| Research Article | ||
Open Vet. J.. 2025; 15(6): 2729-2749 Open Veterinary Journal, (2025), Vol. 15(6): 2729-2749 Research Article Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidiaAzhar Eltanahy1*, Dima Alkadri2, Mohamed Alaaeldein Elmorsy3, Huda A. EL- Emam1, Nahed A. El-Shall4, Bassem Elmishmishy5, Asmaa Elsayyad6,7, Gehad A. Ezzat8, Marwa A. El-Beltagy9, Mohamed Shaalan10, Radwa R. Elzwahary11, Ahmed I. El Sheikh12 and Ahmed M. Abdellatif131Department of Animal Wealth Development, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 2Department of Nutrition and Food Science, Faculty of Agriculture, Jerash University, Jerash, Jordan 3Department of Poultry and Rabbit Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 4Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt 5Parasitology Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 6Department of Physiology and Pharmacology, Faculty of Veterinary Medicine, King Salman International University, El-Tor, Egypt 7Department of Pharmacology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 8Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 9Biochemistry Department, Faculty of Veterinary Medicine, Suez Canal University, Suez, Egypt 10Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 11Education Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 12Department of Public Health, Faculty of Veterinary Medicine, King Faisal University, Hofuf, Saudi Arabia 13Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt *Correspondence to: Azhar Eltanahy. Department of Animal Wealth Development, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt. Email: azhar_r2011 [at] yahoo.com Submitted: 01/05/2025 Revised: 07/05/2025 Accepted: 08/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
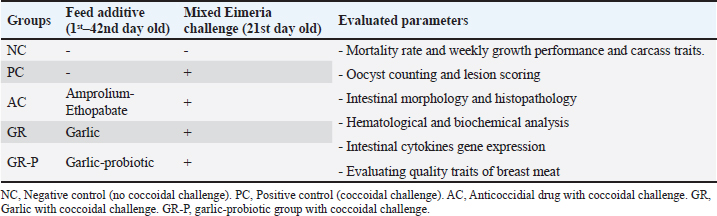
AbstractBackground: Coccidial intestinal infection induces significant economic losses in the production of birds. It also constitutes a major hazard of drug residues to meat consumers if the coccidia is controlled using anticoccidial drugs. Aim: This study aimed to assess the impact of dietary garlic as a natural alternative with or without probiotics compared to the anticoccidial drug against Eimeria infection of broilers. Methods: Growth rate, carcass dressing, blood metabolites, meat quality, intestinal histomorphology, oocyst shedding, and gene expression were assessed. A total of 150 one-day-old Ross broiler chicks were divided into five groups: NC, negative control; PC, positive control; AC, anticoccidial drug; GR, garlic; GR-P, garlic plus probiotics. The coccidia challenge was conducted for all experimental groups except NC. Results: PC birds showed a significantly lower weight gain and worse feed conversion ratio compared to the NC birds. The GR-P and AC groups revealed comparable results regarding WG and were significantly higher than the GR and PC groups. In comparison to the PC, AC, and GR-P groups, the GR group exhibited significantly decreased cecal lesion score, mortality rate, and oocyst shedding. GR-P improved carcass traits compared to other groups. GR-P and GR-P showed the highest scores for appearance, juiciness, tenderness, and the overall acceptability of breast meat. GR-P, GR, and AC reduced breast meat PH, Thiobarbituric acid reactive substances (TBARSs), fat, and ash compared to PC. AC, GR, and GR-P significantly increased jejunal and cecal villus length and crypt depth. Improvements in blood parameters were seen in the AC, GR, and GR-P groups rather than in the PC group. The coccidia challenge significantly induced changes in gene expression levels, while the three additives mainly reversed their expression. Conclusion: The present study reported a promising role of garlic-probiotics dietary supplements in broiler chickens’ growth performance during coccidiosis due to their antioxidant, anticoccidial, and antimicrobial activities. Keywords: Broilers, Coccidiosis, Eimeria, Growth, Gene expression, Phytobiotics.. IntroductionNowadays, broiler production has achieved great success in fulfilling the requirements of animal-origin protein on a large scale (Ata and Al-Masad, 2015). In Egypt, around 7.5 million households produce chicken and eggs. Poultry meat values are recorded annually between 1.3 and 3.5 billion USD (Herforth et al., 2020). Nevertheless, the poultry industry is still challenged by numerous diseases. Among these diseases is coccidiosis, a protozoal disease causing predominant enteric damage and primarily associated with epithelial injury, malabsorption, reduced performance, and increased mortality rates (El-Shall et al., 2022). Resistance to anticoccidial drugs is prevailing. Therefore, an active search for non-chemical sources including plant-and microbial-derived alternatives has progressively increased (Pender et al., 2016). The use of natural substances from various sources in animal feeds is a potential approach to enhance the utilization of nutrients and also to minimize drug residues (Pender et al., 2016). Dietary plants and their extracts have shown beneficial effects on broiler performance by boosting food consumption, digestive activity, and nutrient metabolism (Lipiński et al., 2019). Among several investigated herbs is garlic (Allium sativum), known for its antioxidant, immunomodulatory, and antimicrobial activities (Puvača et al., 2015). Garlic powder includes Fos proto-oncogene, ap-1 transcription factor subunit which has a prebiotic effect (Rivlin, 2001). Garlic has been revealed to contain various beneficial bioactive substances that include organosulfur compounds including alliin, allicin, diallylsulfide, ajoene, dithiin, and S-allylcystine (Khan et al., 2012). Garlic has been recognized as a medicinal herb in many diseases of metabolic and neoplastic origins involving the heart, blood vessels, intestine, and pancreas (Kim et al., 2009). In broiler chickens, boosting growth features, antimicrobial, antioxidative, antithrombotic, antiplatelet aggregation, and antihypertensive properties of garlic have been reported (Ismail et al., 2021). Probiotics, also defined as direct-fed microbials, are live bacteria and yeasts that are associated with health benefits upon their intake. They are usually served in single or multiple microbial strains and could affect the host’s performance by influencing the density of gut microbiota, suppressing the growth of pathogens, enhancing the intake and digestion of feed, and boosting immunity (Gewaily et al., 2021). Supplementing broiler diets with probiotics containing single bacterial species, Lactobacillus sp or Bacillus subtilis, significantly enhanced growth performance, intestinal morphology (Sen et al., 2012), and colonization of useful microorganisms to the intestinal wall (Jacquier et al., 2019). In addition, probiotics have also been found to lessen the invasion of pathogens of the avian intestinal tract to protect against enteric infections including Salmonella enteritidis (Park and Kim, 2014) and Escherichia coli (Cao et al., 2013). A multispecies probiotic preparation composed of Lactobacillus acidophilus, Lactobacillus plantarum, and Enterococcus faecalis minimized the effects of heat stress on growth performance and intestinal integrity in broilers from 22 to 42 days of age (Li et al., 2020). The different mechanisms by which probiotics act include the production of antibacterial substances and competition with the bacteria via occupation of the adhesion sites on host cells (Plaza-Diaz et al., 2019). Among the bacteria investigated as probiotics to mitigate the negative impacts of coccidia infection on livestock are Bacillus amyloliquefaciens and Clostridium butyricum, either in diets or water (Tsukahara et al., 2018; Sureshkumar et al., 2021). Broiler chickens that received multispecies probiotic preparation during the 2–36 days of life revealed a better response to coccidial vaccines compared to those without probiotic supplementation (Ritzi et al., 2016). Indeed, coccidial vaccines when combined with daily probiotic intake resulted in a significant improvement in cecal microbial diversity of the coccidia-challenged broilers (Wang et al., 2019). A synergistic positive effect of probiotics and the anticoccidial drug diclazuril on experimentally induced coccidiosis has been suggested in broilers (Memon et al., 2022). Taken together, these data could indicate a more potent effect for probiotics when combined with different anticoccidial agents. The present study aimed to evaluate the influence of dietary intervention of garlic powder and/or a probiotic mixture of 10 bacterial strains compared to anticoccidial drug on the prevention of mixed Eimeria infection in broiler chickens through examining different parameters related to growth performance, mortality, hematological and biochemical parameters, meat quality and carcass traits, oxidative stress, intestinal morphology, histopathological changes, and gene expression. Material and MethodsHousing and experimental birdsThe present study was licensed by the Ethics Committee of the Faculty of Veterinary Medicine at Mansoura University (VM.R.23.07.112). Birds were maintained in floor pens provided with wood shavings litter. 150 one-day-old (Ross 308) broiler chicks were purchased from a commercial hatchery and were reared under constant lighting and at a starting temperature of 35ºC–32ºC. The temperature was exposed to a gradual decline between 25ºC and 28ºC through the upcoming 2 weeks. All the broiler chicks received experimental starter diets from 0 to 10 days of age, then grower diets were fed from 11 to 24 days of age, and finisher diets were supplied from 25 to 42 days of age. Diets were prepared according to (Aviagen, 2019) and (Behairy et al., 2023). The experiment was performed in a ventilated place to prevent increased humidity and jammed ammonia. Biosecurity measures and sanitary conditions were applied to avoid natural coccidial infection. Birds were fed a non-treated ration and drinking water ad libitum. The chicks were weighed and randomly assigned into five groups having three replicates of 10 birds each, and the birds were raised for 42 days as follows: group 1 (NC): unchallenged, unmedicated (negative control); group 2 (PC): coccidia-challenged, unmedicated (positive control); group 3 (AC): coccidia-challenged, diet supplemented with a chemical anticoccidial agent; and group 4 (GR): coccidia-challenged, diet supplemented with garlic; and group 5 (GR-P): coccidia-challenged, diet supplemented with garlic plus probiotic mixture. The experimental design is shown in Table 1. Table 1. The experimental design of the present study.
