| Research Article | ||
Open Vet. J.. 2025; 15(7): 3012-3023 Open Veterinary Journal, (2025), Vol. 15(7): 3012-3023 Research Article Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticusNadia A. H. Al-Shammari1*, Khalidah S. AL-Niaeem2, Adnan B. Al-Hawash3, Mosleh Mohammad Abomughaid4, Aaser M. Abdelazim4, Preetham Elumalai5, El-Sayed Hemdan Eissa6, Heba H. Mahboub7* and Heba Allah M. Elbaghdady81Department of Natural Marine Science, College of Marine Sciences, University of Basrah, Basrah, Iraq 2Department of Fisheries and Marine Resources, College of Agriculture, University of Basrah, Basrah, Iraq 3Department of Biology, College of Education-Qurna, University of Basrah, Basra, Iraq 4Department of Medical Laboratory Sciences, College of Applied Medical Sciences, University of Bisha, Bisha, Saudi Arabia 5Department of Marine Biology, Microbiology and Biochemistry, School of Marine Sciences, Lakeside Campus, Cochin University of Science and Technology (CUSAT), Fine Arts Avenue Cochin, Kochi, India 6Fish Research Centre, Faculty of Environmental Agricultural Sciences, Arish University, El-Arish, Egypt 7Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 8Zoology Department, Faculty of Science, Mansoura University, Mansoura, Egypt *Corresponding Authors: Nadia A. H. Al- Shammari. Department of Natural Marine Science, College of Marine Sciences, University of Basrah, Basrah, Iraq. Email: hhhmb [at] yahoo.com and Heba H. Mahboub. Department of Aquatic Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: hhhmb [at] yahoo.com Submitted: 01/05/2025 Revised: 13/05/2025 Accepted: 13/05/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Aeromonas hydrophila is a pathogenic bacterial infection threatening the aquaculture industry. Aim: The current work was performed to scrutinize the impacts of A. hydrophila infection on the occurrence of mortalities, altering the clinical picture of fish, antioxidants-associated gene expression, and histopathological architecture of the Cyprinus carpio and Oreochromis niloticus. Methods: Juvenile fishes including C. carpio, n=60, and O. niloticus, n=60 were haphazardly alienated into the control group (uninfected) and infected group with 100 μl of A. hydrophila. Mortalities and clinical signs were recorded during the experiment. Samples of liver, kidney, and spleen were collected post-infection for 7 days to monitor the expression of superoxide dismutase, glutathione peroxidase, and catalase genes, plus the assessment of histopathology. Results: The rate of mortalities was higher in C. carpio compared to O. niloticus. Additionally, infected O. niloticus revealed red eyes and erythema, while C. carpio showed exophthalmia and severe skin ulceration with exposure to viscera. The gene expression indicators showed that A. hydrophila significantly declined on the 1st day and the 7th day after infection, but significantly increased (p < 0.05) on the 3rd day compared to their respective control groups for C. carpio. Meanwhile, O. niloticus significantly regulated gene expression (p < 0.05) in the control groups. The histological picture indicated that the liver is the most affected organ. Moreover, C. carpio exhibited more disruption in histological architecture related to O. niloticus. Conclusion: Overall, A. hydrophila is extremely virulent and results in higher mortalities, profound clinical manifestations, down-regulation in the gene expression, and histopathological alterations in the hepato-renal and splenic tissues of C. carpio and O. niloticus. Cyprinus carpio was more adversely affected by the infection compared to O. niloticus. However, O. niloticus