes, oxidative stress, and antioxidant defense in fish farming: current insights and future perspectives. Front Mar Sci. 11, 1368436. Ampuero-Riega, J. and Morales-Cauti, S. 2021. Determinación de residuos de antibióticos en músculo, hígado y riñón de cuyes comercializados en cuatro ciudades del Perú. Rev. Investig. Vet. Perú 32(1), e19508. Andía, V. and Ángeles, A.M. 2021. Efecto de alimento suplementado con una mezcla probiótica sobre los parámetros productivos de Cavia porcellus, cuy. Tayacaja 4(2), 13–21. Angulo-Tisoc, J.M., Jara, L.M., Pacheco, J.I. and Pezo, D. 2021. Frecuencia de agentes bacterianos asociados a mortalidad en cuyes de centros de crianza familiar-comercial en Canchis, Cusco. Rev. Investig. Vet. Peru. 32(3), e20415. Aphrodita, A., Sentono, D.N. and Fitria, L. 2024. Analysis of carcass weight and proximate composition as guinea pig [Cavia porcellus (Linnaeus, 1758)] meat quality indicator. BIO. Web. Conf. 94(06004), 9. Awad, W.A., Ghareeb, K., Abdel-Raheem, S. and Böhm, J. 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88(1), 49–56. Barrera B.M., Rada, D.A. and Mollericona, M.D. 2022. Efecto de los probióticos en el comportamiento productivo en cuyes (Cavia porcellus). Rev. Estud. AGRO - VET, 6, 71. Cáceres, F.C., Howard, F.S.M., Gómez, M.A., Quintana, S.B., Méndez, A.A., Ruiz-García, L., Sandoval-Monzón, R., Jiménez, R., Perales, R., Santillán, G. and Guevara, J. 2021. Inclusion of different levels of inulin on productive parameters and intestinal morphology in fattening guinea pigs (Cavia porcellus). Cienc. Rural 51(11), e20200961. Canto, F., Bernal, W. and Saucedo, J. 2018. Efecto de suplementación con probiótico (Lactobacillus) en dietas de alfalfa y concentrado sobre parámetros productivos de cuyes mejorados en crecimiento y engorde. Revista científica UNTRM. 1(2), 39–44. Carcelén, F., López, M., San Martín, F., Ara, M., Bezada, S., Ruiz-García, L., Sandoval-Monzón, R., López, S. and Guevara, J. 2021. Effect of probiotics administration at different levels on the productive parameters of guinea pigs for fattening (Cavia porcellus). Open Vet. J. 11(2), 222–227. Carcelén, F. 2021. Morfometría intestinal y desempeño productivo de cuyes (Cavia porcellus) de engorde suplementados con probiótico, prebiótico y simbiótico. Doctoral thesis, UNMSM, Lima, Peru. Carcelén, F., San Martín, F., Ara, M., Bezada, S., Asencios, A., Jimenez, R., Santillán, G., Perales, R. and Guevara, J. 2020. Efecto de la inclusión de diferentes niveles de probiótico sobre los parámetros productivos y morfología intestinal en cuyes de engorde (Cavia porcellus). Rev. Investig. Vet. Peru. 31(3), e18735. Chende, A., Martonos, C., Gal, A. F., Rus, V., Miclăuş, V., Pivariu, D., Vlasiuc, I., Andrei, S. and Damina, A. 2021. Anatomical, histological and histochemical features of the guinea pig (Cavia porcellus). Caecum Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Vet. Med. 78(1), 58–62. Enriquez, K.Y. 2019. Evaluación de la calidad de la carne de cuy (Cavia porcellus) suplementada con un simbiótico natural en la etapa de crecimiento. B. S. thesis, UNMSM, Lima, Peru. Evangelista, A.G., Nazareth, T.D.M., Luz, C., Dopazo, V., Moreno, A., Riolo, M., Meca, G. and Luciano, F.B. 2024. The probiotic potential and metabolite characterization of bioprotective Bacillus and Streptomyces for applications in animal production. Animals 14(3), 388. FAO/WHO. 