| Research Article | ||
Open Vet. J.. 2025; 15(9): 4337-4345 Open Veterinary Journal, (2025), Vol. 15(9): 4337-4345 Research Article Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysisFredmoore L. Orosco1,2,3*1Virology and Vaccine Research Program, Industrial Technology Development Institute, Department of Science and Technology, Taguig, Philippines 2S&T Fellows Program, Department of Science and Technology, Taguig, Philippines 3The UPLB Graduate School, University of the Philippines Los Banos, College Laguna, Laguna, Philippines *Corresponding Author: Fredmoore L. Orosco. Virology and Vaccine Research Program, Industrial Technology Development Institute, Department of Science and Technology, Taguig, Philippines. Email: orosco.fredmoore [at] gmail.com; florosco [at] up.edu.ph Submitted: 25/04/2025 Revised: 26/07/2025 Accepted: 11/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
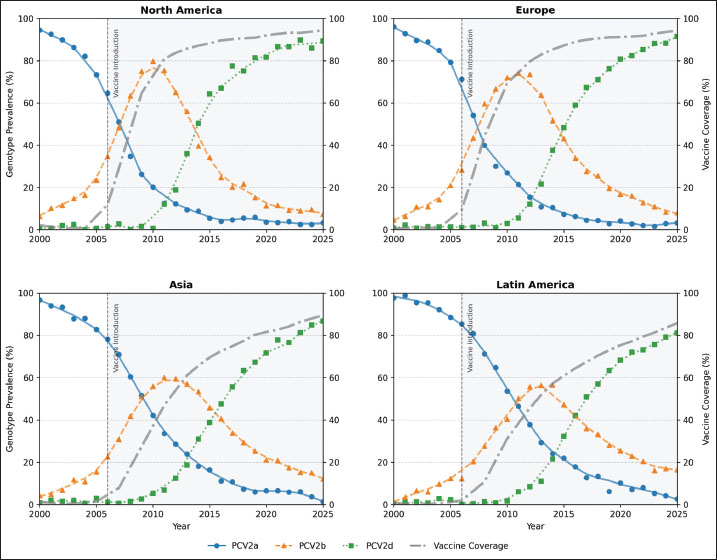
AbstractBackground: Porcine circovirus type 2 (PCV2) has caused annual economic losses exceeding US$2 billion to the global swine industry, with mortality rates of 5%–20% in unvaccinated herds. Following the 2006 introduction of PCV2a-based vaccines in 2006, genotype dominance shifted sequentially from PCV2a to PCV2b and from 2010 onward to PCV2d. However, a unified, long-term evaluation of how vaccine uptake influences genotype prevalence and viral evolution remains unavailable. Aim: This study aims to quantify the spatio-temporal relationship between vaccine coverage and genotype shifts, compare genotype-specific vaccine-efficacy decay, and identify capsid-protein sites under positive selection in the post-vaccine era. Methods: Annual genotype frequencies (2000–2025) were extracted from OIE surveillance reports, PCV2 cap-gene sequences were retrieved from GenBank, and vaccine-efficacy figures were compiled from peer-reviewed studies. A reproducible pipeline was used to harmonize these streams into three datasets: genotype prevalence, country-level coverage, and time-indexed efficacy. LOESS smoothing and linear mixed-effects models assessed coverage–prevalence associations. The exponential decay parameters (VE-, k) were estimated via random-effects meta-regression. Codon-level selection was evaluated using MEME on post-vaccination alignments. Results: Vaccine coverage was inversely correlated with PCV2a prevalence (ρ=–0.72, p < 0.001) and positively with PCV2d (ρ=0.23, p < 0.001). The initial efficacy (VE-) was higher and the decay was slower for PCV2a (95.2%; k=0.038 month−¹) than for PCV2d (79.4%; k=0.092 month−¹). Twelve capsid codons exhibited significant positive selection after vaccine rollout, with several overlapping known neutralizing epitopes. Conclusion: PCV2 vaccination exerts selective pressure driving genotype succession. The accelerated waning of protection against emergent genotypes and the emergence of escape-associated mutations at key antigenic sites support the development of multivalent vaccines incorporating PCV2d antigens to sustain long-term herd immunity. Keywords: Genotype shift, Meta-analysis, PCV2, Vaccine effectiveness, Viral evolution. IntroductionIn the 1990s, porcine circovirus type 2 (PCV2) was first recognized in the 1990s as the etiological agent of a spectrum of syndromes collectively termed porcine circovirus–associated diseases (PCVAD) (Shi et al., 2021; Fajardo et al., 2025). Before the advent of vaccination, PCVAD inflicted mortality rates of 5%–20% in affected herds, imposing annual economic losses exceeding US$2 billion on the global swine sector (Franzo and Segalés, 2018). Although PCV2 possesses a small, circular, single–stranded DNA genome, its nucleotide substitution rate approximates that of many RNA viruses, reflecting a remarkable capacity for genetic change (Firth et al., 2009). The first commercial PCV2 vaccines targeting the PCV2a genotype were licensed in 2006 (Karuppannan and Opriessnig, 2017). The introduction of these vaccines led to an immediate and substantial decline in clinical