revealed higher gene expression, particularly in the spleen, and the genes were more expressed in comparison to C. carpio. Keywords: Gene expression, Histopathology, Aeromonas hydrophila, Cyprinus carpio, Oreochromis niloticus. IntroductionCyprinus carpio (common carp) and Oreochromis niloticus (Nile tilapia) are among the most economically important aquaculture species globally, with commercial cultivation established in over 100 countries, including natural populations in Iraqi waters. Common carp, renowned for its exceptional adaptability, has achieved a widespread distribution across diverse freshwater ecosystems and has become a cornerstone species in global aquaculture production systems (WHO, 2021; Moaheda et al., 2023). However, the escalating challenges of disease resistance and the urgent need to enhance fish immunocompetence and antioxidant defense mechanisms have become critical priorities for sustainable aquaculture development (Hoseinifar et al., 2021; Hendam et al., 2023; Eissa et al., 2024). The pervasive presence of pathogenic bacteria in aquatic environments induces oxidative stress responses, leading to significant alterations in the histological architecture of vital organs, including the kidney, liver, and gills (Faheem et al., 2016; Rashidian et al., 2022; Alzahrani et al., 2023). Among these pathogens, Aeromonas species are particularly notable for their ability to cause severe histopathological disruptions, oxidative damage, and modulation of immune-associated gene expression in both C. carpio and O. niloticus (Abdel Rahman et al., 2022a,b). In C. carpio, Aeromonas infection significantly impairs immune-antioxidant functions, reduces growth performance, and alters cytokine expression profiles in splenic tissue (Mahboub et al., 2022a). Bacterial pathogenesis, particularly involving Aeromonas species, triggers oxidative stress through increased free radical production and compromised cellular detoxification mechanisms, ultimately resulting in widespread cellular dysfunction (Li et al., 2015). Fish exposed to bacterial infections exhibit significant disruptions in their immune-antioxidant defense systems (Ahmad et al., 2022). Cellular responses to these challenges involve the coordinated activation of immune system components and primary enzymatic antioxidants, including catalase (CAT) and superoxide dismutases (SODs) (Hong et al., 2020). Oxidative stress induced by bacterial contamination primarily manifests through the impaired capacity of antioxidant systems to neutralize reactive oxygen species (ROS) (Pizzino et al., 2017). This dysfunction alters cellular defense pathways and modulates gene expression profiles (Bayır et al., 2022). Notable changes include the downregulation of key antioxidant enzymes, specifically SOD, glutathione peroxidase (GPx), and CAT (Baldissera et al., 2018). While oxygen is essential for aerobic metabolism in aquatic organisms and plays a critical role in cellular functions, it also serves as a primary electron acceptor in the generation of ROS, encompassing both free-radical and non-radical species (Chowdhury and Saikia, 2020). Elevated stress conditions compromise the fish’s antioxidant defense capabilities, rendering cellular components vulnerable to oxidative damage, particularly affecting lipids, proteins, and DNA integrity (Sidorczuk et al., 2009). Quantitative real-time polymerase chain reaction (QRT-PCR) has emerged as the gold standard for bacterial identification and quantification, offering superior sensitivity, rapid processing times, and an expanded dynamic quantification range. The