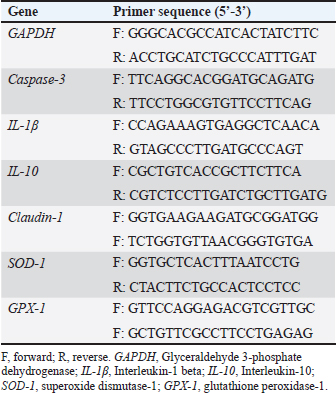
Feed additives and Eimeria challengeDietary additives were applied to the feed from 1 to 42 days old. The chicks were inoculated on the 21st day via intracrop injection using a coccidial live vaccine (FORTEGRA®, Intervet Inc. Omaha, NE, USA). It was composed of Eimeria acervulina, Eimeria maxima, Eimeria tenella, and Eimeria mivati, and each bird received a dose of 20x. G1 was sham-challenged with phosphate buffer saline. The anticoccidial drug (AC) is ATOPROL P LUS®, each kg of which contains Amprolium HCL 254.47 gm, equivalent to 225 gm Amprolium base, and Ethopabate 16 gm was added to the basal diet of group 3 by dose 0.55 kg/ tone feed, manufactured by ATCO pharma for pharmaceutical industries for Abo Elnaga trading Co., Egypt. The garlic powder (GR) was obtained from a local pharmacy in the form of tablets, Tomax plus®, 300 mg dried garlic powder, containing not less than 0.45% Allicin, produced by Atos Pharma, Egypt, M.O.H. Reg.NO.746/2018. It was added by 0.75 g/kg diet for groups 4 and 5 according to Ismail et al. (2021). The probiotic mixture (Physician’s CHOICE, Wheat Ridge, CO, USA) consists of ten bacterial strains of the genera Lactobacillus and Bifidobacterium, Lactobacillus casei, Lactobacillus paracasei, L. acidophilus, Lactobacillus salivarius, L. plantarum, Lactobacillus bulgaricus, Bifidobacterium lactis, Bifidobacterium longum, Bifidobacterium bifidum, and Bifidobacterium breve, that was included in the diet at a dose of 1 × 109 CFU/kg in the view of previously published studies (Li et al., 2020; Summers et al., 2022). Growth performance and mortality rateThe chicks were weighed individually on the first day and then every week till the end of the experiment at 42 days. The same was done to the feed intake. The weight gain of chicks and feed conversion ratio (FCR) were also calculated. They were observed daily for deaths. Lesion scoring and oocyst counting On days 5, 6, and 7 post-coccidia-challenge (pch), 3 birds per group (a bird per replicate) were euthanized by rapid cervical dislocation, and the intestinal and cecal lesion scores were assessed. Sections of the small intestine, including the duodenum, jejunum, and ileum, and the ceca were assigned a score depending on a 0–4 scale (Johnson and Reid, 1970). Dropping samples will be collected from day 4 to day 9 pch for oocyst counting using the McMaster slide (Nematollahi et al., 2009). Gross dissection, histology, and morphometryBirds (n = 6 per group) were randomly chosen and exposed to dissection on the fifth day pch. Birds were euthanized via cervical dislocation followed by whole-blood exsanguination (Abdellatif, 2021; Abdellatif et al., 2022). Immediately following death confirmation, the gastrointestinal tract was reached via a ventral midline incision, and the various segments of the digestive tract were located, exposed, and examined. The sampled parts of the digestive tract included the small intestine (duodenum, jejunum, and ileum) and the large intestine (ceca and rectum). All collected samples were cleaned with 0.9% saline and fixed in 10% neutral buffered formalin for 72 hours. Fixed samples were processed for paraffin embedding through dehydration in ascending grades of ethanol (50, 80, 90, 95, 100), clearance in xylene (2x), infiltration in xylene-paraffin mixture, and finally embedding in molten paraffin (58°C). Four μm-thick sections were cut, mounted on clean glass slides, and stained using hematoxylin and eosin (H&E) for general histological evaluation (Suvarna et al., 2019). The H&E-stained sections were photographed using an Olympus CH30 microscope (Olympus, Tokyo, Japan). 15 randomly captured 100x microscopic images from the jejunum and cecum were used for the morphometric analysis using ImageJ2 software (ver. 2.14/1.54f). The evaluated intestinal morphometric parameters of the broiler chickens were villus length, villus width, and crypt depth. Carcass traitsOn the last day of the growing period, 6 birds from each dietary treatment were randomly collected, starved overnight, and allowed only an ample supply of drinking water. Each bird was weighed individually and then slaughtered by puncturing the jugular and carotid veins, after which they were bled. In each group, the birds were eviscerated and weighed without feathers and heads. The dressing percentages were computed as described by Brake et al. (1993). Dressing %=Dressed carcass weight/live weight *100. Gizzard and liver were recorded and their percentage relative to live body weight; the most major carcass parts included breast, thighs, and drumsticks. Following slaughtering, cold carcasses were processed based on poultry meat regulations and cut into breasts, thighs, and drumsticks, then partitioned and weighed separately as the most important edible parts, and their proportions were expressed as percentages of live body weight according to (Nikolova and Pavlovski, 2009). Muscle thickness of breast muscle was measured by using a metal digital Pachometer Caliper (Huangyuxing Group). Co. Ltd China, free-range (0–15 cm). RNA Isolation and quantitative real-time PCR analysisSix chickens per group were randomly selected. Intestinal tissues were harvested and kept at –80°C till RNA was done. RNA extraction was performed using the QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH) based on the manufacturer’s method. The concentration and integrity of the collected RNA were detected using a NanoDrop spectrophotometer (NanoDrop ND-1000TM, Waltham, USA). RNA was reverse transcribed to cDNA using the QuantiTect Reverse Transcription kit (Qiagen, Heidelberg, Germany) following the manufacturer’s guides. The relative messenger RNA (mRNA) expression levels of caspase-3, IL-1β, IL-10, claudin-1, SOD-1, and GPX-1 were detected for every sample with real-time PCR (Qiagen, Heidelberg, Germany) by SYBR Green QuantiTect PCR kits (Qiagen, Heidelberg, Germany). The sequence of the used primers is shown in Table 2. The reaction mixture was as follows: 12.5 μl of 2x SYBR Green PCR Mastermix, 0.5 μl of each primer at 20 pmol concentration, 0.25 μl of RevertAid Reverse Transcriptase (200 U/μl) (Thermo Fisher), 3 μl of RNA template, and 8.25 μl of water. The reaction was carried out in a Stratagene MX3005P real-time PCR machine. The conditions of the thermal cycle included initial denaturation at 95°C for 15 minutes for 40 cycles, followed by initial heat activation at 94°C for 15 seconds; primer annealing was 60°C for 1 minute for all genes, and; eventually, elongation at 72°C for 30 seconds. Relative fold changes in the mRNA expression of the studied target genes were computed using the comparative 2–ΔΔCt method (Ct: cycle threshold) with the GAPDH as an internal reference gene to normalize levels of target gene expression. Table 2. Primer sequences of the investigated genes.
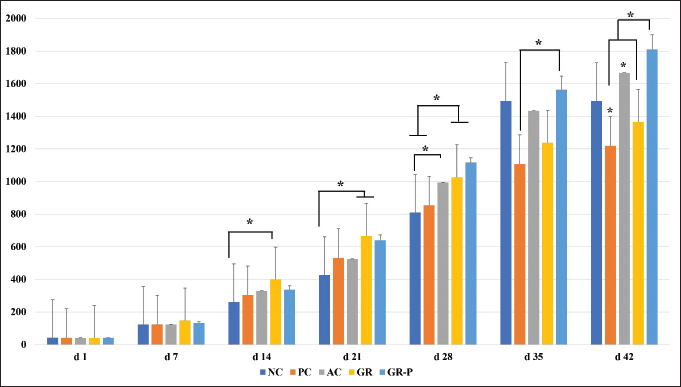
Hematological and biochemical analysisOn day 7 pch, individual blood samples (1 ml) (n = 3/group) were drained from the wing vein in EDTA vials for hematology assessment. Calculation of the total leucocyte count white blood cells (WBCs) and the total erythrocyte count red blood cells (RBCs) was performed (Natt and Herrick, 1952). Hemoglobin was measured following the cyanomethemoglobin protocol (Van Kampen and Zijlstra, 1961). Differential leucocyte count was carried out through a peripheral blood smear stained with Wright-Giemsa stain (Benjamin, 1985). Evaluation of quality traits of broilers` breast meatSamples of broilers` breast muscles were collected from six birds in each treatment group on the last day of the experiment after slaughtering, de-feathering, and evisceration, and then the sensory and physicochemical properties of the breast meat were analyzed as follows. Sensory evaluationThe cooked breast meat samples (n = 6/treatment) were chopped into cubes (2 cm) immediately after slaughter. Broiler chicken cuts were cooked in a convection oven (Heraeus, D-63450 Hanau, Germany) at 150ºC for 20 minutes until a core temperature of 75ºC was obtained. Twenty-five experienced and well-trained panelists from the Food Hygiene and Control Department, Faculty of Veterinary Medicine, Cairo University, Egypt staff members were selected based on their previous experience in sensory evaluation of chicken meat outlined by ISO-11035 (Benjamin, 1985). Additionally, they received a preparatory session related to descriptive analysis of cooked chicken breast. Three sessions were held on every evaluation occasion on 3 different days (3 × 3 = 9 sessions total). Cooked breast meat samples were placed in foam plates coded with random 3 digits. Each panelist assessed the cooked chicken breast meat samples in a randomized order and was asked to give a numerical value between 1 (denotes extremely unacceptable) and 7 (denotes extremely acceptable) following the descriptive sensory assessment carried out using a variation of (Kenawi, 2005), the schemes of (Sumarmono and Rahardjo, 2008), and (Baston and Barna, 2010) for the following criteria: appearance, color, flavor, tenderness, and juiciness. At the end of evaluating the samples, each panelist was asked to provide a score for overall acceptability. Tap water was provided between sessions to alter the mouth feel. Before the analysis, panelists were also trained in the definition and intensities of all investigated sensory parameters. Physicochemical quality pH valueSamples (five grams per sample) were homogenized with 20 ml of distilled water for 10–15 seconds. The pH of the slurry was calculated using a digital pH meter (Lovibond Senso Direct) with a probe-type electrode (Senso Direct Type 330). Thiobarbituric acid reactive substancesThiobarbituric acid reactive substances were estimated following the assay of Du and Ahn (2002). Five grams of breast meat were exposed to homogenizing with 15 ml of deionized distilled water. One milliliter of the homogenate was moved to a test tube and 2 ml of thiobarbituric acid (TBA), 50 μl of butylated hydroxytoluene (7.2 g/100 g), and trichloroacetic acid (TCA) (15 mM TBA, 15 g/100 g TCA) were included. The mixture was subjected to a vortex and then incubated using a bath of boiling water for 15 minutes to acquire color. Then samples were left to cool in cold water for 10 minutes and then vortexed before centrifugation for 15 minutes at 2,500 × g. The absorbance of the concluded supernatant was evaluated at 531 nm against a blank carrying 1 ml of deionized water and 2 ml solution of TBA and TCA. The contents of TBARS were determined as mg of malonaldehyde per kg of meat. Chemical compositionProximate composition (moisture, fat, protein, and ash) contents of all breast muscle samples from each treatment were determined from each replicate based on the protocol performed by AOAC (1995) using the guidelines provided by the Association of Analytical Chemists. For the evaluation of the contents of moisture (g % sample), 10 g of the sample was dried at 100ºC until a fixed weight was achieved. Protein content (g % sample) was recorded according to the Kjeldahl protocol. For the conversion of nitrogen into crude protein, a factor of 6.25 was applied. Fat (g % sample) was measured by 6-cycle extraction with petroleum ether in a Soxhlet apparatus and calculating the weight loss. Ash (g % sample) was estimated by ignition at 500ºC for 5 hours. Statistical analysisShapiro–Wilk’s test was used to verify the normality, and Levene’s test was used to verify the homogeneity of variance components between experimental treatments. Parameters related to growth performance, hematology, blood chemistry, carcass traits, and oocyst count were analyzed using the Statistical Analysis System SAS (SAS Institute Inc., Cary, NC, USA) by means of the general linear model procedure. Parameters related to lesion scoring, intestinal morphometry, and breast muscle characteristics were analyzed using GraphPad Prism 7 (GraphPad Software Inc.; San Diego, CA, USA). Variances between groups were compared using ANOVA with Tukey`s post hoc tests. p-values < 0.05 were applied to evaluate statistical significance. ResultsEffect of dietary garlic and garlic-probiotic on the growth performance of coccidia-infected broilersSupplementation of broiler diets with AC, GR, or GR-P revealed a non-significant increase (p > 0.05) in the live body weight during the early pre-coccidia-challenge period (0–21 days old) compared to the NC group. However, a significant increase was observed beginning from the 14th day old (d) by GR and the 21st day by GR-P till the 28th day (p < 0.05). During the pch period (28–42 days), compared to the PC group, a significant increase in broilers’ live body weight was observed in groups that were fed either GR (on the 28th day), GR-P (on the 28th, 35th, and 42nd day), or AC (on the 42nd day) (p < 0.05). A superior effect on the live body weight of coccidia-infected broilers was observed by GR-P over the GR on the 35th day (p < 0.05), and 42nd day (p < 0.05) and AC from the 28th day till the experimental end (p < 0.05). The live body weights of GR and AC-supplemented groups were comparable (p > 0.05) (Fig. 1).