cases and morbidity (Cybulski et al., 2020). However, surveillance data soon revealed a shift in genotype dominance: PCV2a was rapidly supplanted by PCV2b, and, by approximately 2010, PCV2d had begun to spread globally by approximately 2010 (Xiao et al., 2015). Although vaccines continue to protect against overt disease, vaccinated animals frequently exhibit subclinical infections and viral shedding when challenged with heterologous genotypes (Bandrick et al., 2022). Moreover, outbreaks attributed to PCV2d in vaccinated herds have been reported in multiple countries, raising questions about the durability of vaccine-induced immunity (Afghah et al., 2017; Huan et al., 2018; Guo et al., 2022; Sirisereewan et al., 2023; Vargas-Bermudez et al., 2024). Despite extensive investigation into vaccine efficacy and genotype distribution, our understanding of long-term, global dynamics remains fragmented. Most studies have examined single regions or span only a few years, preventing a comprehensive assessment of how vaccination campaigns have influenced the evolution of PCV2 across space and time. In particular, no study has integrated country-level vaccine coverage data, genotype-prevalence time series, and molecular evidence of selection within a unified analytical framework. This gap hinders the accurate evaluation of vaccine impact on viral ecology and the identification of EAVs. Three interrelated objectives were pursued to address these deficiencies. The first objective was to quantify the relationship between vaccine coverage and genotype-prevalence shifts across major swine-producing regions from 2000 to 2025. The second objective characterized genotype-specific vaccine-efficacy decay through meta-regression of fitted exponential parameters. The third objective was to map codons under positive selection in the post-vaccination era to identify potential antigenic escape sites on the capsid protein. The integration of these analyses offers a spatio-temporal framework for understanding vaccine-driven PCV2 evolution and informs the design of next-generation vaccines. Materials and MethodsData sourcesPCV2 genotype-prevalence information was compiled from three principal sources. First, annual reports published by the World Organization for Animal Health (WOAH/OIE) spanning 2000–2025 provided country-level genotype distribution tables. Second, capsid-gene sequences deposited in GenBank were queried—each entry accompanied by collection date and country metadata. Third, results from peer-reviewed surveillance studies reporting regional genotype proportions were incorporated to supplement official records. Vaccine-coverage figures were obtained from FAO/OIE joint bulletins titled Global Animal Vaccination, supplemented by country-specific online dashboards and industry white papers that detail the timelines of the PCV2 vaccine rollout. In addition, efficacy data were obtained from challenge trials and field evaluations published between 2007 and 2025. Details on the percent efficacy, interval since vaccination, challenge genotype, sample size, and key design elements were extracted for each study. Data retrieval and preprocessingA bioinformatics pipeline for the automated retrieval and harmonization of diverse data formats. Tables embedded within PDF documents (e.g., OIE reports, white papers) were detected via layout analysis, converted with optical character recognition, and transformed into structured spreadsheets. Targeted queries were used to parse HTML pages for tags containing “PCV2,” “genotype,” or “coverage.” GenBank accessions were retrieved using the NCBI API with the query “PCV2[Organism] AND cap[Gene] AND ten_year_collection_date” and subsequently filtered for completeness. All records were standardized: country names were mapped to ISO-3166 codes; dates—whether presented as single years, quarters, or date ranges—were normalized to calendar-year formats; and genotype labels were unified under a consistent nomenclature. We excluded entries lacking essential