selection of appropriate reference genes remains crucial, as their stability can vary significantly across species and experimental conditions (Liang et al., 2022). This study represents the first systematic molecular and histopathological investigation in Iraq to examine the pathogenic effects of Aeromonas infection through multiple parameters, including mortality rates, clinical manifestations, oxidative stress markers, gene expression profiles, and cellular pathways of antioxidant enzymes in C. carpio and O. niloticus. Furthermore, this study evaluates the consequent impact on internal tissue architecture and histological alterations, particularly elucidating the genetic pathways underlying these pathological features. Materials and MethodsExperimental design and bacteria preparationHealthy fingerlings of C. carpio (common carp) and O. niloticus (Nile tilapia) (n=60 per species), with an average body weight of 30–35 g, were collected from the Al-Mashab River in Basra Governorate, Iraq. Before the bacterial challenge, all fish underwent a 1-week acclimatization period in continuously aerated water maintained at 30°C ± 1°C, pH 7.5 ± 0.3, and ammonia nitrogen levels below 0.2 mg/l. The two fish species were randomly distributed into nine tanks (60 × 40 × 50 cm). The pathogen Aeromonas hydrophila was previously isolated from infected carp and identified through molecular isolation of the 16S ribosomal gene (OR398683.1) using BLAST for homology search, available at NCBI (http://www.ncbi.nlm.nih.gov/). The isolate was stored as a frozen stock at −80°C in 50% (v/v) glycerol in the Central Laboratory, Department of Life Sciences, College of Qurna, University of Basrah (Al Shammari et al., 2023). For the challenge trial, the frozen stock culture was resuscitated. The A. hydrophila strain ASS-4 was inoculated into LB Broth Medium and incubated with constant shaking at 30°C for 24 hours. The broth culture was centrifuged at 3,000 rpm for 15 minutes at 4°C to collect the bacterial pellet, which was then washed three times with sterile phosphate buffer saline (PBS). The median lethal dose was determined, and the treatment groups (six tanks) were intraperitoneally injected with 0.2 ml of bacterial suspension (1.6 × 107 CFU/ml), while control fish (three tanks) received sterile PBS. Mortalities and clinical signs were monitored throughout the experiment (Mahboub et al., 2022b). Sample preparationThree fish from each species were randomly selected post-injection (each experiment was conducted separately). The fish were euthanized and dissected, and internal organs (liver, kidney, and spleen) were collected at intervals of 1, 3, 5, and 7 days post-injection. The organs were preserved at −80°C (in liquid nitrogen) until RNA extraction. All animal procedures adhered to the guidelines of the Animal Experiment Ethics Committee of Basrah University and followed the ARRIVE guidelines. Total RNA extraction from tissueTotal RNA was extracted from tissues of C. carpio and O. niloticus infected with A. hydrophila. Three replicates were prepared for each sample at specific time intervals (1, 3, 5, and 7 days post-injection). Fish were dissected using sterile scalpels and scissors to obtain internal organs (liver, kidney, and spleen). The tissues were homogenized and mixed with GENEzol™ Tri RNA Pure reagent according to the manufacturer’s protocol (Sun et al., 2019a,b). Total RNA isolation and cDNA synthesisTen milligrams of each fish’s internal organs (liver, kidney, and spleen) were weighed, homogenized, and preserved in liquid nitrogen. Total RNA was isolated using the GENEzol™ Total RNA Kit