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina: Food and Agriculture Organization of the United Nations; World Health Organization, pp: 1–4, 1–34. Fijan, S. 2023. Probiotics and their antimicrobial effect. Microorganisms 11(2), 528. Furlan, L.R., Macari, M. and Luquetti, B.C. 2004. Como avaliar os efeitos do uso de prebióticos, probióticos e flora de exclusão competitiva. In Simpósio técnico de incubação, matrizes de corte e nutrição, Balneário Camboriú. Anais, pp: 6–28. Gāliņa, D., Ansonska, L. and Valdovska, A. 2020. Effect of probiotics and herbal products on intestinal histomorphological and immunological development in piglets. Vet. Med. Int. 2020(1), 3461768. Goicochea-Vargas, J., Salvatierra-Alor, M., Acosta-Pachorro, F., Rondón-Jorge, W., Herrera-Briceño, A., Morales-Parra, E. and Mialhe, E. 2024. Genomic characterization and probiotic potential of lactic acid bacteria isolated from feces of guinea pig (Cavia porcellus). Open Vet. J. 14(2), 716. Guevara-Vásquez, J.E., Carcelén-Cáceres, F.D. and García-Zapata, T.D. 2021. Comportamiento productivo de cuyes (Cavia porcellus L.) en crecimiento suplementados con prebióticos y probióticos naturales. Cienc. Tecnol. Agropecuaria. 22(3), e1920. Hadi, J.A., Gutierrez, N., Alfaro, A.C. and Roberts, R.D. 2014. Use of probiotic bacteria to improve growth and survivability of farmed New Zealand abalone (Haliotis iris). N. Z. J. Mar. Freshwater. 48(3), 405–415. Hashemitabar, S.H. and Hosseinian, S.A. 2024. The comparative effects of probiotics on growth, antioxidant indices and intestinal histomorphology of broilers under heat stress condition. Sci. Rep. 14(1), 23471. Haupenthal, L.A., Caramori, J.G., Corrêa, G.D.S.S. and Silva, B.A.N. 2020. Oral supplementation of probiotics on the performance and gut histo-morphology of suckling piglets. Cienc. Rural. 50(10), e20190602. Herrera, E., Petrusan, J.I., Salvá-Ruiz, B., Novak, A., Cavalcanti, K., Aguilar, V., Heinz, V. and Smetana, S. 2022. Meat quality of Guinea pig (Cavia porcellus) fed with black soldier fly larvae meal (Hermetia illucens) as a protein source. Sustainability 14(3), 1292. Hill, D.R., Huang, S., Nagy, M.S., Yadagiri, V.K., Fields, C., Mukherjee, D., Bons, B., Dedhia, P.H., Chin, A.M., Tsai, Y., Thodla, S., Schmidt, T.M., Walk, S., Young, V.B. and Spence, J.R. 2017. Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6, e29132. Hossain, M.K., Hossain, M.M., Mim, Z.T., Khatun, H., Hossain, M.T. and Shahjahan, M. 2022. Multi-species probiotics improve growth, intestinal microbiota and morphology of Indian major carp mrigal Cirrhinus cirrhosus. Saudi J. Biol. Sci. 29(9), 103399. Hoste, H., Kerboeuf, D. and Parodi, A.L. 1988. Trichostrongylus colubriformis: effects on villi and crypts along the whole small intestine in infected rabbits. Exp. Parasitol. 67(1), 39–46. Imam, J., Hambolu, J.O., Onyeanusi, B.I., Ayo, J.O. and Sulaiman, M.H. 2021. Morphological and morphometric studies of the gastro-intestinal tract of the guinea pig (Cavia porcellus-Linnaeus, 1758). J. Vet. Anat. 14(1), 1–12. Jumazhanova, M., Kakimova, Z., Zharykbasov, Y., Kassymov, S., Zhumadilova, G., Muratbayev, A., Tasybayeva, M. and Suychinov, A. 2023. Effect of the encapsulation process on the viability of probiotics in a simulated gastrointestinal tract model medium. Processes 11(9), 2757. Kalita, A., Talukdar, M., Sarma, K., Kalita, P.C., Gautam, C., Choudhary, O.P., Doley, P.J., Keneisenuo, K. and Sarkar, R. 2021. Alterations of small intestinal morphology on villi and crypts after feeding probiotic and zinc in pre and post-weaned piglets. Indian J. Anim. Res. 55(10), 1167–1176. Kiela, P.R. and Ghishan, F.K. 2016. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 30(2), 145–159. Li, Y., Jia, D., Wang, J., Li, H., Yin, X., Liu, J., Wang, J., Guan, G., Luo, J., Yin, H., Xiao, S. and Li, Y. 2021. Probiotics isolated from animals in Northwest China improve the intestinal performance of mice. Front. Vet. Sci. 8, 750895. Li, Y., Wan, H., Ma, R., Liu, T., Chen, Y. and Dong, Y. 2023. Chronic stress that changed intestinal permeability and induced inflammation was restored by estrogen. Int. J. Mol. Sci. 24(16), 12822. Lobionda, S., Sittipo, P., Kwon, H.Y. and Lee, Y.K. 2019. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 7(8), 271. López, B.G. 2018. Efecto de la suplementación oral de una mezcla probiótica en cuyes (Cavia porcellus) de engorde desafiados con Salmonella typhimurium sobre la morfología intestinal. B.S. thesis, UNMSM, Lima, Peru. Mendoza-Rodriguez, N.J. 2022. Prevalencia de Salmonella spp. en cuyes en el Anexo de Roldan-distrito de Quilmaná-provincia de Cañete-región Lima. B. S. thesis, UNICA, Ica, Peru. Mitjans, M. and Ferrer, R. 2004. Morphometric study of the guinea pig small intestine during development. Microsc. Res. Tech. 63(4), 206–214. Moreno, E.J. 2021. Uso de la bacteria probiótica Bacillus amyloliquefaciens en el alimento, sobre la respuesta productiva y morfometría intestinal del Cuy. B.S. thesis, UNALM, Lima, Perú. Mousa, W.K., Mousa, S., Ghemrawi, R., Obaid, D., Sarfraz, M., Chehadeh, F. and Husband, S. 2023. Probiotics modulate host immune response and interact with the gut microbiota: shaping their composition and mediating antibiotic resistance. Int. J. Mol. Sci. 24(18), 13783. Nayel, U.A., Baraghit, G.A., Ahmed, B.M., and Elmeshtawy, M.A. 2019. Suckling calves performance and immune status as affected by Lactococcus lactis as a probiotic source. MJAPFP. 3(5), 119–133. Obianwuna, U.E., Qiu, K., Wang, J., Zhang, H.J., Qi, G.H., Huang, L.L. and Wu, S.G. 2023. Effects of dietary Clostridium butyricum and fructooligosaccharides, alone or in combination, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function, and antioxidant capacity of laying hens. Front. Microbiol. 14, 1125897. Obregón, R., Serrano-Martínez, E. and Chauca-Francia, L.J. 2018. Causas de mortalidad neonatal en cobayos (Cavia porcellus) durante la estación fría en el Instituto Nacional de Innovación Agraria, Lima-Perú. Salud Tecnol. Vet. 2, 93–99. Ortiz, J. 2016. Lactobacillus spp. como aditivo sobre parámetros productivos en cuy (Cavia porcellus). B. S. thesis, URP, Lima, Peru. Pearson, J.P. and Brownlee, I.A. 2010. The interaction of large bowel microflora with the colonic mucus barrier. J. Inflamm. 2010(1), 321426. Peuhkuri, K., Vapaatalo, H. and Korpela, R. 2010. Even low-grade inflammation impacts on small intestinal function. World J. Gastroenterol. 16(9), 1057. Preidis, G.A., Saulnier, D.M., Blutt, S.E., Mistretta, T.A., Riehle, K.P., Major, A.M., Venable, S.F., Finegold, M.J., Petrosino, J.F., Conner, M.E. and Versalovic, J. 2012. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB. 26(5), 1960. Puente, J., Carcelén, F., Ara, M., Bezada, S., Huamán, A., Santillán, G., Perales, R., Guevara, J. and Asencios, A. 2019. Efecto de la suplementación con niveles crecientes de probióticos sobre la histomorfometría del intestino delgado del cuy (Cavia porcellus). Rev. Investig. Vet. Perú. 30(2), 624–633. Quijano, W., Andia, V. and Peña, G. 2023. Efecto de la suplementación con bacterias ácido-lácticas sobre los parámetros productivos en cuyes (Cavia porcellus) de engorde, Ayacucho-Perú. Rev. Vet. 34(2), 101–105. Raheem, A., Liang, L., Zhang, G. and Cui, S. 2021. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 12, 616713. Rehman, H., Rosenkranz, C., Böhm, J. and Zentek, J. 2007. Dietary inulin affects the morphology but not the sodium-dependent glucose and glutamine transport in the jejunum of broilers. Poult. Sci. 86, 118–122. Shaker, A. and Rubin, D.C. 2010. Intestinal stem cells and epithelial–mesenchymal interactions in the crypt and stem cell niche. Transl. Res. 156(3), 180–187. Sopla, H. 2024. Uso de probióticos y ácido butírico en el comportamiento productivo de cuyes (Cavia porcellus) región Amazonas. Master thesis, UNTRM, Amazonas, Perú. Valdizán, C., Carcelén, F., Ara, M., Bezada, S., Jiménez, R., Asencios, A. and Guevara, J. 2019. Efecto de la inclusión de probiótico, prebiótico y simbiótico en la dieta sobre los parámetros productivos del cuy (Cavia porcellus). Rev. Investig. Vet. Peru. 30(2), 590–597. Valdizán, C. 2018. Efecto de la inclusión de probiótico, prebiótico y simbiótico en la dieta del cuy (Cavia porcellus) sobre parámetros productivos. Doctoral thesis, UNMSM, Lima, Peru. Walton, K.D., Freddo, A.M., Wang, S. and Gumucio, D.L. 2016. Generation of intestinal surface: an absorbing tale. Development 143(13), 2261–2272. Wang, L., Yan, S., Li, J., Li, Y., Ding, X., Yin, J., Xiong, X., Yin, Y. and Yang, H. 2019. Rapid communication: the relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. Anim. Sci. J. 97(1), 353–358. Wang, X.L., Liu, Z.Y., Li, Y.H., Yang, L.Y., Yin, J., He, J.H., Hou, D.X., Liu, Y.L. and Huang, X.G. 2021. Effects of dietary supplementation of Lactobacillus delbrueckii on gut microbiome and intestinal morphology in weaned piglets. Front. Vet. Sci. 8, 692389. Xu, Z.R., Hu, C.H., Xia, M.S., Zhan, X.A. and Wang, M.Q. 2003. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 82(6), 1030–1036. Yan, F. and Polk, D.B. 2020. Probiotics and probiotic-derived functional factors—mechanistic insights into applications for intestinal homeostasis. Front. Immunol. 11, 1428. Yan, F., Liu, L., Cao, H., Moore, D.J., Washington, M.K., Wang, B., Peek, R.M., Acra, S.A. and Polk, D.B. 2017. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal. Immunol. 10(1), 117–127. Yang, J., Yang, Y., Ishii, M., Nagata, M., Aw, W., Obana, N., Tomita, M., Nomura, N. and Fukuda, S. 2020. Does the gut microbiota modulate host physiology through polymicrobial biofilms? Microbes Environ. 35(3), ME20037. Zhang, M., Feng, Y., Zhong, Z., Du, Q., Yu, W., Wu, J., Huang, X., Huang, Z., Xie, G. and Shu, H. 2024. Host gut-derived probiotic, Exiguobacterium acetylicum G1-33, improves growth, immunity, and resistance to Vibrio harveyi in hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Microorganisms. 12(8), 1688. Zhao, Z., Sun, M., Cui, X., Chen, J., Liu, C. and Zhang, X 2023. Bacillus coagulans MZY531 alleviates intestinal mucosal injury in immunosuppressive mice via modulating intestinal barrier, inflammatory response, and gut microbiota. Sci. Rep. 13(1), 11181. Zuo, Z.H., Shang, B.J., Shao, Y.C., Li, W.Y. and Sun, J.S. 2019. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 86, 160–168. | ||