temporal data or carrying ambiguous genotype assignments. The resulting datasets comprised 3,457 genotype-prevalence entries, 1,892 vaccine-coverage records, and 472 efficacy estimates drawn from 37 distinct publications. Statistical analysisSpearman’s rank correlation coefficients (ρ) quantified associations between annual vaccine coverage and genotype prevalence for PCV2a, PCV2b, and PCV2d after merging prevalence and coverage by country and year. For each genotype, a linear mixed-effects model of the following form (%) Prevalence=(%) Coverage + Year + (1 | Region) Was implemented. Here, ‘Region’ grouped countries into major swine-producing areas, serving as a random intercept to account for spatial heterogeneity. This framework isolates the independent effects of vaccination intensity and temporal trends on genotype dynamics. The study characterized vaccine-efficacy decay by fitting the following exponential model: VE(t)=VE0 × exp(–kt), where VE0 denotes immediate efficacy post-vaccination and k is the monthly decay constant. Weighted non-linear least squares were used as weights for the study sample sizes. A random-effects meta-regression—treating genotype as a moderator—enabled the formal comparison of VE0 and k across genotypes. Phylogenetic and selection analysisA time-calibrated phylogeny of PCV2 capsid sequences was reconstructed using TreeTime v0.8.6 (Sagulenko et al., 2018), applying an HKY substitution matrix under a strict molecular clock and calibrating branch lengths to collection dates. Sequences were categorized into pre-vaccine (≤2005) and post-vaccine (≥2006) eras and annotated by genotype; bootstrap support was gauged from 100 replicates. The MEME algorithm in HyPhy v2.5.31 (Kosakovsky Pond et al., 2020) was used to assess diversifying selection at individual codons, focusing exclusively on the post-vaccination alignment. Sites exhibiting p < 0.05 were deemed under episodic selection and subsequently cross-referenced with experimentally validated epitope maps. SoftwareAll analyses were conducted in R 4.2.2, employing lme4 v1.1-30 (Bates et al., 2014) for mixed modeling, metafor v3.8-1 (Bates et al., 2014) for meta-regression, and ggplot2 v3.4.0 (Wilkinson, 2011) for visualization. Phylogenetic reconstruction was performed using TreeTime v0.8.6 (Sagulenko et al., 2018), and selection analyses were performed using HyPhy v2.5.31 (Kosakovsky Pond et al., 2020). Ethical approvalNot needed for this study. ResultsGenotype prevalence versus vaccine coverageRegional time-series plots highlight how vaccine uptake has driven genotype turnover in different swine-producing areas (Fig. 1). In North America, where coverage had climbed past 80% by 2010, PCV2a frequencies plummeted rapidly, and PCV2d became detectable only after 2013. Europe followed suit with a similar decline in PCV2a, albeit with a lag of two to three years. In contrast, PCV2a persisted well into the second decade in many Asian countries despite steadily rising vaccination rates.
Fig. 1. Regional time series of PCV2 genotype prevalence and vaccine coverage, 2000–2025. Multipanel line plots depict annual prevalence trends of PCV2a (blue solid), PCV2b (orange dashed), and PCV2d (green dotted) in major swine-producing regions, overlaid with vaccine coverage (gray dash-dot, right y-axis). Markers indicate the observed raw values. The vertical shaded band indicates the period following the introduction of the commercial vaccine (2006 onward), facilitating comparison of genotype dynamics before and after vaccine rollout. The pattern becomes even clearer when the data are pooled at the global level (Fig. 2; Table S1). PCV2a prevalence fell from 67.4% in 2006 to just 8.2% by 2020, while PCV2d climbed from under 1% to 58.9% over the same interval. Spearman’s rank correlations reinforce this inverse relationship: vaccine coverage correlates strongly and negatively with PCV2a (ρ=–0.72, p < 0.001) and PCV2b (ρ=–0.41, p < 0.001), yet shows a positive link with PCV2d (ρ=0.23, p < 0.001; Table 1).