and quantified using a NanoDrop spectrophotometer (Thermo Scientific™ Multiskan™, USA) with absorbance measured at 260/280 nm (Okamoto and Okabe, 2000). RNA was reverse-transcribed into cDNA using the AccuPower® RocketScript™ RT PreMix Kit. The reaction mixture included: Total RNA (2 µl), Oligo (dT15) primer (1 µl), Random Hexamer primer (1 µl), and DEPC-water (16 µl), for a total reaction volume of 20 µl. The reaction served as a template for RT-qPCR (Bioneer, Korea) following the manufacturer’s instructions (Cai et al., 2018). The thermal cycler conditions for cDNA synthesis consisted of a single cycle with three steps: primer annealing at 25°C for 10 minutes, cDNA synthesis at 42°C for 60 minutes, and heat inactivation at 95°C for 5 minutes. Samples were stored at −86°C until further analysis (Sun et al., 2019a,b). qPCR primersGene expression analysis utilized specific primers for antioxidant enzymes (SOD, GPX, and CAT) and housekeeping genes. For C. carpio, β-actin served as the reference gene (Sielska et al., 2024) with forward primer 5΄-CCTGTATGCCAACACCGTGCTG -3΄ and reverse primer 5΄-CTTCATGGTGGAGGGAGCAAGG-3΄. For O. niloticus, GAPDH was used as the reference gene (Jiang et al., 2023) with forward primer 5΄-TAACTTTGCTCTTCCCCACT-3΄ and reverse primer 5΄-ATACCGACTTTCACCATTTTG-3΄. The antioxidant enzyme primers for C. carpio (Mahboub et al., 2022a; Mahboub et al., 2022) included: SOD (F: 5΄-TGAGCTGTCGGAAGCCATCAAG-3΄, R: 5΄-TTGGTTCCCACATGCAGCAATCC-3΄), GPX (F: 5΄-CTCAACAGGAGAATGCCAAGAATG-3΄, R: 5΄-CCTTGAGGAACACGAACAGAGG-3΄), and CAT (F: 5΄-AGACGACACCATCGCTGTTCG-3΄, R: 5΄-AAGGTCCCAGTTGCCCTCATCG-3΄). For O. niloticus, the antioxidant enzyme primers (Abdelazim et al., 2019) were: SOD (F: 5΄-CGCCTTTTACATGACCAT-3΄, R: 5΄-GTGTCGCTGGATGCTAAGA-3΄), GPX (F: 5΄-AAAATGTGGCGTCTCTCTG-3΄, R: 5΄-GCACACCCAAAATAACGAG-3΄), and CAT (F: 5΄-ATGGAAGGCGAATAGAGGCT-3΄, R: 5΄-AACATCTTGAACCAGCAGCG-3΄). The expression of antioxidant genes (SOD, GPX, and CAT) in two different fish (C. carpio and O. niloticus) was determined using QRT-PCR. Fish were experimentally injected with A. hydrophila and their expression genes in internal organs (liver, kidneys, and spleen) for each fish were assessed using housekeeping gene; (B-actin) for C. carpio; and (GAPDH) for O. niloticus. Reaction real-time PCR qPCRThe RT-PCR reaction was carried out on (RT-Per Mix) real-time PCR system (7500 Fast System) with optimization. PCR mix in a total volume of 20 µl consisted of Go Taq ® qPCR Master Mix (2×) (20 µl), forward primer (0.5 µl), reverse primer (0.5 µl), cDNA (5 µl), and nuclease-free water (4 µl). The PCR amplification was done according to Abdelazim et al. (2019). HistopathologyThe hepatic, renal, and spleen tissues were washed in a solution containing 1.2% saline, stationed in a solution of 4% paraformaldehyde for 2 days, splashed in 70% ethanol solution, and lastly moved to 70% ethanol solution for packing until treated into histological slides. Embedding in paraffin and collection of images were achieved following Torrecillas et al. (2007). For lesion scoring, three non-repeated, randomly chosen microscopic fields (40×) were examined in one slide per group of two fish. The mean scores in the examined five microscopic fields were considered the final lesion score per fish. The reported histopathological lesions in the liver, kidney, and spleen in all groups were scored according to the following scoring system (+, ++, +++), corresponding to no change, mild change, moderate change, and severe change, respectively. Statistical analysisThe results were compiled by one-way ANOVA followed by all pairwise