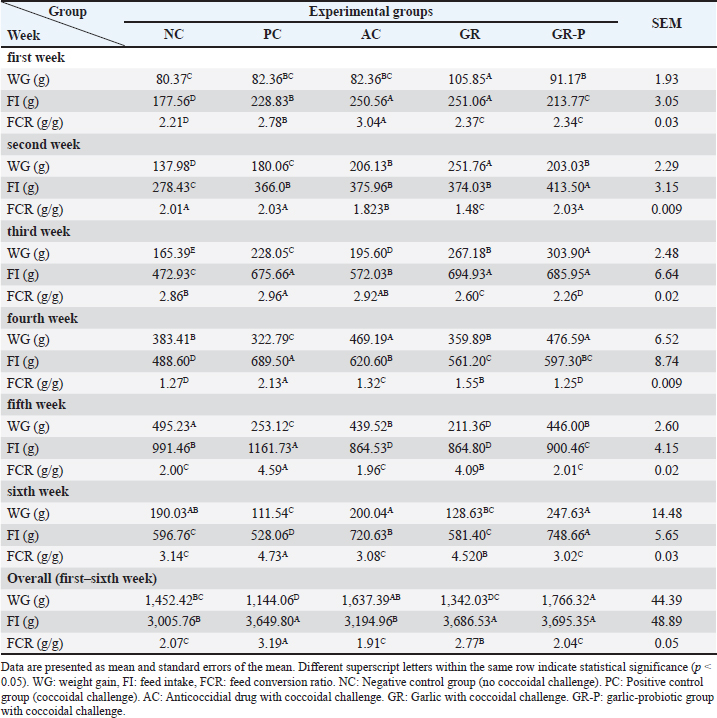
Fig. 1. Live body weight (g) of coccidia-challenged broilers fed diets enriched with garlic only or garlic-probiotics. Asterisk or asterisk over a bar indicates significant difference between those groups (p < 0.05). d: day. NC: Negative control group (no coccoidal challenge). PC: Positive control group (coccoidal challenge). AC: Anticoccidial drug with coccoidal challenge. GR: Garlic with coccoidal challenge. GR-P: garlic-probiotic group with coccoidal challenge. Regarding weight gain in the pre-coccidia-challenge period, the significant positive effect of GR and GR-P was observed beginning from the first week and second week, respectively, compared to non-supplemented groups (p < 0.05). Coccidia challenge induced a significantly lower weight gain in PC birds than NC ones that continued to the 42nd d (p < 0.05) with a total weight loss of 21.2%. In the first–third week pch, GR-P and AC supplementation resulted in significantly higher weight gain than GR and PC groups (p < 0.05) as well as the subsequent overall weight gain. GR supplementation induced a higher, though insignificant (p > 0.05), increase in weight gain than PC birds. The weekly weight gain and overall growth performance of the GR-P and AC groups were comparable (p > 0.05) (Table 3). Table 3. The effect of dietary garlic or garlic-probiotics on the growth performance of coccidia-challenged broilers.
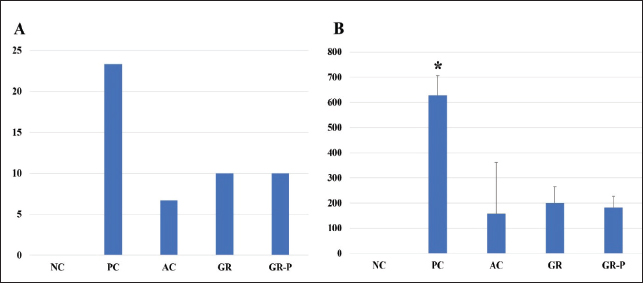
The lowest total feed intake was recorded by the NC and AC groups when compared to the PC, GR, and GR-P groups (p < 0.05) (Table 3). Coccidia-challenge induced a significant increase in the weekly and overall FCR of PC birds compared to NC birds (p < 0.05). However, all tested supplements (AC, GR, and GR-P) improved the FCR significantly compared to PC birds (p < 0.05). The best results of FCR were obtained by GR-P and AC supplementations that are comparable to NC birds (p > 0.05), while the FCR of the GR group was worse than the NC group (p < 0.05) (Table 3). Effect of dietary garlic and garlic-probiotics on mortality rate, intestinal lesion scoring, and oocyst counting in coccidia-challenged broilersCoccidia challenge of broiler chickens in the PC group induced the death of 7 birds (23.33%), while supplementation of AC, GR, and GR-P decreased the mortality rate to 6.67% (2 birds), 10% (3 birds), and 10% (3 birds), respectively. The NC group showed no mortality (Fig. 2A). The number of coccidia oocysts in birds’ droppings was significantly higher in the PC group (627.6 × 102 ± 79) than AC (158.2 × 102 ± 203), GR (204 × 102 ± 64), and GR-P (182.2 × 102 ± 45) groups (p < 0.05) (Fig. 2B).
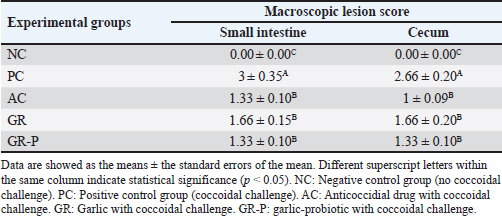
Fig. 2. (A) Mortality rate of experimental groups. (B): Total oocyst count (x102) from the fourth–ninth day post coccidia-challenge.NC: Negative control group (no coccoidal challenge). PC: Positive control group (coccoidal challenge). AC: Anticoccidial drug with coccoidal challenge. GR: Garlic with coccoidal challenge. GR-P: garlic-probiotic with coccoidal challenge. Asterisk means significant difference of this group compared to other groups (p < 0.05). The reduction % of oocyst shedding is 74.8%, 67.5%, and 71% by AC, GR, and GR-P supplementation, respectively. No oocysts were detected in the droppings of non-challenged birds. Post-mortem examination and lesion scoring revealed that the NC group showed zero lesion scores in the small intestine and cecum. The small intestinal lesion scores were lower in different treated groups compared to the PC group (p < 0.05). Similarly, the cecal lesion scores were decreased by AC (p < 0.05), GR (p < 0.05), and GR-P (p < 0.05) treatment in comparison with untreated PC birds. Noteworthy, the lesion scores of all supplemented groups were of comparable values (p > 0.05) (Table 4). Table 4. Macroscopic lesion score in coccidia-challenged broilers fed diets enriched with garlic or garlic-probiotics.
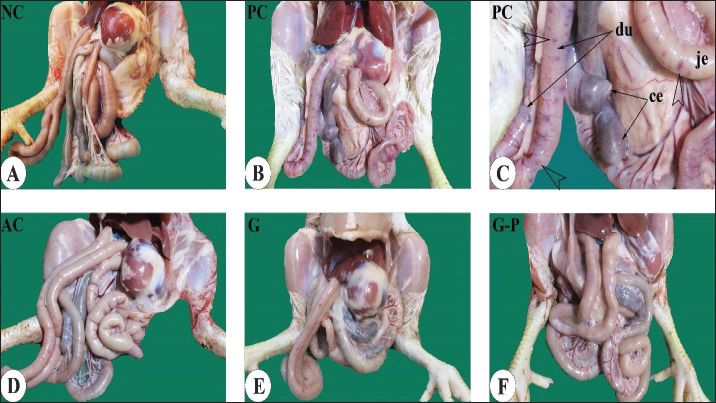
Effect of dietary garlic and garlic-probiotic on gross morphology of the intestine of broilersGross dissections of birds from different experimental groups 5 days after challenge with coccidial oocysts revealed more enlarged and swollen duodenum and jejunum together with more distended and impacted ceca in the positive control group compared to the GR and the GR-P groups, which appeared of comparable appearance to that of the NC and AC groups (Fig. 3).