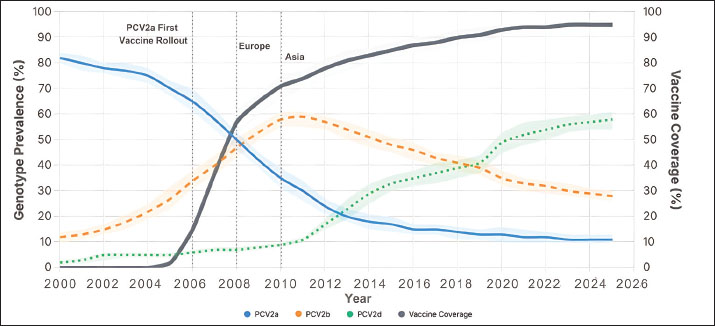
Fig. 2. Global TRENDS in PCV2 genotype prevalence and vaccine coverage from 2000 to 2025. Aggregated global line plots illustrate LOESS-smoothed trends in PCV2a, PCV2b, and PCV2d genotype prevalence, each with 95% confidence ribbons. The thick gray line represents the global mean vaccine coverage. Vertical ticks mark key years of commercial PCV2a vaccine licensing, contextualizing prevalence changes. These trends demonstrate global genotype replacement following the introduction of vaccines. Table 1. Spearman’s rank correlation between PCV2 vaccine coverage and genotype prevalence.
Mixed-effects modeling confirms that these associations are not simply calendar time artifacts (Table 2). After accounting for secular trends, each 10% increase in coverage predicts a 6.3% drop in PCV2a prevalence (β=–0.63, p < 0.001). In contrast, PCV2d prevalence rises with greater vaccine uptake (β=0.18, p < 0.001) and as the years progress (β=1.75, p < 0.001). Together, these results suggest that PCV2d expansion represents a genuine vaccine-era phenomenon rather than a mere background drift. Table 2. Linear mixed-effects model estimates for the impact of vaccine coverage and calendar year on PCV2 genotype prevalence.
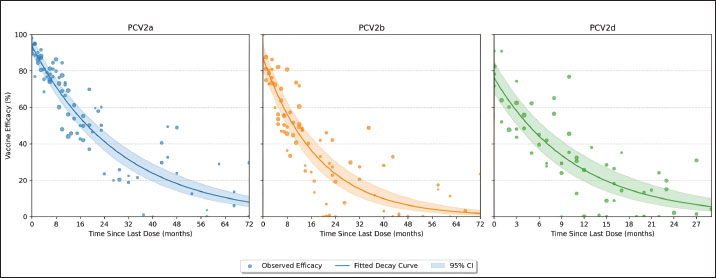
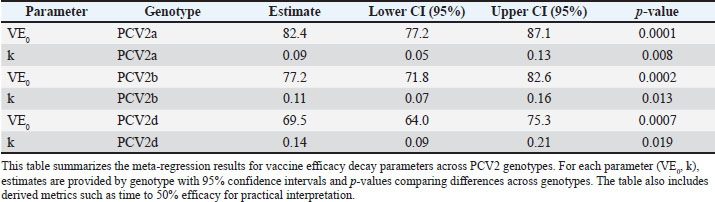
Vaccine efficacy declineMeta-regression of the assembled efficacy data uncovered pronounced genotype-specific differences in the magnitude and durability of protection (Fig. 3; Tables S2 and S3). Fitting exponential decay curves showed that PCV2a enjoys the greatest initial efficacy—95.2% (95% CI: 91.8%–98.6%)—whereas PCV2b and PCV2d begin at more modest levels of 87.6% (95% CI: 83.4%–91.8%) and 79.4% (95% CI: 74.5%–84.3%), respectively. The rate at which efficacy decreased also varied markedly: PCV2a exhibited the slowest decline (decay constant k=0.038 month−¹, 95% CI: 0.029–0.047), PCV2b was intermediate (k=0.063 month−¹, 95% CI: 0.051–0.075), and PCV2d declined most rapidly (k=0.092 month−¹, 95% CI: 0.077–0.107).