comparisons according to the Tukey test to distinguish the difference among means. The statistical analysis was conducted using SPSS V. 21, and graphs were prepared by GraphPad Prism V7. The data were presented as mean ± SD. Ethical approvalAll experimental trials with live fish were agreed by the animal welfare and ethical review committee of the College of Agriculture, University of Basrah, Basrah, Iraq. All experimental procedures were directed in obedience to the ethical guidelines approved by the National Institutes of Health for Use and Treatment of Laboratory Animals following the ARRIVE guidelines.
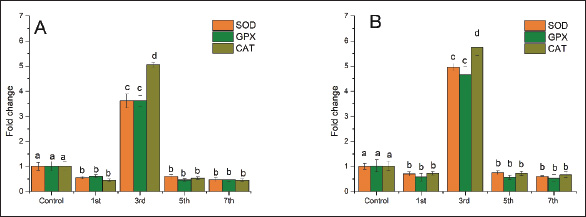
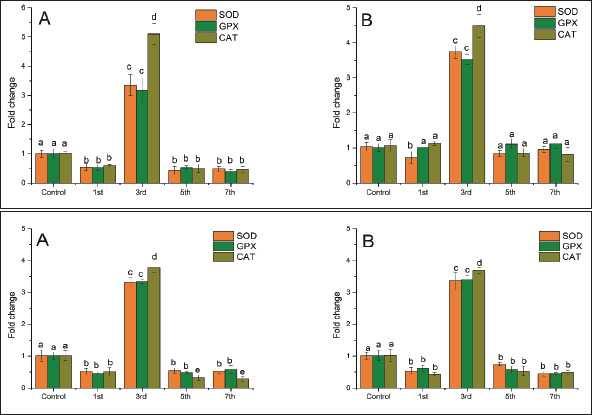
Fig. 1. Mortality of Cyprinus carpio and Oreochromis niloticus during LD 50-7th of Aeromonas hydrophila infection. ResultsResults of mortalitiesAeromonas infection results in the occurrence of skin ulcers and lesions, exophthalmos, inflammation of the intestines, bleeding, and eroded fins, so death is rapid when fish are infected with A. hydrophila. Death rates in two species of fish were high in C. carpio from the 1st day of bacterial injection, the fish began affected by injections, reflecting irregular swimming and interrupted feeding, then the death rates on the third day increased. The percentage of deaths increased, and C. carpio showed higher mortalities compared to O. niloticus and continued to gradually decrease by the 5th day, and then on the 7th day, there were very few deaths in two C. carpio and O. niloticus (Fig. 1). Clinical manifestationsInfected O. niloticus revealed red eyes, scales loss, fin rot, and erythema (Fig. 2). However, C. carpio showed bilateral exophthalmia and severe skin ulceration with exposure to viscera (Fig. 3). Quantitative real-time PCRThe primer sequences for a housekeeping gene (β_actin) to assess relative mRNA transcript levels of gene expression for the three antioxidant enzymes (SOD, GPX, and CAT) in C. carpio. According to Hoseini et al. (2022), the housekeeping gene (On_ GAPDH) to assess relative mRNA transcript levels of gene expression for the three antioxidant enzymes (SOD, GPX, and CAT) in O. niloticus according to Livak and Schmittgen (2001). Relative gene expression levels were calculated using the 2−ΔΔCt method after obtaining the threshold cycle (Ct) values of each sample (Livak and Schmittgen, 2001). The antioxidants-related gene expression in the liver, kidney, and spleenAeromonas hydrophila infection showed a significant effect on the expression of antioxidants-related gene expression in fish. Expression profiles of antioxidant-related genes were examined in the liver, kidney, and spleen tissues of infected C. carpio and O. niloticus (Fig. 4A and B). The SOD, GPx, and CAT were determined in the liver (Fig. 5A and B). The SOD, GPx, and CAT were assessed in the kidney (Fig. 6A and B). Levels of SOD, GPx, and CAT were estimated in the C. carpio at 1, 3, 5, and 7th post-A. hydrophila infection which were largely differed from controls during the whole period, except CAT, at 3rd post-infection and GPX and SOD, at 3rd post-infection (Fig. 4A). In O. niloticus, levels of SOD, GPx, and CAT showed an obvious antioxidant response gene in the liver samples of A. hydrophila infection as compared with those of the controls (Fig. 4B).
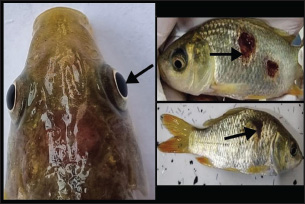
Fig. 2. Oreochromis niloticus reveals red eye, scales loss, fin rot, and erythema in the skin.
Fig. 3. Cyprinus carpio shows exophthalmia and severe ulceration in the skin with exposure of viscera. Table 1. Histological changes and pathological lesions in two species of fish.