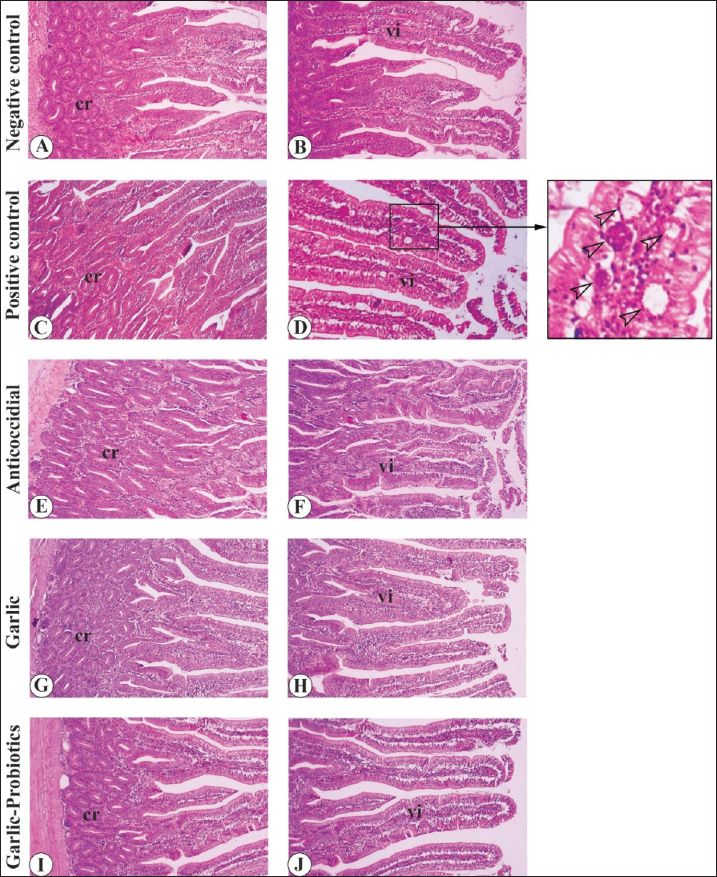
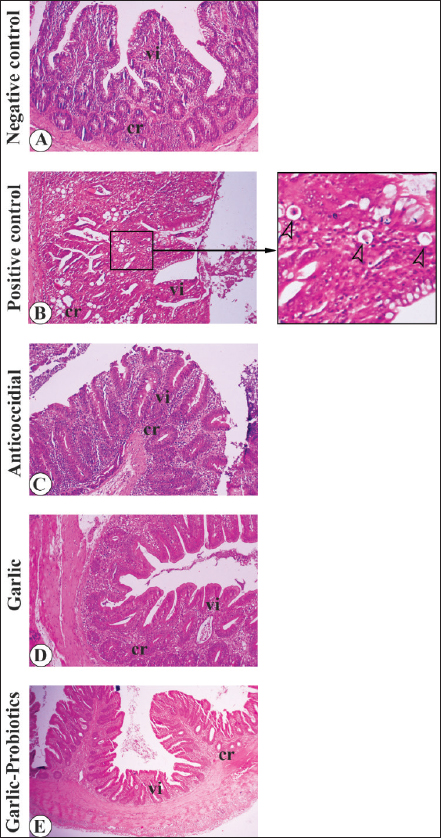
Fig. 3. Gross appearance of the gastrointestinal tract in different broiler groups 5 days after challenge with coccidial oocysts. A. Negative control group (no coccoidal challenge) (NC). B, C. Positive control group (coccoidal challenge) (PC). D. Anticoccidial drug with coccoidal challenge (AC). E. Garlic with coccoidal challenge (GR). F. Garlic-probiotic with coccoidal challenge (GR-P). Compared to other groups, the PC group revealed numerous focal areas of inflammation (empty arrowheads in C) in the duodenum (du) and jejunum (je) together with distended ceca (ce). Effect of dietary garlic and garlic-probiotic on microscopic appearance of the intestine of broilersLight microscopic examination of H&E-stained sections from the jejunum and ceca 5 days after challenge with coccidial oocysts revealed more distortion of the tissue architecture of the intestinal mucosa in the PC group compared to the GR and GR-P groups, which appeared of equivalent appearance to that of the NC and AC groups (Figs. 4 and 5). A large number of developmental stages of coccidia were seen within the villus mucosa of the jejunum and cecum in the PC group (Figs. 4C, D and 5B). These coccidial developmental stages were seldom seen in other experimental groups.
Fig. 4. Light microscopic appearance of the jejunum in different broiler groups 5 days after challenge with coccidial oocysts. A,B. Negative control group (no coccidial challenge). C, D. Positive control group (coccidial challenge). Note the presence of developmental stages of coccidia within the jejunal mucosa (empty arrowheads in the magnified inset). E,F. Anticoccidial drug group (coccidial challenge followed by administration of anticoccidial drug). G,H. Garlic group (coccidial challenge with garlic-enriched diets). I,J. Garlic-probiotics group (coccidial challenge with garlic-probiotic-enriched diets). cr, crypts; vi, villi. Magnification = x 100.
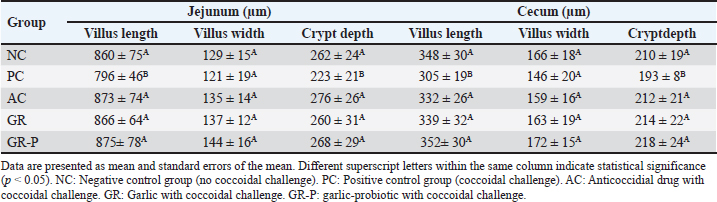
Fig. 5. Light microscopic appearance of the cecum in different broiler groups 5 days after challenge with coccidial oocysts. A. Negative control group (no coccidial challenge). B. Positive control group (coccidial challenge). Note the presence of developmental stages of coccidia within the cecal mucosa (empty arrowheads in the magnified inset). C. Anticoccidial drug group (coccidial challenge followed by administration of anticoccidial drug). D. Garlic group (coccidial challenge with garlic-enriched diets). E. Garlic-probiotics group (coccidial challenge with garlic-probiotic-enriched diets). cr, crypts; vi, villi. Magnification = x 100. Morphometric analysis of the intestinal wall 5 days after challenge with coccidial oocysts revealed a decrease in villus length and crypt depth in the PC group in comparison to the GR and GR-P groups, which displayed similar dimensions to those of the NC and AC groups (Table 5). Table 5. Intestinal morphometry in coccidia-challenged broilers fed diets enriched with garlic or garlic-probiotics.
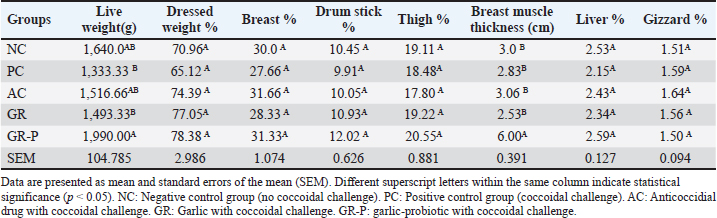
Effect of dietary garlic and garlic-probiotic on Carcass traits in coccidia-challenged broilersExcept for breast muscle thickness that showed a significant increase (p < 0.05) in the GR-Pgroup compared to other experimental groups, limited differences were observed in all evaluated carcass traits including dressed weight percentage, breast muscle percentage, drumstick percentage, thigh muscle percentage, liver percentage, and gizzard percentage, as shown in Table 6. Table 6. Carcass traits in coccidia-challenged broilers fed diets enriched with garlic or garlic-probiotics as a percentage of pre-slaughter (live) weight.
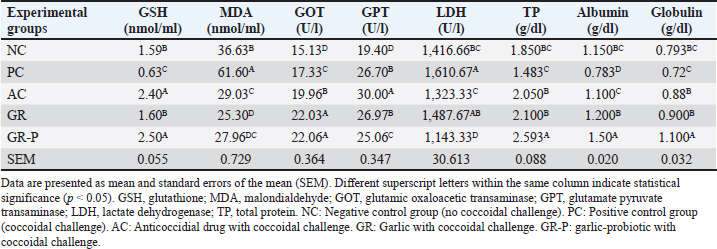
Effect of dietary garlic and garlic-probiotic on serum antioxidant and biochemical parameters of coccidia-challenged broilersCoccidia challenge increased MAD and decreased glutathione (GSH), albumin, and total protein significantly related to negative control birds (p < 0.05). AC, GR, and GR-P supplemented groups induced a reverse situation (p < 0.05). glutamate oxaloacetate aspartate aminotransferase (GOT), glutamate pyruvate alanine aminotransferase (GPT), and lactate dehydrogenase (LDH) were significantly increased by coccidia challenge (PC birds) compared to NC birds (p < 0.05). However, serum GOT was significantly higher by AC, GR, and GR-P supplementation than both control birds (NC and PC) (p < 0.05). While GR-P group showed a significant decrease in GPT, while the AC group showed a significant decrease in GPT and LDH compared to PC birds (p < 0.05). GR showed no significant effect of GPT and LDH compared to PC birds (p > 0.05). Significantly higher levels of TP, albumin, and globulin (p < 0.05) were noted in AC, GR, and GR-P groups with a higher magnitude for the latter (Table 7). Table 7. Serum antioxidants and biochemical parameters of broilers fed diets enriched with garlic or garlic-probiotics.
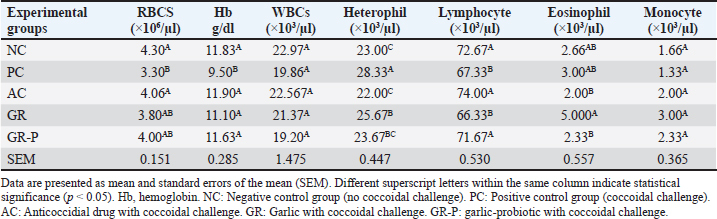
Effect of dietary garlic and garlic-probiotics on hematological parameters of broilersThe RBC count, Hb%, and lymphocytes were significantly decreased, while the heterophil count was significantly increased by coccidia-challenge (PC group) compared to the NC group (p < 0.05). Hematological analysis of all groups demonstrated a significant elevation (p < 0.05) in hemoglobin content and a decrease in heterophil count in AC, GR, and GR-P groups than in PC birds. The change in the total number of WBCs and the percentage of monocytes between the groups were not statistically significant. A significantly increased lymphocyte count was seen in the AC and GR-P groups (p < 0.05) compared to PC and GR. However, the percentages of heterophils in the PC group significantly differed between unchallenged birds and challenged birds (p < 0.05) (Table 8). Table 8. Hematological parameters of coccidia-challenged broilers fed diets enriched with garlic or garlic-probiotics.
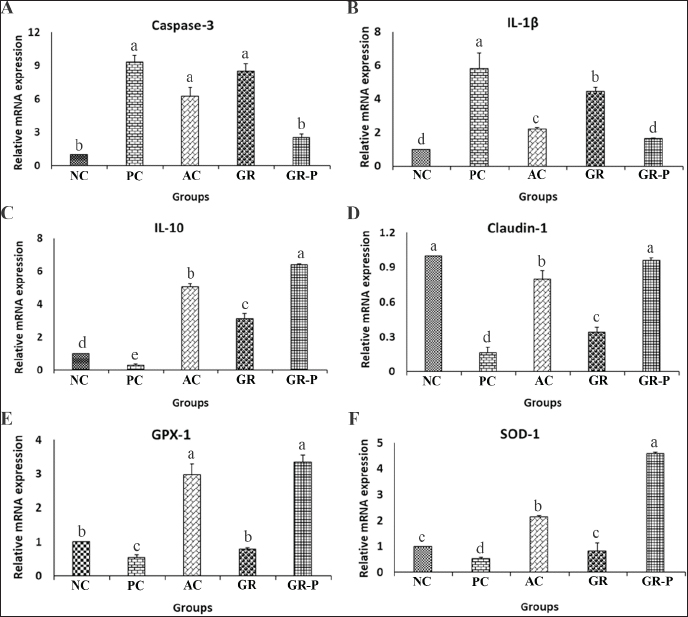
Effect of dietary garlic and garlic-probiotic on intestinal gene expression of coccidia-challenged broilersThe mRNA expression level of the caspase-3 gene related to apoptosis was examined to investigate its responses to different treatments. The mRNA expression level of caspase-3 was noticeably upregulated in the PC, AC, and GR groups relative to the NC group (p < 0.05). However, there was a significant downregulation in the GR-P group compared to the NC group (p > 0.05) (Fig. 6A). The mRNA expression level of the proinflammatory cytokine IL-1β revealed a similar trend to that of caspase-3, though with comparable expression levels in both the NC and the GR-P groups (Fig. 6B). Conversely, the mRNA expression level of the anti-inflammatory cytokine IL-10 was significantly higher in the AC, GR, and GR-P groups than in the PC group. Notably, the IL-10 gene expression level of the supplemented groups significantly exceeded that of the NC group (Fig. 6C). Regarding intestinal permeability, there was a significant upregulation of the claudin-1 gene in all supplemented groups relative to the challenged PC group (p < 0.05). However, only GR-P supplementation was able to restore the gut wall integrity to a level similar to that of NC birds (Fig. 6D). The mRNA expression levels of the antioxidant factors glutathione peroxidase 1 (GPX-1) and superoxide dismutase 1 (SOD-1) were heightened in both AC and GR-P groups with more pronounced effects for the latter (Fig. 6E, F).