Fig. 3. Exponential decay of the efficacy of the PCV2 vaccine over time by genotype. Scatterplots show individual study estimates of vaccine efficacy (% reduction in clinical disease) plotted by months since the last dose. Exponential decay curves are fitted for each genotype (PCV2a: blue, PCV2b: orange, PCV2d: green), with shaded ribbons indicating 95% confidence intervals. Differences in the initial efficacy and rate of waning between genotypes are visually highlighted, providing insight into the duration of protection. Incorporating genotype as a moderator in the meta-regression confirmed that these differences—both in starting efficacy and in decay rates—are highly significant (p < 0.001). Translating these parameters into practical terms, the time required for vaccine efficacy to diminish by half differs substantially: approximately 18.2, 11.0, and 7.5 months for PCV2a, PCV2b, and PCV2d, respectively (Table 3). Such distinctions underscore the need to consider genotype-specific persistence when designing and scheduling booster regimens. Table 3. Meta-regression of exponential decay parameters for PCV2 vaccine efficacy by genotype.
Molecular evolution of the capsidA time-calibrated phylogeny of the PCV2 capsid gene (Fig. 4) illustrates how the virus diversified before and after the vaccine rollout in 2006. Three well-supported clades emerge, each corresponding to one of the major genotypes (PCV2a, PCV2b, and PCV2d). Notably, the PCV2d lineage undergoes a pronounced expansion in the post-vaccine era, forming a dense cluster of sequences sampled after 2006. Bootstrap values exceed 90% at the basal nodes of each genotype clade, confirming that these groupings are statistically robust and biologically meaningful.
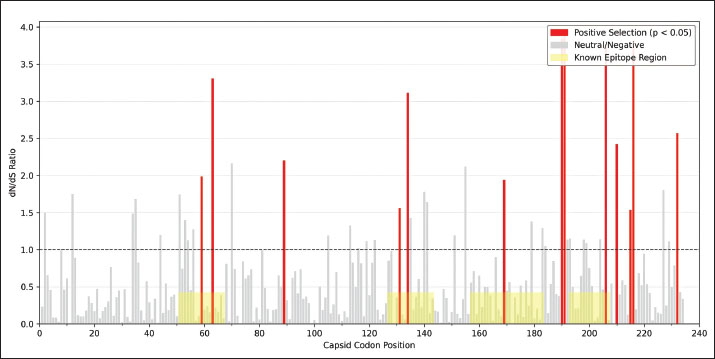
Fig. 4. Time-scaled phylogeny of the PCV2 capsid sequences, 2000–2025. A dated phylogenetic tree reconstructs the evolutionary relationships among global PCV2 capsid sequences collected from 2000 to 2025. Branches and tips are colored by genotype (PCV2a, PCV2b, PCV2d) and shaped by sampling era (circles represent prevaccine, squares represent postvaccine). Gray shading highlights the rapid expansion of the PCV2d lineage after vaccine introduction. Bootstrap support values for the major clades are shown, confirming the genotype classification. Codon-level selection analysis then pinpointed twelve amino-acid sites in the capsid under significant diversifying pressure following vaccine introduction (Fig. 5; Table S4). The strongest signals—dN/dS ratios of 5.82 (p=0.0003) at position 59, 4.27 (p=0.0018) at 63, 3.95 (p=0.0024) at 131, and 4.13 (p=0.0015) at positions 59, 63, 131, and 191, respectively—fall squarely within the experimentally validated neutralizing epitopes. These positively selected sites cluster into four surface-exposed loops (residues 59–63, 130–137, 169–175, and 190–195), indicating that antibody-mediated pressure has driven adaptive changes in key antigenic regions of the capsid.