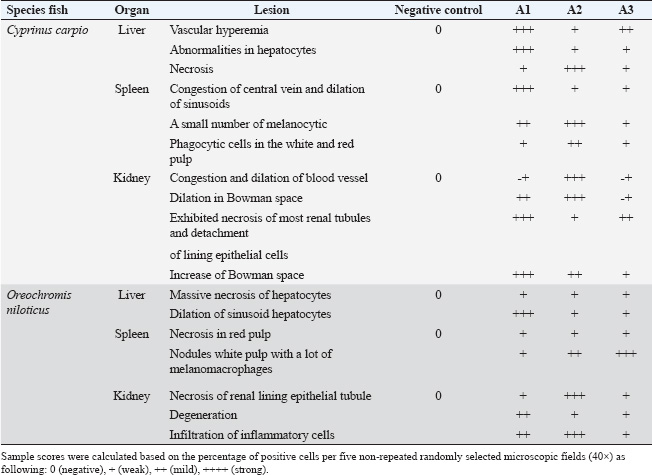
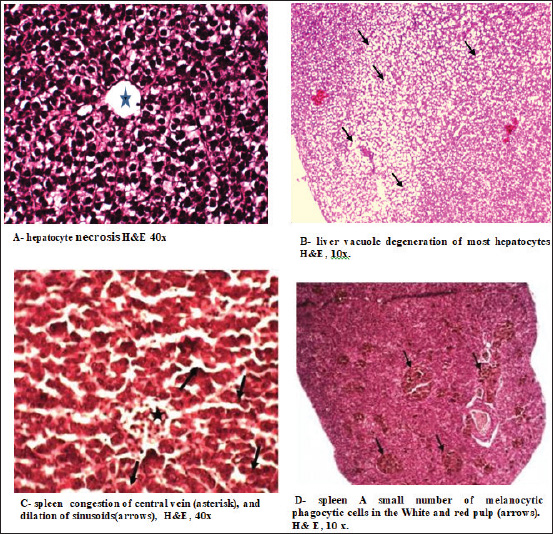
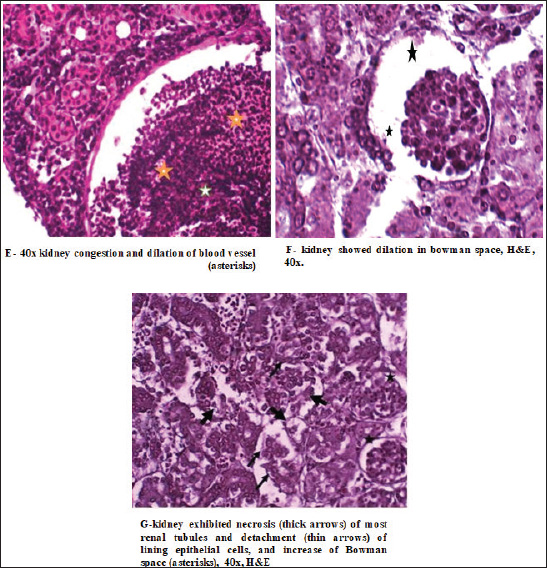
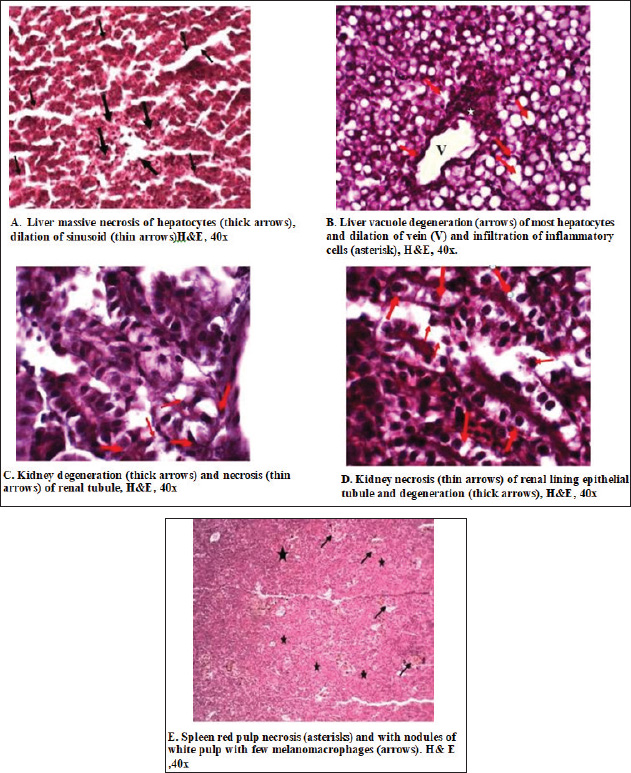
In the kidney, the infected C. carpio showed that the activity of CAT peaked on the 3rd day post-infection. The activities of SOD and GPx peaked on the 3rd day (Fig. 5A). However, the levels of both enzymes decreased remarkably on the 7th day post-infection (p < 0.05). In O. niloticus, the controls at the first day post-infection, there was a significant difference (p < 0.05) and increased substantially with respect to the controls at the 3rd day post-infection. For SOD, GPx, and CAT (p < 0.05), levels in the groups peaked on the 7th day post-infection and were decreased (Fig. 5B). In the spleen (Fig. 6A and B), the activity levels of SOD, GPx, and CAT fluctuated in the spleen peaked at the 3rd third day of infection and were lowest on the 7th day. Both fish markedly differed from the control on the 7th day post-infection (p < 0.05). Histopathological findings post-infection by A. hydrophilaThe histological changes were illustrated in the internal organs of C. carpio (Fig. 7). The liver showed necrosis in the hepatocytes and vacuole degeneration of most hepatocytes. The spleen showed congestion of central vein (asterisk) and dilation of sinusoids. Additionally, the spleen revealed red pulp and white pulp nodules with more pigmented masses of melanomacrophages. Kidney showed congestion and dilation of blood vessels (asterisks) and dilation in Bowman space. Kidney exhibited necrosis (thick arrows) of most renal tubules, detachment (thin arrows) of lining epithelial cells, and increase of Bowman space (asterisks). In the case of histological changes in O. niloticus (Fig. 8), the liver showed massive necrosis of hepatocytes, dilatation of sinusoid, liver vacuole degeneration (arrows) of most hepatocytes and dilation of vein (V), and infiltration of inflammatory cells. The kidney showed degeneration and necrosis and, also, revealed necrosis (thin arrows) of the renal lining epithelial tubule and degeneration. Spleen revealed red pulp necrosis and nodules of white pulp with few melanomacrophages. For lesion scoring, all lesions of the liver, kidneys, and spleen were recorded in all groups of two species of fish and are shown in Table 1.
Fig. 4. Relative gene expression of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), in the liver of common carp (A) and Nile tilapia (B) after 1st, 3rd, 5th, and 7th days of Aeromonas hydrophila infection. The experiment was performed in triplicate, and the data are shown as the mean ± SD. Values with a different letter superscript are significantly different (p < 0.05).