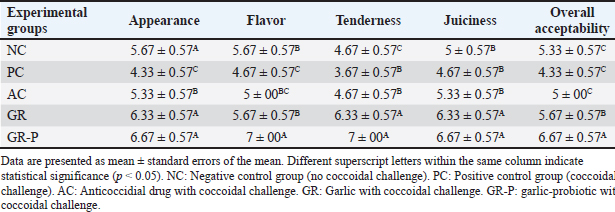
Fig. 6. Expression of genes related to apoptosis, inflammation, permeability, and antioxidant response in small intestinal tissues of different coccidia-challenged broiler groups. A. Caspase-3. B. IL-1β (Interleukin-1 beta). C. IL-10 (Interleukin-10). D. Claudin-1. E. GPX-1 (glutathione peroxidase-1). F. SOD-1 (superoxide dismutase-1). NC: Negative control group (no coccoidal challenge). PC: Positive control group (coccoidal challenge). AC: Anticoccidial drug with coccoidal challenge. GR: Garlic with coccoidal challenge. GR-P: garlic-probiotic with coccoidal challenge. Effect of dietary garlic and garlic-probiotic on sensory quality attributes of breast meat of broilersThe impacts of dietary garlic and probiotic supplementation on broilers’ meat sensory quality attributes are presented in Table 9. It was noticed that the dietary supplementation of both GR and GR-P improved the sensory panel scores of broiler’s breast meat; however, the effect became clearer and stronger in the case of the garlic probiotics. The highest scores for appearance, flavor, tenderness, juiciness, and overall acceptability were presented in groups supplemented by GR and GR-P with significant differences (p < 0.05), especially when compared to the PC group. Table 9. Sensory quality attributes of breast meat of coccidia-challenged broilers fed diets enriched with garlic or garlic-probiotics.
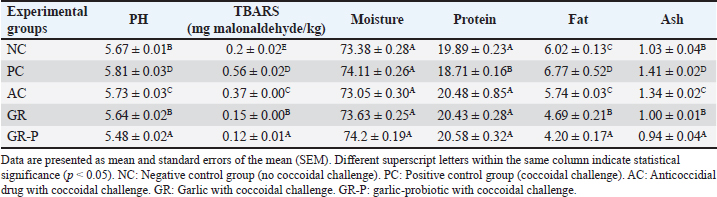
Effect of dietary garlic and garlic-probiotic on physicochemical and nutritional quality characteristics of breast meat of coccidia-challenged broilersThe influences of dietary garlic and probiotic supplementation on the physicochemical and nutritional quality characteristics of broiler breast meat are presented in Table 10. The results of proximate chemical analysis of breast meat revealed a modest difference between the groups in terms of the moisture content of meat. However, a significant elevation in protein content (p < 0.05) was obtained in the groups supplemented with GR and GR-P, with a relatively lower elevation in the group supplemented with AC, especially when compared to the PC group. However, there was a significant reduction in the content of fat and ash of the breast muscle in the group supplemented with the GR-P, GR, and PC groups, respectively, while being elevated in the PC and AC groups. Table 10. Physicochemical and nutritional quality characteristics of breast meat of broilers fed diets enriched with garlic or garlic-probiotics.
DiscussionProbiotics are single or mixed cultures of live microorganisms that positively influence the host by enhancing the balance of their intestinal microbiota (Ahmed et al., 2022). Potentiating the colonization of beneficial bacteria via non-drug dietary additives is essential to improve the intestinal microbiome, and boost the function of the gut barrier (El-Bouhy et al., 2021). Supplementing herbals and their extracts to broiler diets has been verified to have a promising influence on their performance by activating feed consumption, enhancing the release of digestive enzymes, and augmenting intestinal uptake of nutrients. Plant-based substances are preferred to chemicals in control of coccidiosis in broiler chickens due to their short life span; thus avoiding the presence of drug resides in the meat of slaughtered birds (Saeed and Alkheraije, 2023). The performance of broilers and scoring of intestinal lesions are crucial biomarkers used to assess the severity of enteric diseases such as coccidiosis. Therefore, the present study highlighted the impact of dietary treatment with garlic powder and/or a probiotic mix of ten bacteria compared to the anticoccidial drug for preventing mixed Eimeria infection in broiler chickens through assessment of various indices including growth, mortality, hematological and biochemical indicators, meat quality and carcass traits, oxidative damage, and intestinal morphology, histopathological alterations, and gene expression. In this study, during the pre-challenge period (till the 21st day of age), the best FCR was observed in GR and GR-P groups. Previous studies reviewed by Rusli et al. (2022) revealed a growth-promoting impact of garlic supplementation on broiler chickens. This improvement in weight gain, feed intake, and FCR can be directly attributed to the high quantity of amino acids, minerals, enzymes, and sulfur-containing compounds (allicin and diallyl sulfide) in garlic. In direct effects include garlic’s action on digestive enzymes (Brzóska et al., 2015) and the count of intestinal pathogens (Ibrahim et al., 2021) that could boost digestion and improve performance. Regarding probiotics, several studies reported high productivity induced by their dietary inclusion (Timmerman et al., 2006; Awad et al., 2009; Awad et al., 2010; Ipek et al., 2016; Deng et al., 2020). The data of the present study are consistent with that of the aforementioned studies where probiotic intake significantly improved growth performance and intestinal barrier functions. However, birds fed anaerobic bacteria, lactose-fermenting enterobacteria, Enterococcus spp., and L. acidophilus during the first 21 days of age presented similar weight gain as control birds (Timmerman et al., 2006). In general, according to Jha et al. (2020), probiotics have the power to replace antibiotic growth promoters by altering the intestinal microbiota, stimulating the immune system, reducing inflammatory reactions, preventing pathogen colonization, improving growth performance, modifying the apparent digestibility coefficient, and lowering the excretion of ammonia and urea in poultry production. However, these outcomes could be impacted by several variables including birds’ intestinal health, incubation conditions, feed, and water supply, probiotic dose, nature, and method of intake (feed or drinking water). Upon Eimeria mixed infection, the birds displayed weight loss of 21.2% and a significantly lower FCR, in addition to decreased serum total protein, RBCs count and Hb% compared with non-challenged birds. Although the main cause for this weight loss wasn’t investigated, the impaired intestinal integrity induced by coccidia could be the main cause of poor growth performance. Epithelial cell tight junctions, such as Claudin-1, OCLDN, JAM, and ZO are the primary constituents of the intestinal mucosal barrier. Previous studies revealed that chickens infected with Eimeria tenalla (Mohsin et al., 2022) or E. maxima (Su et al., 2014; Su et al., 2015) exhibited down-regulation of nutrient transporter gene expression in the small intestine. Therefore, coccidiosis action might involve both paracellular and transcellular translocation by rupturing the tight junctions connecting enterocytes and degrading intestinal epithelial cells (Teng et al., 2021). Moreover, coccidiosis-induced oxidative stress was indicated by increasing serum Malondialdehyde and decreasing GSH as well as the downregulating antioxidant enzyme, SOD-1, and GPX-1, gene expression. Oxidative damage can produce molecular lesions and activate apoptosis (Kasprzak, 1991). Therefore, the activation of Caspase-3, an important marker of apoptosis, mRNA expression observed in coccidia-challenged birds could indicate apoptosis mediated by excessive numbers of coccidia within the intestinal lumen. As a result, the cellular destruction, morphologic alterations in the intestinal mucosa, and impaired epithelial barrier functions resulted in the reduction of absorptive surface area (Nabian et al., 2018), impaired digestion and protein absorption, as well as increased cell permeability and leakage of nutrient and plasma protein (Nabian et al., 2018; Madlala et al., 2021), and thus inducing the retarded growth performance. Regarding intestinal morphometry, a significant decline in the intestinal villus length and crypt depth was noted in the PC (coccidia-challenged) group on the fifth day post challenge. The decline in these parameters is most probably driven by the large number of coccidia developmental stages retained within the mucosa of the intestine as revealed by light microscopic study. Notably, probiotics and/or garlic protected the epithelium of both the small and large intestines from the stunting effects of coccidia as detected in the GR and GR-P groups of the present study. These findings correspond with those of Wang et al. (2021) in which diets supplemented with garlic and probiotics revealed similar enhancement of the mentioned parameters in coccidia-challenged broiler chickens. GR and AC induced a significantly improved effect on intestinal integrity through up-regulation of claudin-1, with a substantial difference among the treated groups. Meanwhile, a synergetic effect was observed by GR-P to be comparable to non-challenged birds. Similar findings were reported for occludin and JAM-2 through dietary inclusion of garlic nano hydrogel at 100–400 mg/kg in Clostridium perfringens-challenged broilers which accordingly resulted in enhanced growth performance indices (Ibrahim et al., 2021). Feeding E. tenella-infected broiler chickens with L. plantarum increased mRNA expression of ZO-1 and Claudin-1 (Mohsin et al., 2022). Also, supplementation of B. subtilis, Bacillus licheniformis, and L. plantarum to heat-stressed broilers (Song et al., 2014) or L. plantarum and Lactobacillus reuteri to broilers exposed to feed restriction as a stress factor (Meyer et al., 2020) increased the intestinal barrier integrity. superoxide dismutase and glutathione peroxidase are essential markers for evaluating the antioxidant efficiency and the effect of scavenging free radicals. Our study proved that the mRNA expression levels of the antioxidant factors GPX-1 and SOD-1 were heightened in both GR and GR-P groups. These results were consistent with those obtained by Gbore et al. (2020) indicating the antioxidant activity of significant compounds of garlic such as diallyl sulfide and allicin (Jang et al., 2018). In addition, probiotics are a key factor influencing the oxidative trait of the gut by triggering direct antioxidant