| Research Article | ||
Open Vet. J.. 2025; 15(7): 3024-3034 Open Veterinary Journal, (2025), Vol. 15(7): 3024-3034 Research Article Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattleSri Mulyati1*, Imam Mustofa1, Pudji Srianto1, Aswin Rafif Khairullah2, Fedik Abdul Rantam3, Adeyinka Oye Akintunde4, Tita Damayanti Lestari1, Lili Anggraini5, Riza Zainuddin Ahmad2, Latifah Latifah5, Bima Putra Pratama6 and Ulvi Fitri Handayani51Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo State, Nigeria 5Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Research Center for Agroindustry, National Research and Innovation Agency (BRIN), Tangerang, Indonesia *Corresponding Author: Sri Mulyati. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga. Surabaya, Indonesia. Email: sri-m [at] fkh.unair.ac.id Submitted: 22/04/2025 Revised: 03/06/2025 Accepted: 04/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
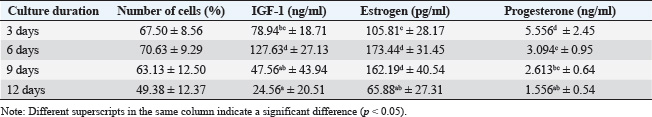
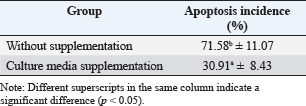
ABSTRACTBackground: Ovaries have the ability to produce insulin-like growth factor-1 (IGF-1) and estrogen, but both of these factors can also be produced through monolayer cell cultures of cumulus oophorus tissue with the addition of precursor materials such as fetal calf serum (FCS) or bovine serum albumin (BSA). Aim: This study aimed to determine the bioactivity of IGF-1 and estrogen obtained from bovine cumulus cells in the context of in vitro fertilization (IVF). Methods: Cumulus cells were harvested from ovarian follicles measuring 2–6 mm and cultured in TCM 199 supplemented with 10% FCS and 10% BSA. The IGF-1, estrogen, and progesterone levels were quantified using the immunoradiometric assay and radioimmunoassay techniques. To lower progesterone concentrations within the culture, a binding technique using antiprogesterone (anti-P4) coated tubes was applied. The culture media served as both a supplement for IVF and an environment for embryo culture. The study assessed the mitogenic and antiapoptotic properties of IGF-1 and estrogen by measuring the reduction of apoptosis in embryos. Findings revealed that incubating for 6 days resulted in the greatest cell monolayer confluency. Results: The anti-P4 effectively reduced progesterone levels in the culture of cumulus cells. The cleavage rates for embryos with and without progesterone absorption were 27.48% and 53.61%, respectively, whereas the rates of morula embryos increased from 5.73% to 27.59%. Additionally, supplementation decreased apoptosis rates from 70.58% to 31.01%. Conclusion: In summary, the monolayer culture of bovine cumulus cells generated IGF-1 and estrogen, which functioned as mitogenic agents, positively influencing IVF outcomes and embryo development while significantly decreasing apoptosis in the embryos. Keywords: Abattoir waste product, Apoptosis, Food production, Ovarian, Morula. IntroductionThe growth of the cattle population in Indonesia plays a crucial role in supporting the Free Nutritious Meals initiative, which aims to improve the health and nutrition of communities, especially children and vulnerable groups (Hilmiati et al., 2024). Beef is a source of animal protein rich in nutrients, such as iron, vitamin B12, and essential amino acids that are essential for optimal growth and development (Geiker et al., 2021). By increasing the cattle population, Indonesia can ensure the availability of sufficient beef to meet the nutritional needs of the community. The Free Nutritious Meals initiative will be able to provide appropriate portions of beef, thereby contributing to increasing individual protein and nutrient intake (Sarjito, 2024). In addition, increasing the cattle population can reduce the dependence on imported meat. Indonesia, as a country with a large population, faces challenges in meeting its meat needs (Kusumaningrum et al., 2024). By developing local livestock farming, Indonesia can achieve food independence that supports price stability and product quality, which is very important for the sustainability of nutritious food supplies (Rozaki, 2021). In addition, the government must ensure that an increase in the cattle population has a positive impact on the welfare of local farmers (Fathan et al., 2024). Supporting farmers in investing in better livestock farming can improve their incomes and quality of life (Banda and Tanganyika, 2021). Therefore, Free Nutritious Meals not only provide nutritional benefits but also help improve the economy of livestock farming communities through the demand for local beef. The program also raises awareness of the importance of local products. By emphasizing domestic food products, people will recognize and appreciate products from local farms more (Hilmiati et al., 2024). This will contribute to the development of a more sustainable livestock industry, as well as having a positive impact on the environment and agricultural ecosystems. Collaboration between the government, farmers, and communities is essential in supporting the Free Nutritious Meals initiative (Sarjito, 2024). Policies that support increasing cattle populations, education on good livestock practices, and access to modern technology will form an ecosystem that supports the success of the program (Martyniuk, 2021). Thus, increasing the cattle population in Indonesia is an important step in line with the goal of creating free nutritious meals. This not only helps the availability of nutritious food but also contributes to food independence, farmer welfare, and sustainable local economic development. In terms of improving livestock quality, the application of biotechnology is very relevant. An effective method to improve reproduction is through embryo transfer techniques (D’Angelo et al., 2022). The success of this technique depends on the quality of the embryos produced; however, in vivo embryo production is limited by the capacity of donor female livestock (Salek et al., 2025). Therefore, embryo production using in vitro methods is a promising alternative. Growth factors such as insulin-like growth factor-1 (IGF-1) and estrogen hormones play an important role in stimulating and regulating cell growth (Ipsa et al., 2019). IGF-1 is a polypeptide produced by the liver and other tissues in response to growth hormone, whereas estrogen is produced by follicles in the ovaries (Khan et al., 2025). Both have paracrine, autocrine, and endocrine functions in stimulating mitogenic activity in cells. The ovaries have the ability to produce IGF-1 and estrogen, but both of these factors can also be produced through monolayer cell cultures of cumulus oophorus tissue with the addition of precursor materials such as fetal calf serum (FCS) or bovine serum albumin (BSA) (Vandaele and Van Soom, 2011). Based on this, there is still a rare research on the production of growth factors and sex steroids, such as estrogen and progesterone, from cumulus cell monolayer culture fluid using cow ovaries from slaughterhouses. This study aimed to determine the bioactivity of IGF-1 and estrogen obtained from cow cumulus cells in the context of in vitro fertilization (IVF). Materials and MethodsResearch designThe study was conducted from April 2024 to September 2024. Cow ovary samples were collected from the Pegirian Slaughterhouse in Surabaya. Monolayer culture and IVF were performed at the Bovine IVF Laboratory, Veterinary Reproduction Division, Faculty of Veterinary Medicine, Airlangga University. Examination of IGF-1, estrogen, and progesterone levels at the Endocrinology Laboratory, Veterinary Reproduction Division, Faculty of Veterinary Medicine, Airlangga University. Research replication was conducted with six repetitions for each pteridis (research group). Maturation and embryo culture media were made in the form of drops with a volume of 50 µl/drop, with as many as five drops for each pteridis (research group). Each drop contained 4–5 oocytes with cumulus oocyte complex (COC) or mature oocytes. Each research group contained 20–25 fertile embryos whose development was observed for 2 days (2–8 cell stage) and 6 days (Morula stage). Research sampleCumulus cell culture was performed by taking six times the number of cow ovaries, each containing 20–30 ovaries. A number of ovaries from each ovary taking, cumulus cells from follicle aspiration are divided into six parts (as a repeat) for making monolayer cultures. In IVF, six times of cow ovaries are taken, each containing 20–30 ovaries. A number of ovaries from each ovary taking, cumulus oocyte complexes (COCs) from follicle aspiration, are divided into six parts (as a repeat) in the treatment without absorption or with absorption, without supplementation or with supplementation of monolayer culture media (for apoptosis examination), each containing 6–10 COCs in a drop containing in vitro oocyte maturation media. Research stagesThe study was conducted in several stages. In the first stage, the main focus was the preparation and collection of the cumulus cell monolayer culture fluid. Cumulus cells with diameters between 2 and 6 mm were taken from the follicle aspiration. After collection, the cumulus cells were cultured in TCM 199 medium mixed with 10% FCS and 10% BSA. The cell concentration in the culture was set at 1.9 × 106 cells/ml and then incubated in incubator conditions at 38.5°C and 5% CO2 for 3, 6, 9, and 12 days. The next stage focuses on measuring IGF-1 levels in culture fluid using the immunoradiometric assay (IRMA) method. Simultaneously, estrogen and progesterone levels were measured using the radioimmuno assay (RIA) technique. In the third stage, the process of separation or absorption of progesterone, which acts as an antimitogenic agent, from IGF-1 and estrogen, which act as mitogenic agents, is carried out. This separation process is performed by binding progesterone using antiprogesterone (anti-P4) placed in a polystyrene tube coated with anti-P4. The bioactivity of the IGF-1 and estrogen growth factors produced from the study was tested as a media supplement in IVF and embryo culture. Testing was performed by monitoring the cleavage and development of the embryo until it reached the morula stage 6 days after IVF. The fourth stage includes further testing of the bioactivity of IGF-1 and estrogen to determine their mitogenic and antiapoptotic properties. The goal of this test is to detect a decrease in apoptosis in embryos produced by IVF. Cumulus culture is makingCow ovaries taken from the slaughterhouse were cleaned, soaked in Dulbecco’s Phosphate-buffered saline (PBS) solution, placed in a plastic bag, and placed into a thermos filled with warm water at a temperature of 36°C–37°C. The ovaries were then taken to the laboratory for the cell culture process. In the laboratory, the ovaries were cleaned of hanging tissue and washed with PBS solution. To obtain cumulus cells, follicle aspiration was performed using a 10-ml syringe and an 18-G needle. Aspiration was performed on follicles with a diameter of between 2 and 6 mm, through the medulla of the ovary, and repeated several times without removing the needle from the ovary. The aspirated fluid was collected in a glass tube and then centrifuged at 3,000 rpm for 10 minutes. After the centrifugation process, the supernatant was discarded, and the remaining sediment (pellet) was washed by adding 3 ml of TCM 199 containing 10% FCS and then centrifuged once more at the same speed and time. The supernatant was discarded again, and the cells were made into a suspension by adding TCM 199 containing 10% FCS at a ratio of (1:1). Subsequently, dilution was performed by adding TCM 199 containing 10% FCS at a ratio of (1:9), and the number of cells in each ml of media was measured. The cell concentration was adjusted to 1.9 × 106 cells/ml. Next, cell culture was carried out by taking 50 μl of the cell suspension and placing it into five places in a 36-mm Petri dish, where each place was given 50 μl of culture media consisting of TCM 199 and 10% FCS. The total volume in each Petri dish was 500 μl of cell suspension and culture media, which were then incubated at 38.5°C in an atmosphere containing 5% CO2. Every day, the cell culture was observed, shaken gently, and the media was replaced every 3 days after 90 μl for each place from the five places in the Petri dish, so that the total cell product that could be collected from each Petri dish was 450 μl until a confluent single-layer cell culture was formed. Monolayer cell harvesting was performed on days 3, 6, 9, and 12. IGF-1, estrogen, and progesterone examinationMeasurement of IGF-1 concentration was measured in cumulus cell monolayer culture products after incubation for 3, 6, 9, and 12 days using the IRMA method. On the other hand, estrogen and progesterone hormone levels were measured on days 3, 6, 9, and 12 by applying the solid-phase RIA technique (Skenandore et al., 2017). The samples used for both analyses were fluids from the cumulus cell culture media. The bioactivity test of growth factors (IGF-1) and estrogen hormones as mitogenic materials was carried out by adding 40% of the cumulus cell monolayer culture fluid (after being absorbed with anti-P4) into the fertilization culture medium and the embryo culture medium resulting from IVF, incubated for 2 days after fertilization (stage 2–8 cells); then, the number of embryos that developed into the Morula stage was calculated after 6 days of incubation after fertilization. For the progesterone separation or absorption procedure, a polystyrene tube coated with carbonate buffer (pH 9.2) was prepared first. This buffer functions to help the bond between anti-P4 and the inner surface of the tube. Then, 100 µl of anti-P4 was added to the tube, mixed with a vortex for 1 minute, and left for 24 hours at room temperature, or 3 hours in a 5% CO2 incubator at 38.5°C. After this process, the remaining liquid was removed by washing the tube with 2 ml of deionized water. The tube was cleaned by inverting it on tissue paper and then covering it to prevent moisture from entering. If not used immediately, the tube can be stored in a refrigerator for up to 2–3 months. Next, 100 µl of the monolayer culture fluid was added to the tube and reincubated in a 5% CO2 incubator at 38.5°C. This process provides an opportunity for progesterone to bind to anti-P4. Incubation was carried out twice, each time for 3 hours. In vitro bioactivity testing of cumulus cell monolayer cultures in the cleavage and development of bovine embryosThe cow embryos for which development was examined were embryos created through IVF. In vitro oocyte collection and maturationOocytes were collected from the ovaries of cows slaughtered at the Pegirian Slaughterhouse in Surabaya and immediately transported to the laboratory for IVF. The oocytes were then soaked in a physiological NaCl solution enriched with antibiotics (penicillin and streptomycin) at 37°C in a thermos provided. Upon arrival at the laboratory, the ovaries were placed in a glass beaker and washed with physiological NaCl solution before being soaked again in the same solution. The ovaries were then placed in a water bath at 30°C. The ovaries were then taken one by one using tweezers, and the surface was dried with sterile tissue paper. Oocytes were obtained by aspirating follicular fluid from follicles measuring 2–6 mm, using a 10 ml disposable syringe and an 18G needle. The follicular