| How to Cite this Article |
| Pubmed Style Goicochea-vargas J, Salvatierra-alor M, Acosta-pachorro F, Rondón-jorge W, Caro-magni C, Cajacuri-aquino J, Morales-parra E, Mialhe E, Silva M, Ratto M. Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Vet. J.. 2025; 15(7): 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 Web Style Goicochea-vargas J, Salvatierra-alor M, Acosta-pachorro F, Rondón-jorge W, Caro-magni C, Cajacuri-aquino J, Morales-parra E, Mialhe E, Silva M, Ratto M. Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). https://www.openveterinaryjournal.com/?mno=251515 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.8 AMA (American Medical Association) Style Goicochea-vargas J, Salvatierra-alor M, Acosta-pachorro F, Rondón-jorge W, Caro-magni C, Cajacuri-aquino J, Morales-parra E, Mialhe E, Silva M, Ratto M. Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Vet. J.. 2025; 15(7): 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 Vancouver/ICMJE Style Goicochea-vargas J, Salvatierra-alor M, Acosta-pachorro F, Rondón-jorge W, Caro-magni C, Cajacuri-aquino J, Morales-parra E, Mialhe E, Silva M, Ratto M. Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 Harvard Style Goicochea-vargas, J., Salvatierra-alor, . M., Acosta-pachorro, . F., Rondón-jorge, . W., Caro-magni, . C., Cajacuri-aquino, . J., Morales-parra, . E., Mialhe, . E., Silva, . M. & Ratto, . M. (2025) Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Vet. J., 15 (7), 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 Turabian Style Goicochea-vargas, José, Max Salvatierra-alor, Fidel Acosta-pachorro, Wilson Rondón-jorge, Cesar Caro-magni, Julissa Cajacuri-aquino, Edson Morales-parra, Eric Mialhe, Mauricio Silva, and Marcelo Ratto. 2025. Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Veterinary Journal, 15 (7), 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 Chicago Style Goicochea-vargas, José, Max Salvatierra-alor, Fidel Acosta-pachorro, Wilson Rondón-jorge, Cesar Caro-magni, Julissa Cajacuri-aquino, Edson Morales-parra, Eric Mialhe, Mauricio Silva, and Marcelo Ratto. "Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus)." Open Veterinary Journal 15 (2025), 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 MLA (The Modern Language Association) Style Goicochea-vargas, José, Max Salvatierra-alor, Fidel Acosta-pachorro, Wilson Rondón-jorge, Cesar Caro-magni, Julissa Cajacuri-aquino, Edson Morales-parra, Eric Mialhe, Mauricio Silva, and Marcelo Ratto. "Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus)." Open Veterinary Journal 15.7 (2025), 2972-2981. Print. doi:10.5455/OVJ.2025.v15.i7.8 APA (American Psychological Association) Style Goicochea-vargas, J., Salvatierra-alor, . M., Acosta-pachorro, . F., Rondón-jorge, . W., Caro-magni, . C., Cajacuri-aquino, . J., Morales-parra, . E., Mialhe, . E., Silva, . M. & Ratto, . M. (2025) Effect of dietary inclusion of indigenous probiotics on the growth and intestinal histomorphology of guinea pigs (Cavia porcellus). Open Veterinary Journal, 15 (7), 2972-2981. doi:10.5455/OVJ.2025.v15.i7.8 |