Fig. 5. Relative expression of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), in the kidney of common carp (A) and Nile tilapia (B) after 1st, 3rd, 5th, and 7th days of Aeromonas hydrophila infection. The experiment was performed in triplicate, and the data are shown as the mean ± SD. Values with a different letter superscript are significantly different (p < 0.05). DiscussionBacterial infection is a life-and-death contest between the pathogen and the host, in which the host must organize all available resources to conquest (Sun et al., 2019a,b). Bacterial infection has a significant effect on the expression of genes in fish. Aeromonas hydrophila is a zoonotic virulent bacterial disease that infects both humans and animals resulting in higher mortalities (Al Shammari et al., 2023; El Gamal et al., 2023). To help prevent an outbreak in carp and tilapia and to improve our understanding of Aeromonas infection disease in fish, the current study is the first trial that aims to compare mortalities, clinical signs, antioxidant-associated genes, and histopathological changes upon experimental infection by A. Hydrophila in C. carpio and O. niloticus.
Fig. 6. Relative expression of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), in the spleen of common carp (A) and Nile tilapia (B) after 1st, 3rd, 5th, and 7th days of Aeromonas hydrophila infection. The experiment was performed in triplicate, and the data are shown as the mean ± SD. Values with a different letter superscript are significantly different (p < 0.05). Monitoring mortalities is essential to indicate the pathogenicity of A. hydrophila. The present perspective showed that the moral level of the affected groups was significantly increased on the 3rd day compared to the control, and C. carpio showed higher mortalities compared to O. niloticus. This could be returned to the occurrence of oxidative stress, which stimulates pro-inflammatory pathways. In line with Mohammadi et al. (2020), this study demonstrated the beginning of mortalities after 1 day from A. hydrophilia injection, the cumulative mortality rate exhibited an increase in the fish population until the 10th day after in O. niloticus. Concurrent with Ünver and Bakıc (2021), similar signs in infected C. carpio were obtained, represented in small bleedings on fish skin and fin bases, swollen abdomen, and prominent eyes. Infection by A. hydrophila has a varied temporal distribution in fish; it is most common in summer in fish. Infection rates were 6% in summer, 2% in spring, and 0% in autumn and winter. The water temperature changes, and unfavorable conditions in the water the majority of injuries occurred. Fish become more sensitive to stress in addition to other environmental conditions, and the prevalence of A. hydrophila in the current study was high and caused clinical symptoms. The incubation period of the disease depends on the species of fish and their resistance, environmental conditions, and season. This period varies from 2–4 days in natural infections and 8–48 hours in experimental infection models in the acute form of the disease (Aboelgalagel, 2015). When there are clinical signs of infection, infected fish may show exophthalmos, redness of the skin, and accumulation of fluid in the abdomen (Ammar et al., 2023).