fluid was carefully collected in a test tube to avoid damage to the cumulus cells and then sedimented in a water bath at 30°C for 15 minutes. After this process, the liquid at the bottom was poured into a Petri dish and examined under a bisecting microscope at 100× magnification. The oocytes selected were those with complete cumulus cells and were then washed twice using oocyte washing solution (OWS) and once with TCM 199 containing 10% FCS and gonadotropin [Follicle-stimulating hormone (FSH)/Luteinizing hormone (LH)]. The oocyte maturation process uses TCM 199 media, which is formed into drops with a volume of 50 µl per drop, with as many as five drops in a Petri dish. The drops were then covered with mineral oil and placed in a CO2 incubator with 95% humidity for at least 2 hours before being used for oocyte maturation. After the oocytes are washed once with OWS and once with TCM 199, 4–5 oocytes that have complete cumulus cells are inserted into the drops of maturation media and incubated for 24 hours in a 5% CO2 incubator with 95% humidity at a temperature of 38.5°C. Sperm preparationSperm used for IVF were prepared using the swim-up method. The first step was to thaw the frozen semen by immersing it in a hot water bath at 30°C for 30–60 seconds. After the semen had thawed, the liquid was poured into a 15 ml sterile centrifuge tube. Next, 3 ml of Earle’s Balanced Salt Solution (EBSS) medium was slowly added on top of the semen (0.5 ml) before centrifugation at 1,800 rpm for 10 minutes. After the centrifugation process, approximately 1 ml of the supernatant was carefully removed using a pipette. The motile spermatozoa cell sediment was then resuspended in 2 ml of EBSS medium and centrifuged again at the same speed for 10 minutes, at which point the supernatant was discarded again. After that, spermatozoa were resuspended in 2 ml of EBSS medium once more and incubated in a laminar flow chamber, with the tube tilted at a 45° angle at 37°C for 20 minutes. This process allows motile spermatozoa to swim upward. After the incubation period, sperm were collected from the upper third of the tube and placed in the previously prepared rosette medium. As a final step, the medium was incubated in an incubator with 5% CO2 and 95% air pressure at 38.5°C for 30 minutes for the capacitation process. IVF and embryo developmentIVF was performed using EBSS medium. Mature oocytes are transferred into EBSS medium formed into drops or rosettes, where each drop has a volume of 25 µl and contains 25 µl of a spermatozoa suspension placed in the center of the rosette. The oocytes are then placed in the drops on the edge, with each drop containing 4–5 cumulus oocyte complexes (COCs) or mature oocytes. Furthermore, the drops were covered with liquid paraffin or mineral oil and incubated in an incubator with 5% CO2 and 95% air pressure at a temperature of 38.5°C for 24 hours. After the incubation process, observations were made to determine whether fertilization had occurred. After fertilization, the oocytes were transferred to a medium consisting of cumulus cell monolayer culture fluid, TCM 199, and 10% FCS. The medium was then incubated in an incubator at 5% CO2 and 95% air pressure at a temperature of 38.5°C. After 48 hours (2 days), observations were made to determine whether cleavage had occurred in the embryo. The process of observing embryo development was carried out by transferring cow embryos produced from IVF and having undergone cleavage at the 2–8 cell phase into a petri dish containing cumulus cell culture fluid, TCM 199, and 10% FCS. The embryos were then incubated in an incubator with 5% CO2, 95% air pressure, and a temperature of 38.5°C. Embryo development was observed daily, while the media were replaced every 3 days. The final assessment of the embryo was carried out after the embryo reached the morula phase, which was 6 days after fertilization. Apoptosis examination using the tunnel assayThe bioactivity test of cumulus cell monolayer culture was conducted to evaluate the occurrence of apoptosis in bovine embryos produced through IVF using a TUNEL Assay and Apoptosis Detection Kit. IVF embryos at the two-cell to morula stage were taken using a micropipette and placed on a glass object (microslides) that had been coated with Poly-L-Lysine. The glass object was then covered with a cover glass, and both sides were glued with liquid paraffin. The embryos were then fixed in 4% paraformaldehyde or 10% formalin solution. This process was left for several days at 4°C to ensure that the embryos were completely attached to the glass object. If the embryos had not attached properly, the slides were stored at 37°C until complete attachment and drying. After the embryo was successfully attached to the glass object, the slide was washed with PBS solution by flowing and sucking the PBS solution around the cover glass using tissue or filter paper. After washing, the embryo was dripped with pronase for 30 minutes at 37°C and washed with PBS three times. Next, proteinase K 200 μg/ml was added for 30 minutes at room temperature, followed by washing with PBS three times. Then, 3% H2O2 in PBS was added and left for 15 minutes at room temperature, before being washed once with PBS. After that, give DNA labeling solution as much as 50 μl for 1–1.5 hours at 37°C and wash with PBS once again. The glass object was then covered with blocking buffer (BSA) 100 μl for 10 minutes at room temperature. Add 100 μl of antibody solution in a dark room, cover the slide with aluminum foil, and let it sit for 1–1.5 hours at room temperature before washing with PBS once. Next, add 100 μl of blocking buffer (BSA) and conjugate solution for 30 minutes at room temperature, and wash with PBS once. After that, followed by washing with dH2O, adding DAB solution for 15 minutes at room temperature, and washing with PBS once, ending with dH2O. After all the processes are complete, the slide is dried and examined under an inverted microscope. To calculate the results, perform a counterstain by dropping 100 μl of methyl green counterstain solution and storing it for 3 minutes at room temperature and then re-examine it under an inverted microscope. Data analysisBefore statistical analysis, the collected data were tested for normality using the one-sample Kolmogorov–Smirnov method and for homogeneity using Levene’s test. Data analysis for the first, second, and fourth stages was conducted using the univariate analysis of variance (ANOVA), while for the third stage, the use of the unpaired t-test (independent samples t-test) was applied. In the fifth stage, the analysis was conducted using one-way ANOVA. After all the analysis, to determine whether there was a significant difference, the high significant difference test at a significance level of 5% was applied. All statistical calculations were performed using SPSS 14.0 for Windows. Ethical clearanceThis research was approved by the ethics commission of Airlangga University Faculty of Dental Medicine Health Research Ethical Clearance with certificate number: 1119/HRECC.FODM/IX/2023. ResultsSix-day-old cumulus cell monolayer culture produced the highest percentage of cell numbers, with the highest levels of IGF-1 and estrogen (p < 0.05). However, the highest levels of progesterone in the culture medium were observed in 3-day-old cultures (Table 1). Embryos cultured in media with the addition of cumulus cell culture media with progesterone absorption produced a higher percentage of development of 2–8 cell stage embryos (day 2 of culture) and morula stage embryos (day 6 of culture) (p < 0.05) compared with culture in media with the addition of cumulus cell culture without progesterone