characteristics and inducing the intrinsic organisms signaling antioxidant defense (Zolotukhin et al., 2018). Moreover, among the treatments, only GR-P decreased the apoptosis-related protein caspase-3 significantly. Gharib-Naseri et al. (2020) reported mitigation of the undesirable impacts of necrotic enteritis on intestinal cell death by B. amyloliquefaciens administration. Intestinal epithelial cell expression of IL-10 is crucial in sustaining intestinal homeostasis and barrier integrity (Hyun et al., 2014). IL-10 is a potent anti-inflammatory cytokine that controls the host immune response to inhibit the production of pro-inflammatory cytokines such as IL-1β, reducing the inflammatory immune response (Arendt et al., 2016). The current investigation showed a noticeable up-regulation and down-regulation of the mRNA expression level of IL-10 and IL-1β, respectively, in the GR and GR-P groups compared to the PC group, with top effects for GR-P. Taken together, a combination of GR and probiotics is recommended to achieve maximal anti-inflammatory and immunomodulatory effects during coccidian infection. The three additives used in the present study significantly restored the normal intestinal architecture. Similar findings were reported for garlic administration to coccidia-infected broilers (Gotep et al., 2016; Ali et al., 2019). Allicin has been documented to enhance and regenerate the physiological status of the intestinal epithelium layer (Adibmoradi et al., 2006). Therefore, regeneration of the physiological structure of the intestinal epithelium by garlic supports the digestive capacity through enhanced absorption of nutrients and assimilation (Adibmoradi et al., 2006). In the jejunum, duodenum, and ileum, the greatest villus height values were found by a mixture of probiotics compared to infected control birds and fairly similar improvement to lasalocid as an approved anticoccidial during a mixed Eimeria infection (Giannenas et al., 2012). Jha et al. (2020) suggested that longer villi reflect enhanced feed efficiency and growth-promoting activity. Therefore, it has been suggested that the addition of probiotics can induce an enhancement of the villi length and intestinal nutrient absorption and subsequently feed efficiency and growth-promoting efficiency. The present study indicates a possible anticoccidial effect exerted by GR and GR-P. This was deducted from the alleviated lesion score in addition to the reduced oocyst shedding from the infected birds by 67.5% and 71%, respectively, compared to AC (74.8%). Garlic supplementation by 15 g/kg feed to cecal coccidiosis-infected broilers induced significantly lower oocyst counts than non-treated birds (Ali et al., 2019). Many other studies recorded the role of garlic against coccidiosis such as Toulah and Al-Rawi (2007) who recorded that garlic has anti-coccidial activity in rabbits. In another study, this effect was attributed to allicin and phenolic constituents in garlic, which change the cytoplasmic penetrability and eventually destroy the Eimeria cells (Ali et al., 2019). Kim et al. (2013) reported protective immunity during avian coccidiosis could be induced by the secondary metabolites of garlic, propyl thiosulphinate oxide, and propyl thiosulphinate which killed the invasive E. acervulina sporozoites in a dose-dependent manner, stimulated higher spleen cell proliferation, and enhanced E. acervulina profilin antibody responses. Probiotics consisting of Enterococcus faecium, Bifidobacterium animalis, and L. salivarius groups found lesion score records and oocyst numbers that were less than in control infected birds but did not differ from the approved anticoccidial drugs, lasalocid (Giannenas et al., 2012) and salinomycin (Abdelrahman et al., 2014). Also, Pediococcus-and Saccharomyces-based probiotics resulted in lower oocyst output in the treated groups during E. tenella and E. acervulina infection (Lee et al., 2007a; Lee et al., 2007b). Among eight strains of Bamongus-based direct-fed microbials, three strains significantly improved lesion scores in E. maxima infected birds in comparison to infected non-supplemented chickens (Lee et al., 2010). The 10 bacterial strain probiotic mixture, mainly from the genera Lactobacillus and Bifidobacterium, used in the present study successfully preserved the intestinal integrity of supplemented birds as shown in our lesion scoring analysis. One possible explanation such as the effect is the ability of our multistrain bacterial mixture to increase the diversity of gut microbiota which produces a wide range of beneficial molecules essential for gut wall functioning (Liao et al., 2020). Similar to our results, the use of probiotics with phytobiotics (B. subtilis combined with leaves of Artemisia annua Linn., Dichroa febrifuga Lour., or peel of Punica granatum L.) during chicken E. tenella infection significantly reduced the oocysts excretion compared to herb only group (Yang et al., 2021). The carcass yield particularly breast muscle thickness in garlic probiotic-supplemented groups may be due to the significant improvement in final body weight as a result of increased weight gain and proper feed conversion efficiency. Ashour et al. (2024) reported that supplementation with thyme powder, garlic powder, or a combination of both at appropriate levels (2% garlic powder, 2% thyme, or 1% each) improved carcass yield and heart percent. Moreover, Abd El-Hack et al. (2021) demonstrated that all carcass traits were significantly impacted by the dietary treatment supplemented with zinc nanoparticles, curcumin, and B. licheniformis in broilers. For assessment of meat quality, infection with coccidia revealed a clear adverse impact on the sensory quality attributes of breast meat considering flavor, appearance, and tenderness. In addition, there was a significant elevation in TBA, PH, Fat, and Ash and a decline in protein. GR and GR-P exhibited the significantly greatest scores for tenderness, juiciness, appearance, and overall acceptability of breast meat, even in the case of infection conditions in these groups. This could be attributed to the potential phytogenic component in garlic that can improve the qualitative attribute of meat (Oluwafemi et al., 2021). Besides, the strong antioxidant properties of garlic and the potential role of the probiotic in lipid metabolism, which prevents storage-induced oxidative stress, leading to a high ratio between unsaturated fatty acids and saturated fatty acids (Elkhouly et al., 2016). In addition to that, the beneficial metabolites produced by probiotics such as acetate and lactate increase epithelium cell integrity and stop microbial growth and multiplication (Mishra and Jha, 2019). The results of the present study are compatible with Mohammed et al. (2021) who stated that the leg meat from probiotic-fed broilers had better outcomes in the general sensory analysis at 5 hours after slaughter. ConclusionThe present research concluded that garlic alone GR or in combination with probiotics GR-P could be recommended as natural additives in broiler diets. However, GR-P revealed a higher anticoccidial effect than GR in mixed Eimeria species-infected chickens. The coccidial protection mediated by GR-P was coupled with positive effects on growth performance, breast muscle thickness, meat quality, intestinal integrity, oocyte shedding, and blood indices. These results suggest potential utility as a complementary strategy in coccidiosis prevention programs to lower the incidence of coccidiosis and its severity, minimize the usage of chemical anticoccidials, and overcome the issues of chemical residues and long-term anticoccidial resistance, economic feasibility, and scalability. Future research is required to decipher molecular networks governing the anticoccidial action of garlic and probiotics in broiler diets. Conflict of interestThe authors declare no conflict of interest. FundingNo funding was provided for this research. Authors’ contributionAzhar Eltanahy: Conceptualization, Formal analysis, Experimental design, Methodology, Writing-original draft, Writing-review & editing final manuscript. Dima Alkadrib: Formal analysis, Experimental design. Mohamed Elmorsy: Formal analysis, Experimental design, Methodology, Writing-original draft. Huda EL-Emam: Formal analysis, Experimental design, Methodology, Writing-original draft. Nahed El-Shall: Formal analysis, Experimental design, Methodology, Writing-original draft. Bassem Elmishmishy: Formal analysis, Experimental design. Asmaa Elsayyad: Formal analysis, Experimental design. Gehad Ezzat: Formal analysis, Experimental design, Methodology, Writing-original draft. Ahmed I. El Sheikh: Formal analysis, Experimental design, Writing-review final manuscript. Marwa El-Beltagy: Formal analysis, Experimental design. Mohamed Shaalan: Formal analysis, Experimental design, Writing-review & editing final manuscript. Radwa Elzwahary: Formal analysis, Experimental design. Ahmed Abdellatif: Formal analysis, Experimental design, Methodology, Writing-review & editing final manuscript. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data generated or analyzed during this study are included in this article. Ethical approvalThis research was performed in accordance with ethical standards approved by the Animal Care at Mansoura University MU-ACUC (VM.R.23.07.112), Egypt. ReferencesAbd El-Hack, M.E., Alaidaroos, B.A., Farsi, R.M., Abou-Kassem, D.E., El-Saadony, M.T., Saad, A.M., Shafi, M.E., Albaqami, N.M., Taha, A.E. and Ashour, E.A. 2021. Impacts of supplementing broiler diets with biological Curcumin, Zinc Nanoparticles and Bacillus licheniformis on Growth, Carcass Traits, blood indices, meat quality and cecal microbial load. Animals (Basel) 11(7), 1878. Abdellatif, A.M. 2021. Structure of the Eurasian moorhen spleen: a comprehensive study using gross anatomy, light, and transmission electron microscopy. Microsc. Res. Tech. 84(8), 1696–1709. Abdellatif, A.M., Farag, A. and Metwally, E. 2022. Anatomical, histochemical, and immunohistochemical observations on the gastrointestinal tract of Gallinula chloropus (Aves: Rallidae). BMC Zool. 7(1), 61. Abdelrahman, W., Mohnl, M., Teichmann, K., Doupovec, B., Schatzmayr, G., Lumpkins, B. and Mathis, G. 2014. Comparative evaluation of probiotic and salinomycin effects on performance and coccidiosis control in broiler chickens. Poult. Sci. 93(12), 3002–3008. Adibmoradi, M., Navidshad, B., Seifdavati, J. and Royan, M. 2006. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 43(4), 378–383. Ahmed, S.A., Nada, H.S., Elsheshtawy, H.M., Ibrahim, S.M., Fahmy, E.M., Khedr, M.H., Moustafa, S.M., Ismail, T.A., Gesriha, S. and Assayed, M.E. 2022. Comparative antitoxic potency of honey and natamycin-supplemented diets against aflatoxicosis and their influences on growth, serum biochemistry, immunohistochemistry, and residual deposition in Nile tilapia (Oreochromis niloticus). Aquaculture 551, 737934. Ali, M., Chand, N., Khan, R.U., Naz, S. and Gul, S. 2019. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 47(1), 79–84. AOAC. 1995. Official methods of analysis 16th Ed. Association of official analytical chemists. Washington, DC: AOAC. Arendt, M.K., Sand, J.M., Marcone, T.M. and Cook, M.E. 2016. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 95(2), 430–438. Ashour, E.A., Aldhalmi, A.K., Elolimy, A.A., Madkour, M., Elsherbeni, A.I., Alqhtani, A.H., Khan, I.M. and Swelum, A.A. 2024. Optimizing broiler performance, carcass traits, and health: evaluating thyme and/or garlic powders as natural growth promoters in antibiotic-free diets. Poult. Sci. 104(2), 104689. Ata, M. and Al-Masad, M. 2015. Effect of milk powder supplementation on growth performance of broilers. J. Agri. Sci. 7(8), 111–117. Aviagen, R. 2019. Ross 308 nutrition specifications. Midlothian, UK: Aviagen. Awad, W.A., Ghareeb, K., Abdel-Raheem, S. and Böhm, J. 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88(1), 49–56. Awad, W.A., Ghareeb, K. and Böhm, J. 2010. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. (Berl.) 94(4), 486–494. Baston, O. and Barna, O. 2010. Raw chicken leg and breast sensory evaluation. Food Sci. Technol 11(1), 25–30. Behairy, A., Amer, S.A., Gouda, A., Moustafa, A.A., Abdel-Warith, A.W.A., Younis, E.M., Kamal, A.S., Eltanahy, A., Davies, S.J. and EL-Sayed Kamel, A. 2023. Assessment of Lavandula angustifolia L. essential oil as a natural feed additive on broiler chicken’s growth, blood physiological markers, immunological status, intestinal histomorphology, and immunoexpression of CD3 and CD20. Ital. J. Anim. Sci. 22(1), 1230–1245. Benjamin, M.M. 1985. Outline of Veterinary Clinical Pathology, third ed. New Delhi, India: Kalyani Publishers. Brake, J., Havenstein, G., Scheideler, S., Ferket, P. and Rives, D. 1993. Relationship of sex, age, and body weight to broiler carcass yield and offal production. Poult. Sci. 72(6), 1137–1145. Brzóska, F., Śliwiński, B., Michalik-Rutkowska, O. and Śliwa, J. 2015. The effect of garlic (Allium Sativum L.) on growth performance, mortality rate, meat and blood parameters in broilers. Ann. Anim. Sci. 15, 961–975. Cao, G., Zeng, X., Chen, A., Zhou, L., Zhang, L., Xiao, Y. and Yang, C. 2013. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 92(11), 2949–2955. Deng, Q., Shi, H., Luo, Y., Zhao, H. and Liu, N. 2020. Effect of dietary Lactobacilli mixture on Listeria monocytogenes infection and virulence property in broilers. Poult. Sci. 99(7), 3655–3662. Du, M. and Ahn, D. 2002. Effect of antioxidants on the quality of irradiated sausages prepared with turkey thigh meat. Poult. Sci. 81(8), 1251–1256. El-Shall, N.A., Abd El-Hack, M.E., Albaqami, N.M., Khafaga, A.F., Taha, A.E., Swelum, A.A., El-Saadony, M.T., Salem, H.M., El-Tahan, A.M., AbuQamar, S.F., El-Tarabily, K.A. and Elbestawy, A.R. 2022. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 101(1), 101542. El-Bouhy, Z.M., Reda, R.M., Mahboub, H.H. and Gomaa, F.N. 2021. Chelation of mercury intoxication and testing different protective aspects of Lactococcus lactis probiotic in African catfish. Aquac. Res. 52(8), 3815–3828. Elkhouly, M., Khairy, M., Abd- El Alim, A.E.A. and Ali, A. 2016. Effect of phytobiotics, probiotics and toltrazuril on chicken coccidiosis. Zagazig Vet. J. 44(3), 214–223. Gbore, F.A., Oloruntola, O.D., Adu, O.A., Olarotimi, O.J., Falowo, A.B. and Afolayan, E.O. 2020. Serum and meat antioxidative status of broiler chickens fed diets supplemented with garlic rhizome meal, Moringa leaf meal and their composite. Trop. Anim. Health Prod. 53(1), 26. Gewaily, M.S., Shukry, M., Abdel-Kader, M.F., Alkafafy, M., Farrag, F.A., Moustafa, E.M., Doan, H.V., Abd-Elghany, M.F., Abdelhamid, A.F. and Eltanahy, A. 2021. Dietary Lactobacillus plantarum relieves Nile tilapia (Oreochromis niloticus) juvenile from oxidative stress, immunosuppression, and inflammation induced by deltamethrin and Aeromonas hydrophila. Front. Mar. Sci. 8, 621558. Gharib-Naseri, K., de Paula Dorigam, J.C., Doranalli, K., Kheravii, S., Swick, R.A., Choct, M. and Wu, S.B. 2020. Modulations of genes related to gut integrity, apoptosis, and immunity underlie the beneficial effects of Bacillus amyloliquefaciens CECT 5940 in broilers fed diets with different protein levels in a necrotic enteritis challenge model. J. Anim. Sci. Biotechnol. 11, 104. Giannenas, I., Papadopoulos, E., Tsalie, E., Triantafillou, E., Henikl, S., Teichmann, K. and Tontis, D. 2012. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 188(1-2), 31–40. Gotep, J.G., Tanko, J.T., Forcados, G.E., Muraina, I.A., Ozele, N., Dogonyaro, B.B., Oladipo, O.O., Makoshi, M.S., Akanbi, O.B., Kinjir, H., Samuel, A.L., Onyiche, T.E., Ochigbo, G.O., Aladelokun, O.B., Ozoani, H.A., Viyoff, V.Z., Dapuliga, C.C., Atiku, A.A., Okewole, P.A., Shamaki, D., Ahmed, M.S. and Nduaka, C.I. 2016. Therapeutic and safety evaluation of combined aqueous extracts of Azadirachta indica and Khaya senegalensis in chickens experimentally infected with Eimeria Oocysts. J. Parasitol. Res. 2016, 4692424. Herforth, A., Bai, Y., Venkat, A., Mahrt, K., Ebel, A. and Masters, W.A. 2020. Cost and affordability of healthy diets across and within countries: background paper for The State of Food Security and Nutrition in the World 2020. FAO Agricultural Development Economics Technical Study. Rome, Italy: Food & Agriculture Org, vol. 9. Hyun,J.,Romero,L.,Riveron,R.,Flores,C.,Kanagavelu, S., Chung, K.D., Alonso, A., Sotolongo, J., Ruiz, J. and Manukyan, A. 2014. Human intestinal epithelial cells express interleukin-10 through Toll- like receptor 4-mediated epithelial-macrophage crosstalk. J. Innate Immun. 7(1), 87–101. Ibrahim, D., Ismail, T.A., Khalifa, E., Abd El-Kader, S.A., Mohamed, D.I., Mohamed, D.T., Shahin, S.E. and Abd El-Hamid, M.I. 2021. Supplementing garlic nanohydrogel optimized growth, gastrointestinal integrity and economics and ameliorated necrotic enteritis in broiler chickens using a Clostridium perfringens challenge model. Animals (Basel) 11(7), 2027. Ipek, A., Sozcu, A. and Akay, V. 2016. 1012 Effects of dietary inclusion of probiotics and prebiotics (SynerAll) on growth performance and serum biochemical parameters in broilers. J. Anim. Sci. 94(suppl_5), 484–485. Ismail, I.E., Alagawany, M., Taha, A.E., Puvača, N., Laudadio, V. and Tufarelli, V. 2021. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood haematology, immunity and antioxidant status of broiler chickens. Anim. Biosci. 34(3), 363–370. Jacquier, V., Nelson, A., Jlali, M., Rhayat, L., Brinch, K. and Devillard, E. 2019. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 98(6), 2548–2554. Jang, H.J., Lee, H.J., Yoon, D.K., Ji, D.S., Kim, J.H. and Lee, C.H. 2018. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by- products extracted with different solvents. Food Sci. Biotechnol. 27(1), 219–225. Jha, R., Das, R., Oak, S. and Mishra, P. 2020. Probiotics (Direct-Fed Microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Animals (Basel) 10(10), 1863. Johnson, J. and Reid, W.M. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28(1), 30–36. Kasprzak, K.S. 1991. The role of oxidative damage in metal carcinogenicity. Chem. Res. Toxicol. 4(6), 604–615. Kenawi, M. 2005. Technological, chemical, sensory, and microbiological examination of frozen chicken as affected by microwave thawing. Biotechnol. Anim. Husbandry 21(1-2), 99–108. Khan, R., Nikousefat, Z., Tufarelli, V., Naz, S., Javdani, M. and Laudadio, V. 2012. Garlic (Allium sativum) supplementation in poultry diets: effect on production and physiology. World’s Poult. Sci. J. 68(3), 417–424. Kim, D.K., Lillehoj, H.S., Lee, S.H., Lillehoj, E.P. and Bravo, D. 2013. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 109(1), 76–88. Kim, Y., Jin, S. and Yang, H. 2009. Effect of dietary garlic bulb and husk on the physicochemical properties of chicken meat. Poult. Sci. 88(2), 398– 405. Lee, B.H., Kim, W.H., Jeong, J., Yoo, J., Kwon, Y.K., Jung, B.Y., Kwon, J.H., Lillehoj, H.S. and Min, W. 2010. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. J. Vet. Med. Sci. 72(8), 985–989. Lee, S., Lillehoj, H.S., Dalloul, R.A., Park, D.W., Hong, Y.H. and Lin, J.J. 2007a. Influence of Pediococcus- based probiotic on coccidiosis in broiler chickens. Poult. Sci. 86(1), 63–66. Lee, S., Lillehoj, H.S., Park, D.W., Hong, Y.H. and Lin, J.J. 2007b. Effects of Pediococcus- and Saccharomyces-based probiotic (MitoMax) on coccidiosis in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 30(4), 261–268. Li, Q., Wan, G., Peng, C., Xu, L., Yu, Y., Li, L. and Li, G. 2020. Effect of probiotic supplementation on growth performance, intestinal morphology, barrier integrity, and inflammatory response in broilers subjected to cyclic heat stress. Anim. Sci. J. 91(1), e13433. Liao, X., Shao, Y., Sun, G., Yang, Y., Zhang, L., Guo, Y., Luo, X. and Lu, L. 2020. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 99(11), 5883–5895. Lipiński, K., Antoszkiewicz, Z., Kotlarczyk, S., Mazur- Kuśnirek, M., Kaliniewicz, J. and Makowski, Z. 2019. The effect of herbal feed additive on the growth performance, carcass characteristics and meat quality of broiler chickens fed low-energy diets. Arch. Anim. Breed. 62(1), 33–40. Madlala, T., Okpeku, M. and Adeleke, M.A. 2021. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review. Parasite 28, 48. Memon, F.U., Leghari, I.H., Rajput, N., Gadahi, J.A., Sahito, J.Z.A., Yang, Y., Baig, M.B., Laghari, F., Memon, H.A. and Si, H. 2022. Immunomodulatory and ameliorative effects of probiotic in combination with diclazuril on broilers under coccidia infection. J. Appl. Microbiol. 132(4), 3181–3188. Meyer, M.M., Fries-Craft, K.A. and Bobeck, E.A. 2020. Composition and inclusion of probiotics in broiler diets alter intestinal permeability and spleen immune cell profiles without negatively affecting performance. J. Anim. Sci. 98(1), skz383. Mishra, B. and Jha, R. 2019. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 6, 60. Mohammed, A., Zaki, R., Negm, E., Mahmoud, M. and Cheng, H. 2021. Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 100(3), 100906. Mohsin, M., Zhang, Z. and Yin, G. 2022. Effect of probiotics on the performance and intestinal health of broiler chickens infected with Eimeria tenella. Vaccines 10(1), 97. Nabian, S., Arabkhazaeli, F., Seifouri, P. and Farahani, A. 2018. Morphometric analysis of the intestine in experimental coccidiosis in broilers treated with anticoccidial drugs. Iran J. Parasitol. 13(3), 493–499. Natt, M.P. and Herrick, C.A. 1952. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult. Sci. 31(4), 735–738. Nematollahi, A., Moghaddam, G. and Pourabad, R.F. 2009. Prevalence of Eimeria species among broiler chicks in Tabriz (Northwest of Iran). Mun. Ent. Zool 4(1), 53–58. Nikolova, N. and Pavlovski, Z. 2009. Major carcass parts of broiler chicken from different genotype, sex, age and nutrition system. Biotechnol. Anim. Husbandry 25(5-6-2), 1045–1054. Oluwafemi, R., Lawal, A.O., Adetope, A.S. and Alagbe, J. 2021. Effects of dietary inclusion of ginger (Zingiber officinale) and garlic (Allium sativum) oil (GGO) mixtures on carcass characteristics and sensory evalaution of broiler chickens. J. La Medihealtico 2(6), 22–31. Park, J. and Kim, I. 2014. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 93(8), 2054–2059. Pender, C., Kim, S., Potter, T., Ritzi, M., Young, M. and Dalloul, R. 2016. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Beneficial Microb. 7(5), 699–706. Plaza-Diaz, J., Ruiz-Ojeda, F.J., Gil-Campos, M. and Gil, A. 2019. Mechanisms of action of probiotics. Adv. Nutr. 10, S49–S66. Puvača, N., Kostadinović, L., Popović, S., Lević, J., Ljubojević, D., Tufarelli, V., Jovanović, R., Tasić, T., Ikonić, P. and Lukač, D. 2015. Proximate composition, cholesterol concentration and lipid oxidation of meat from chickens fed dietary spice addition (Allium sativum, Piper nigrum, Capsicum annuum). Anim. Prod. Sci. 56(11), 1920–1927. Ritzi, M.M., Abdelrahman, W., Van-Heerden, K., Mohnl, M., Barrett, N.W. and Dalloul, R.A. 2016. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 47, 1–8. Rivlin, R.S. 2001. Historical perspective on the use of garlic. J. Nutr. 131(3), 951S–954S. Rusli, R.K., Sadarman, S., Hidayat, C., Sholikin, M.M., Hilmi, M., Yuniza, A., Mutia, R., Jayanegara, A. and Irawan, A. 2022. A meta-analysis to evaluate the effects of garlic supplementation on performance and blood lipids profile of broiler chickens. Livestock Sci. 263, 105022. Saeed, Z. and Alkheraije, K.A. 2023. Botanicals: a promising approach for controlling cecal coccidiosis in poultry. Front. Vet. Sci. 10, 1157633. Sen, S., Ingale, S.L., Kim, Y.W., Kim, J.S., Kim, K.H., Lohakare, J.D., Kim, E.K., Kim, H.S., Ryu, M.H., Kwon, I.K. and Chae, B.J. 2012. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 93(1), 264–268. Sen, S., Ingale, S.L., Kim, Y.W., Kim, J.S., Kim, K.H., Lohakare, J.D., Kim, E.K., Kim, H.S., Ryu, M.H., Kwon, I.K. and Chae, B.J. 2012. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 93(1), 264–268. Song, J., Xiao, K., Ke, Y., Jiao, L., Hu, C., Diao, Q., Shi, B. and Zou, X. 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 93(3), 581–588. Su, L., Dong, L., Bughio, S., Guo, M. and Wang, L. 2014. Effect of colibacillosis or coccidiosis on expression of breast cancer resistance protein in small intestine and liver of chickens. J. Vet. Pharmacol. Ther. 37(1), 53–58. Su, S., Miska, K., Fetterer, R., Jenkins, M. and Wong, E. 2015. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 150, 13–21. Sumarmono, J. and Rahardjo, A.H.D. 2008. Effects of decontamination using organic acids on total microbial number and qualities of poultry carcasses. Anim. Prod. 10(2), 129–134. Summers, J., Turner, B. and Tillman, N. 2022. Effects of feeding a probiotic blend on live performance of broiler chickens from 0 to 49 days of age. J. Appl. Poult. Res. 31(3), 100273. Sureshkumar, S., Park, J. and Kim, I. 2021. Effects of the inclusion of dietary organic acid supplementation with anti-coccidium vaccine on growth performance, digestibility, fecal microbial, and chicken fecal noxious gas emissions. Braz. J. Poult. Sci. 23, eRBCA-2020-1425. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2019. Bancroft’s Theory and Practice of Histological Techniques. 8th ed. Amsterdam, The Netherlands: Elsevier Health Sciences. Teng, P.-Y., Choi, J., Tompkins, Y., Lillehoj, H. and Kim, W. 2021. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 52(1), 81. Timmerman, H.M., Veldman, A., van den Elsen, E., Rombouts, F.M. and Beynen, A.C. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult. Sci. 85(8), 1383–1388. Toulah, F.H. and Al-Rawi, M.M. 2007. Efficacy of garlic extract on hepatic coccidiosis in infected rabbits (Oryctolagus cuniculus): histological and biochemical studies. J. Egypt. Soc. Parasitol. 37(3), 957–968. Tsukahara, T., Inoue, R., Nakayama, K. and Inatomi, T. 2018. Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim. Sci. J. 89(4), 679–687. Van Kampen, E. and Zijlstra, W. 1961. Standardization of hemoglobinometry II. The hemiglobincyanide method. Clin. Chim. Acta 6(4), 538–544. Wang, X., Farnell, Y.Z., Kiess, A.S., Peebles, E.D., Wamsley, K.G.S. and Zhai, W. 2019. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult. Sci. 98(9), 3839– 3849. Wang, Y., Lv, X., Li, X., Zhao, J., Zhang, K., Hao, X., Liu, K. and Liu, H. 2021. Protective effect of Lactobacillus plantarum P8 on growth performance, intestinal health, and microbiota in Eimeria-infected broilers. Front. Microbiol. 12, 705758. Yang, Y., Memon, F.U., Hao, K., Jiang, M., Guo, L., Liu, T., Lv, F., Zhang, W., Zhang, Y. and Si, H. 2021. The combined use of Bacillus subtilis-based probiotic and anticoccidial herb had a better anti-Eimeria tenella efficiency. J. Appl. Poult. Res.1 30(3), 100181. Zolotukhin, P., Prazdnova, E. and Chistyakov, V. 2018. Methods to assess the antioxidative properties of probiotics. Probiotics Antimicrob. Proteins 10, 589–599. | ||
| How to Cite this Article |
| Pubmed Style Eltanahy A, Alkadri D, Elmorsy MA, Emam HAE, El-shall NA, Elmishmishy B, Elsayyad A, Ezzat GA, El-beltagy MA, Shaalan M, Elzwahary RR, Sheikh AIE, Abdellatif AM. Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Vet. J.. 2025; 15(6): 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 Web Style Eltanahy A, Alkadri D, Elmorsy MA, Emam HAE, El-shall NA, Elmishmishy B, Elsayyad A, Ezzat GA, El-beltagy MA, Shaalan M, Elzwahary RR, Sheikh AIE, Abdellatif AM. Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. https://www.openveterinaryjournal.com/?mno=255489 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.42 AMA (American Medical Association) Style Eltanahy A, Alkadri D, Elmorsy MA, Emam HAE, El-shall NA, Elmishmishy B, Elsayyad A, Ezzat GA, El-beltagy MA, Shaalan M, Elzwahary RR, Sheikh AIE, Abdellatif AM. Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Vet. J.. 2025; 15(6): 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 Vancouver/ICMJE Style Eltanahy A, Alkadri D, Elmorsy MA, Emam HAE, El-shall NA, Elmishmishy B, Elsayyad A, Ezzat GA, El-beltagy MA, Shaalan M, Elzwahary RR, Sheikh AIE, Abdellatif AM. Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 Harvard Style Eltanahy, A., Alkadri, . D., Elmorsy, . M. A., Emam, . H. A. E., El-shall, . N. A., Elmishmishy, . B., Elsayyad, . A., Ezzat, . G. A., El-beltagy, . M. A., Shaalan, . M., Elzwahary, . R. R., Sheikh, . A. I. E. & Abdellatif, . A. M. (2025) Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Vet. J., 15 (6), 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 Turabian Style Eltanahy, Azhar, Dima Alkadri, Mohamed Alaaeldein Elmorsy, Huda A. El- Emam, Nahed A. El-shall, Bassem Elmishmishy, Asmaa Elsayyad, Gehad A. Ezzat, Marwa A. El-beltagy, Mohamed Shaalan, Radwa R. Elzwahary, Ahmed I. El Sheikh, and Ahmed M. Abdellatif. 2025. Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Veterinary Journal, 15 (6), 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 Chicago Style Eltanahy, Azhar, Dima Alkadri, Mohamed Alaaeldein Elmorsy, Huda A. El- Emam, Nahed A. El-shall, Bassem Elmishmishy, Asmaa Elsayyad, Gehad A. Ezzat, Marwa A. El-beltagy, Mohamed Shaalan, Radwa R. Elzwahary, Ahmed I. El Sheikh, and Ahmed M. Abdellatif. "Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia." Open Veterinary Journal 15 (2025), 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 MLA (The Modern Language Association) Style Eltanahy, Azhar, Dima Alkadri, Mohamed Alaaeldein Elmorsy, Huda A. El- Emam, Nahed A. El-shall, Bassem Elmishmishy, Asmaa Elsayyad, Gehad A. Ezzat, Marwa A. El-beltagy, Mohamed Shaalan, Radwa R. Elzwahary, Ahmed I. El Sheikh, and Ahmed M. Abdellatif. "Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia." Open Veterinary Journal 15.6 (2025), 2729-2749. Print. doi:10.5455/OVJ.2025.v15.i6.42 APA (American Psychological Association) Style Eltanahy, A., Alkadri, . D., Elmorsy, . M. A., Emam, . H. A. E., El-shall, . N. A., Elmishmishy, . B., Elsayyad, . A., Ezzat, . G. A., El-beltagy, . M. A., Shaalan, . M., Elzwahary, . R. R., Sheikh, . A. I. E. & Abdellatif, . A. M. (2025) Synergistic effects of garlic powder and probiotics on production and growth performance parameters in broiler challenged with coccidia. Open Veterinary Journal, 15 (6), 2729-2749. doi:10.5455/OVJ.2025.v15.i6.42 |