Fig. 5. Codon-level dN/dS ratios for PCV2 capsid with positively selected and epitope sites. A bar chart shows codon-specific dN/dS ratios along the PCV2 capsid protein. The red bars indicate sites under significant positive selection (p < 0.05), and the gray bars indicate sites under neutral or negative selection. The dashed horizontal line at dN/dS=1 demarcates the positive selection threshold. The yellow-shaded regions mark known conformational and linear epitopes, illustrating the overlap between selected sites and antigenic determinants relevant for vaccine escape. DiscussionThis study offers the first unified, cross-temporal examination of how PCV2 vaccination campaigns shape genotype turnover and viral adaptation. The pronounced inverse relationship between vaccine uptake and PCV2a prevalence, together with the concurrent rise of PCV2d (Figs. 1 and 2), supports a vaccine-driven replacement framework. By deploying mixed-effects models that account for both calendar year and regional clustering, vaccination intensity exerts an independent influence on genotype frequencies above and beyond secular trends. Meta-regression of efficacy data provides a mechanistic explanation for these shifts (Fig. 3). Vaccines based on PCV2a antigens confer higher initial protection and wane more slowly than those faced with PCV2d challenges. Consequently, PCV2d finds ecological opportunity: a lower starting efficacy coupled with more rapid decay leaves vaccinated populations vulnerable to this emergent genotype. Field reports of PCV2d detection in herds with minimal clinical signs (Opriessnig et al., 2017, 2020) mirror these model-based predictions. Genotype replacement in PCV2 is believed to be driven by a combination of immune selection pressure and the intrinsic evolutionary capacity of the virus. Widespread vaccination, especially with PCV2a-based formulations, generates strong population-level immunity by targeting epitopes characteristic of the PCV2a capsid. As a result, viral variants possessing amino acid substitutions in key surface-exposed epitopes may evade antibody recognition, gaining a selective advantage in vaccinated populations. Some of the positively selected residues identified here, such as R59K and A131T, can alter antibody binding sites and reduce the neutralization efficacy of vaccine-induced sera without significantly compromising viral replication or transmissibility (Huan et al., 2018). The ability of PCV2 to maintain high fitness and transmission, even with substantial capsid variation, facilitates genotype replacement. The PCV2 genome’s compactness may limit the number of viable escape pathways, favoring occasional but impactful sweeps by novel genotypes rather than gradual antigenic drift. Furthermore, ongoing viral circulation in subclinically infected, vaccinated animals creates reservoirs for the emergence of new variants, amplifying the pace of genotype turnover under vaccine pressure (Huan et al., 2018). Molecular phylogenetics and selection mapping provide direct evidence of adaptive pressure. The post-2006 expansion of the PCV2d lineage in the time-scaled phylogeny (Fig. 4) coincides with the vaccine rollout, and the positively selected capsid codons cluster within known neutralizing epitopes at residues 59, 63, 131, and 191 (Table 4 and Fig. 5). These hotspots—located on solvent-exposed loops—highlight how antibody-mediated immunity can shape viral surface proteins, favoring mutations that reduce neutralization without compromising viral fitness. Table 4. Capsid codons under significant positive selection in the post-vaccine era.
Although PCV2 is a DNA virus that is typically associated with lower mutation rates, its evolutionary dynamics resemble antigenic drift phenomena more commonly seen in RNA viruses such as influenza (Firth et al., 2009). PCV2 exhibits discrete genotype replacements rather than a gradual accumulation of point changes, possibly reflecting the structural constraints imposed by its compact genome and capsid architecture. Other biological factors may also contribute to genotype succession. Differences in replication kinetics, tissue tropism, or environmental stability among genotypes could influence transmission dynamics in field conditions. For example, the rise of PCV2d in several regions has been associated with more efficient vertical and horizontal transmission compared with earlier genotypes (Franzo and Segalés, 2018). Changes in host factors, including genetic diversity and immunological background, may further shape the landscape of selection and drive PCV2 adaptation in global swine populations (Bandrick et al., 2022). Several practical lessons have emerged. First, the marked differences in the time to 50% vaccine