absorption (Table 2 and Fig. 1). Embryos cultured in media with the addition of cumulus cell culture media with progesterone absorption produced a lower percentage of apoptosis (p < 0.05) compared with culture in media without the addition of cumulus cell culture (Table 3 and Fig. 2). DiscussionCumulus cells play an important role in oocyte maturation in vitro, which has implications for the quality of the resulting embryo (Turathum et al., 2021). If cumulus cells are released before the oocyte reaches maturity, the oocyte maturation process can be hampered or even not occur at all (Anazawa et al., 2025). The presence of cumulus cells is essential for supporting transcription and protein synthesis before germinal vesicle breakdown occurs in sheep and cow oocytes (Farin et al., 2007). Oocytes equipped with full cumulus cells usually show more optimal development than oocytes lacking cumulus cells (Karl et al., 2023). Cumulus cell expansion plays a significant role in facilitating the release of the cumulus oocyte complex (COC) from the follicle wall, which is important for the ovulation process and accelerates the acrosome reaction in spermatozoa (Di Giacomo et al., 2016). In the in vitro maturation (IVM) phase, cumulus cell expansion is influenced by gonadotropin hormones and contributes to the improvement of fertilization ability and future embryo development (Widayati and Pangestu, 2020). Thus, cumulus cell expansion is one of the criteria used to evaluate the success of the IVM process and serves as a basis for selecting oocytes for IVF. Development of culture cells and bioactive material yieldsIn this study, the 3-day culture period produced only a few cells that attached, grew, and developed at the bottom of the Petri dish; thus, the number of monolayer cells obtained remained low. In contrast, in the culture that lasted for 6 days, there was the most significant increase in the number of cells compared with the other culture durations. The 6-day culture duration was considered the optimal duration because at that time, the cells could cover the entire surface of the bottom of the Petri dish where they were attached. However, in the 9-day culture, the cells did not have enough space to grow at the bottom of the Petri dish. After the entire surface of the dish was covered with cells, new cell growth did not get a place to attach, so the cells could not grow and develop properly. The result of this is the possibility of cell death. These results are in line with previous research conducted by Pemayun et al. (2011) on the creation of monolayer cultures of cumulus cells and fallopian tube epithelial cells in Bali cattle to obtain IGF-1, estrogen, and progesterone. IGF-1, estrogen, and progesterone concentrations in cumulus cell monolayer culture resultsGrowth factors are polypeptides that function similarly to hormones and act as paracrine or autocrine in stimulating mitogenic activity in developing tissues (Farooq et al., 2021). IGF, also known as somatomedin C, is one type of polypeptide growth factor secreted by cumulus cells in response to growth hormone (Ipsa et al., 2019). IGF consists of single-chain peptides, where IGF-1 has 70 amino acids and IGF-2 has 67 amino acids (LeRoith et al., 2021). Both proteins play a role in regulating the proliferation and differentiation of various cell types. The main source of IGF is hepatocyte cells, but several other tissues can also produce IGF (Kineman et al., 2018). Estrogen, which belongs to the sex steroid hormone group, is mainly produced by ovarian follicles at various stages of development, from primordial to de Graaf, as well as by the adrenal glands and placenta (Xu et al., 2022). Estrogen production is influenced by the hormones Follicle-stimulating hormone and LH secreted by the anterior pituitary (Mitwally et al., 2005). In addition, other intrafollicular factors such as IGF-1 and gonadotropins also play a role in influencing estrogen production (Orisaka et al., 2021). On the other hand, progesterone is a steroid hormone produced mainly by corpus luteum cells, the placenta, and the adrenal glands, and its secretion is highly dependent on the estrus cycle (Kolatorova et al., 2022). Table 1. Percentage of the number of cumulus cells in culture, IGF-1, estrogen, and progesterone levels in the culture medium.
Table 2. Percentage of embryo development without and with progesterone absorption.
Fig. 1. Results of the development of bovine embryos cultured in media containing IGF-1 and estrogen at the 8-cell (A), 16-cell (B), and morula (C) stages. Magnification 200×. In this study, IGF-1, estrogen, and progesterone were detected in monolayer cell culture products from bovine cumulus cells. Cumulus cells not only function as nutrient providers during the processes of oogenesis and folliculogenesis in the ovaries, but they are also able to produce IGF-1 (Piau et al., 2023). Estrogen is a C18 steroid that does not have an angular methyl group at position 10 or a ∆4-3-keto configuration on the A ring, with a molecular weight of approximately 272 Da (Dembitsky, 2024). During the productive phase, the main form of estrogen found in the blood circulation is estradiol-17β, which is produced at a rate of 80–500 µg per day (Reslan and Khalil, 2012). In the blood, estradiol is found in a free state of 2%, 60%, and 38% bound to albumin, gonadal steroid-binding globulin (Hammond, 2011). Sex hormone-binding globulin (SHBG), a glycoprotein produced by hepatocytes, is the largest sex hormone-binding protein in plasma and plays a role in influencing the bioavailability of estrogen for tissue response to hormones (Winters et al., 2014). In addition, SHBG is influenced by steroid hormones, peptide hormones, T4, nutritional status, and fluctuations in estrogen levels (Narinx et al., 2022). Table 3. Percentage apoptosis in embryos cultured without or with the addition of cumulus cell culture media.
Embryo development on days 2 and 6 without or with absorptionIGF-1 functions as an endocrine factor that mediates the actions of growth hormone and belongs to the group of growth factors that play a role in the process of cell differentiation and growth (Laron, 2001). IGF-1 is synthesized in the liver as a prohormone. This polypeptide has five domains, namely A, B, C, D, and E, in response to stimulation by growth hormone (Livingstone and Borai, 2014). After the translation process, mature IGF-1 consists of domains A, B, C, and D and has a structure similar to that of IGF-2 and insulin (LeRoith et al., 2021). In addition, this growth factor can also be produced by almost all organs and tissues that function autocrine and paracrine to stimulate cell growth. Estrogen is a sex steroid hormone that is mostly produced by ovarian follicles during the follicular development phase (Chauvin et al., 2022). Cumulus cells present in the follicles surround the oocyte and play a role in providing nutrients for oogenesis and folliculogenesis (Xie et al., 2023). In a paracrine context, IGF-1 functions closely with autocrine mechanisms, stimulating mitogenic activity in tissues undergoing proliferation, such as when ovarian follicles transform into the corpus luteum (Hull and Harvey, 2014). IGF-1 also contributes to stimulating the secretion of reproductive hormones. In the uterus, IGF-1 mRNA is found in the stromal cells surrounding the endometrial glands, which are rich in IGF type 1 receptors (Shi et al., 2022). The concentration of this mRNA varies throughout the estrous cycle, with the highest peak occurring in the mucosal layer during estrus, which then decreases during the luteal phase but increases again when an embryo is present (Lopera-Vásquez et al., 2022).
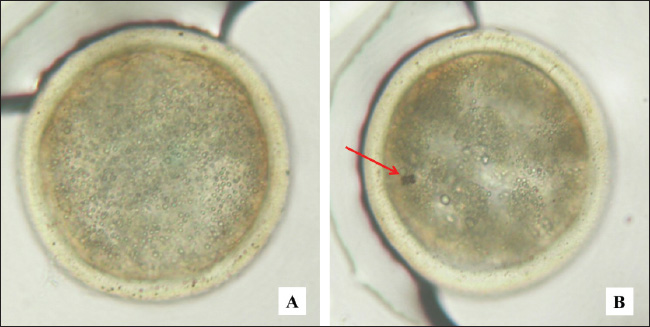
Fig. 2. Bovine embryos resulting from IVF at the blastocyst stage. Magnification 200×. A=normal, B=apoptosis, arrow sign (red): cell fragmentation. IGF-1 stimulates the proliferation and differentiation of granulosa cells in various species, including sheep, rats, pigs, and humans (Dai et al., 2022). In addition, IGF-1 also increases steroidogenesis in theca cells and has been reported to promote the proliferation of granulosa cells in sheep from small follicles with a diameter of 1–3 mm (Ipsa et al., 2019). Lin et al. (2003) reported that the combination of IGF-1 and IGFBP-1 increased blastocyst formation and number via culture and verification in stem cell lines. IGF-binding protein-1 (IGFBP-1) plays an important role in the biological activity of IGF-1, with the presence of IGFBP-1 mRNA in the oviduct lumen mucosa, which increases when there is an embryo (Lewitt and Boyd, 2024). Embryo culture in coculture media can increase the expression of IGFBP-mRNA, thereby strengthening the activity of IGF-1 during embryo development (Thongkittidilok et al., 2014). The results of monolayer cultures of bovine cumulus cells indicate the presence of materials with mitogenic properties that can be used as supplements in in vitro embryo culture media, contributing to increased cleavage rates and embryo development (Zhang et al., 1995). In the media produced from monolayer cell cultures impregnated with anti-P4, the antimitogenic effects of progesterone have been eliminated, allowing IGF-1 and estrogen in the monolayer cell culture fluid to function optimally as mitogenic materials (McDermott et al., 2025). This allows an increase in the number of embryos that undergo cleavage and develop into the morula. This study produced growth factor IGF-1 and steroid hormone estrogen from a monolayer culture of bovine cumulus cells. This product can be used as a supplement in media for IVF and embryo development, with the aim of increasing the cleavage rate and embryo development to the morula phase. In cattle, embryo developmental block, also known as developmental block, is often observed at the 8-cell stage and is related to the quality of the oocyte cytoplasm (Meirelles et al., 2004). The utilization of media supplements from monolayer cultures of bovine cumulus cells can help prevent these developmental obstacles (Hashimoto et al., 1998). The study showed that the addition of IGF-1 and estrogen obtained from culture as a media supplement can increase the cleavage rate and embryo development to the morula phase. IGF-1 and estrogen act as mitogenic agents by inhibiting the release of the proapoptotic protein p53 (Gallagher and LeRoith, 2010). This inhibition causes the suppression of the transcription factor p21, which then activates cyclin-dependent kinases (CDKs). CDK activation then leads to the occurrence of the cell cycle, which culminates in mitosis, proliferation, and cell differentiation (Baker and Reddy, 2012). Thus, the results of embryo cultures that experienced increased cleavage and development when supplemented with IGF-1 and estrogen media showed a significant increase compared with embryos that did not receive the supplements. Embryo apoptosis in cultureResearch by Makarevich and Markkula (2002) reported that IGF-1 functions as a mitogenic and antiapoptotic agent that supports cell proliferation during oocyte maturation, production, and in vitro embryo culture in cattle. In the study, it was found that in the oocyte maturation process using media enriched with IGF-1, the percentage of apoptosis measured by TUNEL assay was 0%, whereas in the control media without IGF-1, it reached 9.87% (p < 0.05). In addition, measurements of active caspase showed a significant difference in the percentage of apoptosis, which was 1.13% in media containing IGF-1, compared with 2.08% in control media (p < 0.05). These findings suggest that IGF-1 functions as an antiapoptotic factor during oocyte maturation, helping to inhibit apoptosis in oocytes during the caspase activation phase, as well as preventing oocyte progression toward apoptosis. In addition, it is known that insulin and IGF-1 can accelerate blastocyst development and prevent apoptosis (Herrler et al., 1998). Quirk et al. (2004) also highlighted the importance of growth factors and steroids in the regulation of ovarian follicle development. In cows, significant differences can be seen, where healthy dominant follicles have a greater ability to produce IGF and estradiol than subordinate follicles that experience atresia. IGF-1 and estradiol have also been shown to promote granulosa cell proliferation in vitro and enhance their survival by increasing resistance to apoptosis. The ability of IGF-1 and estradiol to increase resistance to apoptosis contributes to the enhancement of the cell development cycle (Quirk et al., 2004). Velazquez et al. (2009) reported the important role of IGF-1 in follicle growth, oocyte maturation, embryo viability, and reducing the level of apoptosis in bovine embryos. Apoptosis can occur physiologically or pathologically. Physiological apoptosis is cell death that is programed to occur in a certain period due to the inactivation of telomerase, an enzyme that plays a role in the formation of telomeres at the ends of chromosomes (Bekaert et al., 2004). Meanwhile, pathological apoptosis is caused by environmental factors such as infection (viruses or bacteria), stress, free radicals, hormones, and cytokines, with mechanisms involving various pathways, including the intrinsic pathway (mitochondrial), p53, and extrinsic pathways (Fas and Fas Ligand) (O’Brien and Kirby, 2008). Estrogen and IGF-1 inhibit apoptosis through the intrinsic pathway (mitochondrial) by increasing the production of antiapoptotic proteins such as Bcl-2 and Bcl-x and reducing proapoptotic proteins such as Bak, Bax, and Bim (Chimento et al., 2022). Increased levels of Bcl-2 and Bcl-x inhibit the release of cytochrome c from mitochondria, preventing the complex between cytochrome c and Apaf-1 (apoptosis-activating factor-1) does not occur (Ashraf et al., 2007). In addition, Bcl-2 and Bcl-x also inhibit Apaf-1 activity. Other proteins released from