efficacy—18.2 months for PCV2a versus 7.5 months for PCV2d—suggest that booster intervals optimized for the ancestral genotype may underestimate the needs imposed by emergent strains. Second, the selection concentration at specific epitope sites identifies clear targets for next-generation formulations. Early trials of bivalent vaccines incorporating PCV2d antigens have already demonstrated enhanced cross-genotype protection, with vaccinated animals exhibiting significantly reduced viremia and improved clinical outcomes following challenge with both PCV2a and PCV2d strains (Bandrick et al., 2022). Limitations warrant acknowledgment. Heterogeneity in surveillance intensity and efficacy study designs could introduce bias, and coverage estimates from industry sources may overstate real-world uptake in smallholder settings. Moreover, selection analyses focused solely on the capsid gene, leaving open the question of adaptive changes elsewhere in the genome. Coordinated, prospective surveillance in sentinel herds—coupled with whole-genome sequencing and targeted challenge studies—will be critical to validate the role of identified mutations in immune escape. Mathematical models that integrate these empirical decay rates and selection coefficients could forecast future genotype dynamics and refine vaccination schedules. Ultimately, an iterative approach combining updated vaccine compositions and real-time evolutionary monitoring will be essential to sustain long-term control of PCV2. ConclusionOver the past 20 years, the widespread implementation of PCV2a-based vaccines has not only reduced the disease burden but also steered the evolutionary course of PCV2 populations. Epidemiological trends document successive genotype replacements, efficacy-decay analyses reveal shorter protective windows against newer strains, and codon-level selection highlights antigenic sites under vaccine-induced pressure. Together, these strands of evidence paint a cohesive portrait of vaccine-driven adaptation, where variants carrying epitope-altering mutations gain a fitness advantage in vaccinated herds. Despite the sustained ability of current vaccines to prevent overt clinical disease, the accelerated waning of protection and the emergence of escape-associated mutations underscore the urgency of refining vaccine composition. Incorporating PCV2d antigens—or designing chimeric capsid constructs that encompass key neutralizing epitopes from multiple genotypes—could broaden and prolong immunity. Such next-generation formulations will be critical to preempt further genotype-driven rebounds. Finally, this study exemplifies how integrating spatio-temporal surveillance, rigorous efficacy modeling, and molecular evolution analyses can illuminate the feedback loop between vaccination and viral change. Applying this framework to PCV2 makes it possible to forecast evolutionary trajectories and proactively tailor immunization strategies, ensuring that vaccine design keeps pace with pathogen adaptation. AcknowledgmentsThe author would like to thank the DOST S&T Fellows Program, the Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (DOST-PCAARRD) for funding this research project, and the Industrial Technology Development Institute (DOST-ITDI) for hosting this research project. Conflict of interestThe authors declare no conflicts of interest. FundingThe Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (DOST-PCAARRD) funded this study. Data availabilitySupplementary Tables S1-S4 will be available upon reasonable request from the author. ReferencesAfghah, Z., Webb, B., Meng, X.J. and Ramamoorthy, S. 2017. Ten years of PCV2 vaccines and vaccination: is eradication a possibility? Vet. Microbiol. 206, 21–28; doi:10.1016/j.vetmic.2016.10.002 Bandrick, M., Balasch, M., Heinz, A., Taylor, L., King, V., Toepfer, J. and Foss, D. 2022. A bivalent porcine circovirus type 2 (PCV2), PCV2a-PCV2b, vaccine offers biologically superior protection compared to monovalent PCV2 vaccines. Vet. Res. 53, 12; doi:10.1186/s13567-022-01029-w Bates, D., Mächler, M., Bolker, B. and Walker, S. 2014. Fitting linear mixed-effects models using lme4 [Remark 1]. ArXiv E-Prints arXiv:1406; doi:10.18637/jss.v067.i01 Cybulski, P., Woźniak, A., Podgórska, K. and Stadejek, T. 2020. Vaccination of sows against PCV2 in a subclinically infected herd does not impact reproductive performance. Agriculture 10, 639; doi:10.3390/agriculture10120639 Fajardo, L.E., Banico, E.C., Sira, E.M., Odchimar, N.M.O. and Orosco, F.L. 2025. Computational multi-epitope based design of a multivalent subunit vaccine against co-infecting African swine fever virus and porcine circovirus type 2. Vet. Integr. Sci. 23(1), 1–30; doi:10.12982/VIS.2025.065 Firth, C., Charleston, M.A., Duffy, S., Shapiro, B. and Holmes, E.C. 2009. Insights into the evolutionary history of porcine circovirus 2: an emerging livestock pathogen. J. Virol. 