mitochondria, namely the apoptosis-inducing factor, are also inhibited; thus, there is no interaction that can neutralize apoptosis inhibitors (Susin et al., 1996). As a result, this process inhibits the caspase pathway, preventing cell execution phase does not occur. In this study, growth factor IGF-1 and estrogen produced through cumulus cell monolayer culture were proven effective in reducing the level of apoptosis in embryos obtained through IVF. Embryo developmental block in cattle generally occurs at the eight-cell stage, which is related to the quality of the oocyte cytoplasm (Meirelles et al., 2004). The application of media supplements from cow cumulus cell monolayer culture in the process of IVF and embryo development can prevent this developmental block (Zhang et al., 1995). The study showed that there was a decrease in the level of apoptosis after the addition of the media supplement (P1 and P2), which was lower than that of the control group without supplement (P0), with a very significant difference (p < 0.01). However, this study has limitations regarding the purification of IGF-1 and estrogen as mitogenic materials from progesterone, which functions as an antimitogenic material. The process of separating progesterone through binding with anti-P4 did not produce a 100% binding level, with results still around 60%. Although Konishi et al. (2012) stated that the concentration of progesterone can be considered insignificant if it is bound to more than 50% by anti-P4, this limitation still exists. The results of the purification of IGF-1 and estrogen as mitogenic materials from progesterone as an antimitogenic material by means of absorption/separation of progesterone through binding of progesterone using the absorption technique with anti-P4 have not been able to produce a binding percentage of up to 100% (still ± 60%). According to Paul et al. (2025), the concentration of progesterone can be ignored or considered to have very little effect if it can be absorbed/bound by anti-P4 by >50%. This is shown by the results of embryo development (2–8 cells) and (morula), in which with the addition of embryo culture media from monolayer cumulus cell culture containing IGF-1 and estrogen absorbed with anti-P4, the embryo (2–8 cells) was obtained in 53.614% of cases and the morula stage was 27.588%, significantly different (p < 0.01) with no absorption. The results of this study are in the laboratory stage (in vitro), which aims to produce the growth factor IGF-1 and the steroid hormone estrogen to increase the number of embryos that reach the morula or blastocyst phase. The ultimate goal of this study is to create frozen embryos or a cow embryo bank that can be used for the embryo transfer process. This initiative aims to increase the livestock population, especially cattle. However, application in the field (in vivo) as a growth factor to increase livestock growth is still required. ConclusionMonolayer culture of bovine cumulus cells can produce growth factor IGF-1 and estrogen as mitogenic materials and can be used as a supplement for fertilization media and in vitro embryo development, thereby reducing the incidence of apoptosis in embryos. AcknowledgmentsThanks are extended to Surtinem and Ida Prasetyawati for their assistance in carrying out the endocrine laboratory techniques and IVF laboratory work. In addition, the authors thank Mr Muhamad Lutfi and Mr Waris from the Pegirian Slaughterhouse in Surabaya for facilitating and providing technical assistance in collecting samples for this research. Conflict of interestThe authors declare no conflict of interest. FundingThis study was funded by the authors. Author’s contributionsSM, IM, PS, and FAR: conceived the idea and manuscript drafting. ARK, LL, TDL, and BPP: acquisition, analysis, and interpretation of data. AOA, RZA, LA, and UFH: The manuscript was critically read and revised for intellectual content. All authors have read and approved the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAnazawa, M., Ashibe, S. and Nagao, Y. 2025. Gene expression levels in cumulus cells are correlated with developmental competence of bovine oocytes. Theriogenology 231(1), 11–20. Ashraf, Q.M., Mishra, O.P. and Delivoria-Papadopoulos, M. 2007. Mechanisms of expression of apoptotic protease activating factor-1 (Apaf-1) in nuclear, mitochondrial and cytosolic fractions of the cerebral cortex of newborn piglets. Neurosci. Lett. 415(3), 253–258. Baker, S.J. and Reddy, E.P. 2012. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer 3(11–12), 658–669. Banda, L.J. and Tanganyika, J. 2021. Livestock provide more than food in smallholder production systems of developing countries. Anim. Front. 11(2), 7–14. Bekaert, S., Derradji, H. and Baatout, S. 2004. Telomere biology in mammalian germ cells and during development. Dev. Biol. 274(1), 15–30. Chauvin, S., Cohen-Tannoudji, J. and Guigon, C.J. 2022. Estradiol signaling at the heart of folliculogenesis: its potential deregulation in human ovarian pathologies. Int. J. Mol. Sci. 23(1), 512. Chimento, A., De Luca, A., Avena, P., De Amicis, F., Casaburi, I., Sirianni, R. and Pezzi, V. 2022. Estrogen receptors-mediated apoptosis in hormone-dependent cancers. Int. J. Mol. Sci. 23(3), 1242. Dai, S., Zhang, H., Yang, F., Shang, W. and Zeng, S. 2022. Effects of IGF-1 on the three-dimensional culture of ovarian preantral follicles and superovulation rates in mice. Biology (Basel) 11(6), 833. D’Angelo, A., Panayotidis, C., Alteri, A., Mcheik, S. and Veleva, Z. 2022. Evidence and consensus on technical aspects of embryo transfer. Hum. Reprod. Open. 2022(4), hoac038. Dembitsky, V.M. 2024. Naturally occurring norsteroids and their design and pharmaceutical application. Biomedicines 12(5), 1021. Di Giacomo, M., Camaioni, A., Klinger, F.G., Bonfiglio, R. and Salustri, A. 2016. Cyclic AMP-elevating agents promote cumulus cell survival and hyaluronan matrix stability, thereby prolonging the time of mouse oocyte fertilizability. J. Biol. Chem. 291(8), 3821–3836. Farin, C.E., Rodriguez, K.F., Alexander, J.E., Hockney, J.E., Herrick, J.R. and Kennedy-Stoskopf, S. 2007. The role of transcription in EGF- and FSH-mediated oocyte maturation in vitro. Anim. Reprod. Sci. 98(1–2), 97–112. Farooq, M., Khan, A.W., Kim, M.S. and Choi, S. 2021. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells 10(11), 3242. Fathan, S., Laya, N.K., Dako, S., Handayani, S., Machieu, S.R. and Hippy, M.Z. 2024. The effectiveness of cattle assistance program policy in bone bolango regency, Indonesia. Adv. Anim. Vet. Sci. 12(11), 2175–2184. Gallagher, E.J. and LeRoith, D. 2010. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 21(10), 610–618. Geiker, N.R.W., Bertram, H.C., Mejborn, H., Dragsted, L.O., Kristensen, L., Carrascal, J.R., Bügel, S. and Astrup, A. 2021. Meat and human health-current knowledge and research gaps. Foods 10(7), 1556. Hammond, G.L. 2011. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 85(3), 431–441. Hashimoto, S., Saeki, K., Nagao, Y., Minami, N., Yamada, M. and Utsumi, K. 1998. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology 49(8), 1451–1463. Herrler, A., Krusche, C.A. and Beier, H.M. 1998. Insulin and insulin-like growth factor-I promote rabbit blastocyst development and prevent apoptosis. Biol. Reprod. 59(6), 1302–1310. Hilmiati, N., Ilham, N., Nulik, J., Rohaeni, E.S., de Rosari, B., Basuki, T., Hau, D.K., Ngongo, Y., Lase, J.A., Fitriawaty, F., Surya, S., Qomariyah, N., Hadiatry, M.C., Ahmad, S.N., Qomariah, R., Suyatno, S., Munir, I.M., Hayanti, S.Y., Panjaitan, T. and Yusriani, Y. 2024. Smallholder cattle development in Indonesia: learning from the past for an outcome-oriented development model. Int. J. Des. Nat. Ecodynamics 19(1), 169–184. Hull, K.L. and Harvey, S. 2014. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014(1), 234014. Ipsa, E., Cruzat, V.F., Kagize, J.N., Yovich, J.L. and Keane, K.N. 2019. Growth hormone and insulin-like growth factor action in reproductive tissues. Front. Endocrinol. (Lausanne) 10(1), 777. Karl, K.R., Schall, P.Z., Clark, Z.L., Ruebel, M.L., Cibelli, J., Tempelman, R.J., Latham, K.E. and Ireland, J.J. 2023. Ovarian stimulation with excessive FSH doses causes cumulus cell and oocyte dysfunction in small ovarian reserve heifers. Mol. Hum. Reprod. 29(10), gaad033. Khan, M.Z., Zugaza, J.L. and Aleman, I.T. 2025. The signaling landscape of insulin-like growth factor 1. J. Biol. Chem. 301(1), 108047. Kineman, R.D., Del Rio-Moreno, M. and Sarmento-Cabral, A. 2018. 40 YEARS of IGF1: understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 61(1), T187–T198. Kolatorova, L., Vitku, J., Suchopar, J., Hill, M. and Parizek, A. 2022. Progesterone: a steroid with wide range of effects in physiology as well as human medicine. Int. J. Mol. Sci. 23(14), 7989. Konishi, S., Brindle, E., Guyton, A. and O’Connor, K.A. 2012. Salivary concentration of progesterone and cortisol significantly differs across individuals after correcting for blood hormone values. Am. J. Phys. Anthropol. 149(2), 231–241. Kusumaningrum, R., Darjanto, A., Nurmalina, R., Mulatsih, S. and Suprehatin. 2024. Effect of import policy on beef supply and demand in Indonesia before and after the COVID-19 pandemic. Trop. Anim. Sci. J. 47(2), 242–251. Laron, Z. 2001. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54(5), 311–316. LeRoith, D., Holly, J.M.P. and Forbes, B.E. 2021. Insulin-like growth factors: ligands, binding proteins, and receptors. Mol. Metab. 52(1), 101245. Lewitt, M.S. and Boyd, G.W. 2024. Insulin-like growth factor-binding protein-1 (IGFBP-1) as a biomarker of cardiovascular disease. Biomolecules 14(11), 1475. Lin, T.C., Yen, J.M., Gong, K.B., Hsu, T.T. and Chen, L.R. 2003. IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC Cell Biol. 4(1), 14. Livingstone, C. and Borai, A. 2014. Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin. Endocrinol. 80(6), 773–781. Lopera-Vásquez, R., Uribe-García, F. and Rondón-Barragán, I. 2022. Effect of estrous cycle phases on gene expression in bovine oviduct epithelial cells. Vet. World 15(7), 1665–1675. Makarevich, A.V. and Markkula, M. 2002. Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor I during in vitro maturation and culture. Biol. Reprod. 66(2), 386–392. Martyniuk, E. 2021. Policy Effects on the sustainability of animal breeding. Sustainability 13(14), 7787. McDermott, N., O’Shea, S., Rieger, L., Cox, O.T. and O’Connor, R. 2025. β1-integrin controls IGF-1R internalization and intracellular signaling. J. Biol. Chem. 301(1), 108021. Meirelles, F.V., Caetano, A.R., Watanabe, Y.F., Ripamonte, P., Carambula, S.F., Merighe, G.K. and Garcia, S.M. 2004. Genome activation and developmental block in bovine embryos. Anim. Reprod. Sci. 82–83(1), 13–20. Mitwally, M.F., Casper, R.F. and Diamond, M.P. 2005. Oestrogen-selective modulation of FSH and LH secretion by pituitary gland. Br. J. Cancer 92(2), 416–417. Narinx, N., David, K., Walravens, J., Vermeersch, P., Claessens, F., Fiers, T., Lapauw, B., Antonio, L. and Vanderschueren, D. 2022. Role of sex hormone-binding globulin in the free hormone hypothesis and the relevance of free testosterone in androgen physiology. Cell. Mol. Life Sci. 79(11), 543. O’Brien, M.A. and Kirby, R. 2008. Apoptosis: a review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care (San Antonio) 18(6), 572–585. Orisaka, M., Miyazaki, Y., Shirafuji, A., Tamamura, C., Tsuyoshi, H., Tsang, B.K. and Yoshida, Y. 2021. The role of pituitary gonadotropins and intraovarian regulators in follicle development: a mini-review. Reprod. Med. Biol. 20(2), 169–175. Paul, D., Agrawal, R. and Iqbal, M.A. 2025. An overview of endometriosis and molecular target-based therapeutic approach. Middle East Fertil. Soc. J. 30(1), 6. Pemayun, T.G.O., Trilaksana, I.G.N.B. and Mahaputra, L. 2011. Prostaglandin F2α concentrations of Bali cattle endometrial andseminal vesicle monolayer cells culture products and its in vitrotest on luteal monolayer cells culture. Indones. Vet. J. 12(1), 50–57. Piau, T.B., de Queiroz Rodrigues, A. and Paulini, F. 2023. Insulin-like growth factor (IGF) performance in ovarian function and applications in reproductive biotechnologies. Growth Horm IGF Res. 72–73(1), 101561. Quirk, S.M., Cowan, R.G., Harman, R.M., Hu, C.L. and Porter, D.A. 2004. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J. Anim. Sci. 82(E-Suppl), E40–E52. Reslan, O.M. and Khalil, R.A. 2012. Vascular effects of estrogenic menopausal hormone therapy. Rev. Recent Clin. Trials 7(1), 47–70. Rozaki, Z. 2021. Food security challenges and opportunities in indonesia post COVID-19. Adv. Food Secur. Sustain. 6(1), 119–168. Salek, F., Guest, A., Johnson, C., Kastelic, J.P. and Thundathil, J. 2025. Factors affecting the success of ovum pick-up, in vitro production and cryopreservation of embryos in cattle. Animals 15(3), 344. Sarjito, A. 2024. Free nutritious meal program as a human resource development strategy to support national defence. Int. J. Adm. Bus. Organ. 5(5), 129–141. Shi, J.W., Lai, Z.Z., Yang, H.L., Zhou, W.J., Zhao, X.Y., Xie, F., Liu, S.P., Chen, W.D., Zhang, T., Ye, J.F., Zhou, X.Y. and Li, M.Q. 2022. An IGF1-expressing endometrial stromal cell population is associated with human decidualization. BMC Biol. 20(1), 276. Skenandore, C.S., Pineda, A., Bahr, J.M., Newell-Fugate, A.E. and Cardoso, F.C. 2017. Evaluation of a commercially available radioimmunoassay and enzyme immunoassay for the analysis of progesterone and estradiol and the comparison of two extraction efficiency methods. Domest. Anim. Endocrinol. 60(1), 61–66. Susin, S.A., Zamzami, N., Castedo, M., Hirsch, T., Marchetti, P., Macho, A., Daugas, E., Geuskens, M. and Kroemer, G. 1996. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 184(4), 1331–1341. Thongkittidilok, C., Tharasanit, T., Sananmuang, T., Buarpung, S. and Techakumphu, M. 2014. Insulin-like growth factor-1 (IGF-1) enhances developmental competence of cat embryos cultured singly by modulating the expression of its receptor (IGF-1R) and reducing developmental block. Growth Horm. IGF Res. 24(2–3), 76–82. Turathum, B., Gao, E.M. and Chian, R.C. 2021. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10(9), 2292. Vandaele, L. and Van Soom, A. 2011. Intrinsic factors affecting apoptosis in bovine in vitro produced embryos. Verh. K. Acad. Geneeskd. Belg. 73(1–2), 79–104. Velazquez, M.A., Zaraza, J., Oropeza, A., Webb, R. and Niemann, H. 2009. The role of IGF1 in the in vivo production of bovine embryos from superovulated donors. Reproduction 137(2), 161–180. Widayati, D.T. and Pangestu, M. 2020. Effect of follicle-stimulating hormone on Bligon goat oocyte maturation and embryonic development post in vitro fertilization. Vet. World 13(11), 2443–2446. Winters, S.J., Gogineni, J., Karegar, M., Scoggins, C., Wunderlich, C.A., Baumgartner, R. and Ghooray, D.T. 2014. Sex hormone-binding globulin gene expression and insulin resistance. J. Clin. Endocrinol. Metab. 99(12), E2780–E27888. Xie, J., Xu, X. and Liu, S. 2023. Intercellular communication in the cumulus-oocyte complex during folliculogenesis: a review. Front. Cell. Dev. Biol. 11(1), 1087612. Xu, X.L., Huang, Z.Y., Yu, K., Li, J., Fu, X.W. and Deng, S.L. 2022. Estrogen biosynthesis and signal transduction in ovarian disease. Front Endocrinol. 13, 827032. Zhang, L., Jiang, S., Wozniak, P.J., Yang, X. and Godke, R.A. 1995. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol. Reprod. Dev. 40(3), 338–344. | ||
| How to Cite this Article |
| Pubmed Style Mulyati S, Mustofa I, Srianto P, Khairullah AR, Rantam FA, Akintunde AO, Lestari TD, Anggraini L, Ahmad RZ, Latifah L, Pratama BP, Handayani UF. Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Vet. J.. 2025; 15(7): 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 Web Style Mulyati S, Mustofa I, Srianto P, Khairullah AR, Rantam FA, Akintunde AO, Lestari TD, Anggraini L, Ahmad RZ, Latifah L, Pratama BP, Handayani UF. Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. https://www.openveterinaryjournal.com/?mno=253852 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.13 AMA (American Medical Association) Style Mulyati S, Mustofa I, Srianto P, Khairullah AR, Rantam FA, Akintunde AO, Lestari TD, Anggraini L, Ahmad RZ, Latifah L, Pratama BP, Handayani UF. Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Vet. J.. 2025; 15(7): 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 Vancouver/ICMJE Style Mulyati S, Mustofa I, Srianto P, Khairullah AR, Rantam FA, Akintunde AO, Lestari TD, Anggraini L, Ahmad RZ, Latifah L, Pratama BP, Handayani UF. Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 Harvard Style Mulyati, S., Mustofa, . I., Srianto, . P., Khairullah, . A. R., Rantam, . F. A., Akintunde, . A. O., Lestari, . T. D., Anggraini, . L., Ahmad, . R. Z., Latifah, . L., Pratama, . B. P. & Handayani, . U. F. (2025) Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Vet. J., 15 (7), 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 Turabian Style Mulyati, Sri, Imam Mustofa, Pudji Srianto, Aswin Rafif Khairullah, Fedik Abdul Rantam, Adeyinka Oye Akintunde, Tita Damayanti Lestari, Lili Anggraini, Riza Zainuddin Ahmad, Latifah Latifah, Bima Putra Pratama, and Ulvi Fitri Handayani. 2025. Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Veterinary Journal, 15 (7), 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 Chicago Style Mulyati, Sri, Imam Mustofa, Pudji Srianto, Aswin Rafif Khairullah, Fedik Abdul Rantam, Adeyinka Oye Akintunde, Tita Damayanti Lestari, Lili Anggraini, Riza Zainuddin Ahmad, Latifah Latifah, Bima Putra Pratama, and Ulvi Fitri Handayani. "Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle." Open Veterinary Journal 15 (2025), 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 MLA (The Modern Language Association) Style Mulyati, Sri, Imam Mustofa, Pudji Srianto, Aswin Rafif Khairullah, Fedik Abdul Rantam, Adeyinka Oye Akintunde, Tita Damayanti Lestari, Lili Anggraini, Riza Zainuddin Ahmad, Latifah Latifah, Bima Putra Pratama, and Ulvi Fitri Handayani. "Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle." Open Veterinary Journal 15.7 (2025), 3024-3034. Print. doi:10.5455/OVJ.2025.v15.i7.13 APA (American Psychological Association) Style Mulyati, S., Mustofa, . I., Srianto, . P., Khairullah, . A. R., Rantam, . F. A., Akintunde, . A. O., Lestari, . T. D., Anggraini, . L., Ahmad, . R. Z., Latifah, . L., Pratama, . B. P. & Handayani, . U. F. (2025) Insulin-like growth factor 1 and estrogen from cumulus cell culture increases the success of in vitro fertilization in cattle. Open Veterinary Journal, 15 (7), 3024-3034. doi:10.5455/OVJ.2025.v15.i7.13 |