83, 12813–12821; doi:10.1128/JVI.01719-09 Franzo, G. and Segalés, J. 2018. PCV-2 genotype update and proposal of a new genotyping methodology. PLoS One 13; doi: 10.1371/journal.pone.0208585 Guo, J., Hou, L., Zhou, J., Wang, D., Cui, Y., Feng, X. and Liu, J. 2022. Porcine circovirus type 2 vaccines: commercial application and advances in research. Viruses 14, 92005; doi:10.3390/v14092005 Huan, C., Fan, M., Cheng, Q., Wang, X., Gao, Q., Wang, W., Gao, S. and Liu, X. 2018. Evaluation of the efficacy and cross-protective immunity of live-attenuated chimeric PCV1-2b vaccine against PCV2b and PCV2d subtype challenge in pigs. Front. Microbiol. 9, 455; doi: 10.3389/fmicb.2018.00455 Karuppannan, A. and Opriessnig, T. 2017. Porcine circovirus type 2 (PCV2) vaccines in the context of current molecular epidemiology. Viruses 9, 99; doi:10.3390/v9050099 Kosakovsky Pond, S.L., Poon, A.F.Y., Velazquez, R., Weaver, S., Hepler, N.L., Murrell, B., Shank, S.D., Magalis, B.R., Bouvier, D., Nekrutenko, A., Wisotsky, S., Spielman, S.J., Frost, S.D.W. and Muse, S.V. HyPhy 2.5-A customizable platform for evolutionary hypothesis testing using phylogenies. Mol. Biol. Evol. 2020;37(1):295–299; doi:10.1093/molbev/msz197 Opriessnig, T., Karuppannan, A.K., Castro, A.M.M.G. and Xiao, C.T. 2020. Porcine circoviruses: current status, knowledge gaps and challenges. Virus Res. 286, 198044; doi:10.1016/j.virusres.2020.198044 Opriessnig, T., Xiao, C.T., Halbur, P.G., Gerber, P.F., Matzinger, S.R. and Meng, X.J. 2017. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 35, 248–254; doi:10.1016/j.vaccine.2016.11.085 Sagulenko, P., Puller, V. and Neher, R.A. 2018. TreeTime: maximum-likelihood phylodynamic analysis. Virus. Evol. 4, vex042; doi:10.1093/ve/vex042 Shi, R., Hou, L. and Liu, J. 2021. Host immune response to infection with porcine circovirus. Anim. Dis. 1, 23; doi:10.1186/s44149-021-00027-3 Sirisereewan, C., Nguyen, T.C., Janetanakit, T., Kedkovid, R. and Thanawongnuwech, R. 2023. Emergence of novel porcine circovirus 2d strains in Thailand from 2019 to 2020. Front. Vet. Sci. 10, 1170499; doi: 10.3389/fvets.2023.1170499 Vargas-Bermudez, D.S., Gil-Silva, A.C., Naranjo-Ortíz, M.F., Mogollón, J.D., Gómez-Betancur, J.F., Estrada, J.F., Aldaz, A., Garzón-González, H., Angulo, J., Foss, D., Gutierrez, A.H. and Jaime, J. 2024. Detection of PCV2d in vaccinated pigs in colombia and prediction of vaccine T cell epitope coverage against circulating strains using EpiCC analysis. Vaccines 12, 1119; doi:10.3390/vaccines12101119 Wickham, H. 2011. Ggplot2: elegant graphics for data analysis. Biometrics 67, 678–679; doi:10.1111/j.1541-0420.2011.01616.x Xiao, C.T., Halbur, P.G. and Opriessnig, T. 2015. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 96, 1830–1841; doi:10.1099/vir.0.000100 | ||
| How to Cite this Article |
| Pubmed Style Fredmoore L. Orosco. Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Vet. J.. 2025; 15(9): 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 Web Style Fredmoore L. Orosco. Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. https://www.openveterinaryjournal.com/?mno=254314 [Access: November 22, 2025]. doi:10.5455/OVJ.2025.v15.i9.40 AMA (American Medical Association) Style Fredmoore L. Orosco. Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Vet. J.. 2025; 15(9): 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 Vancouver/ICMJE Style Fredmoore L. Orosco. Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Vet. J.. (2025), [cited November 22, 2025]; 15(9): 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 Harvard Style Fredmoore L. Orosco (2025) Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Vet. J., 15 (9), 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 Turabian Style Fredmoore L. Orosco. 2025. Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Veterinary Journal, 15 (9), 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 Chicago Style Fredmoore L. Orosco. "Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis." Open Veterinary Journal 15 (2025), 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 MLA (The Modern Language Association) Style Fredmoore L. Orosco. "Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis." Open Veterinary Journal 15.9 (2025), 4337-4345. Print. doi:10.5455/OVJ.2025.v15.i9.40 APA (American Psychological Association) Style Fredmoore L. Orosco (2025) Global dynamics of vaccine effectiveness and genotype shift in porcine circovirus 2: A cross-temporal analysis. Open Veterinary Journal, 15 (9), 4337-4345. doi:10.5455/OVJ.2025.v15.i9.40 |