Fig. 7. Histopathological changes caused by Aeromonas hydrophila infection in the liver, kidney, and spleen of common carp. Changes in gene expression in the liver, kidneys, and spleen were also evaluated by injecting fish with pathogenic bacteria. When evaluating the results of Real time- quantitative PCR, it was noted that CAT expression in bacteria-exposed fish can be an immediate response mechanism to stress caused by bacterial contamination. The decrease in the expression of CAT indicated the involvement of oxidative stress in the mechanism of toxicity of bacteria as documented by Bayır et al. (2022). The disparity between (mRNA) abundance and enzyme activity should not be surprising. That is a possible reason for observed changes in the expression of the measured CAT, which is one of the stress response-related genes. As regards the observed down-regulation of CAT in fish, similar trends were obtained in a previous study (Mu et al., 2015). Levels of antioxidant genes, SOD, GPX, and CAT showed an obvious antioxidant response in the liver samples of A. hydrophila-infection in O. niloticus as compared with those of the controls (as illustrated in Fig. 4B). This may be due to its high tolerance and tolerability protection of Tilapia against stressful environmental conditions as recorded by El Asely et al. (2020). Pal et al. (2019) confirmed our findings and indicated that challenged Labeo rohita with A. hydrophila resulted in a high rate of ROS production in the liver of infected fish producing oxidative stress. The expression GPX gene in O. niloticus was analyzed. In comparison to the control group, the liver had the highest level of expression of genes. In line with a new study, Yu et al. (2021) found that the liver showed a rapid speed of response in GPX expression among different tissues. Similar outcomes by Pamplona and Costantini (2011) who measured CAT and GPx in the internal organs (liver, kidneys, and spleen) of common carp and Indigo tilapia and the levels of mRNA in the hepatic tissue were also detected. The results showed increased gene expression of CAT in the liver and kidneys, and the gene expression of antioxidants in both fish was similar during the experiment period, while the spleen had an increase in carp.
Fig. 8. Histopathological changes caused by Aeromonas hydrophila infection in the liver , kidney, and spleen of Nile tilapia. In the current study, the occurrence of oxidative damage reflects tissue architecture which exhibited necrosis of hepatocytes, kidney, and spleen red pulp necrosis. This can be explained by the increased production of ROS due to A. hydrophila infection. The excessive production of free radicals and lipid peroxidation result in the occurrence of necrosis, loss of epidermal, and skin epithelium as mentioned by El Gamal et al. (2023). Additionally, it could be attributed to the destruction of polyunsaturated fatty acids from the cell membrane lipids resulting in lipid peroxidation, which represented significant variances between the expression of genes, GPx and CAT in the tissues. The liver revealed the highest peak which reflects the presence of oxidative stress and cellular damage. In line with Ünver and Bakıc (2021), A. hydrophila induced histopathological changes in C. carpio such as the removal of the epithelium because of hyperplasia and hypertrophy. Overall, this is the first report in Iraq to analyze antioxidant mRNA as a potential biomarker of oxidative stress due to the influence of the bacterium A. hydrophila. Analysis of important enzymatic antioxidant genes, SOD, GPX, and CAT, for defending against ROS, as a result of the attack of pathological bacteria A. hydrophila is crucial to study oxidative stress. Furthermore, studying the negative influence of A. hydrophila via recording mortalities and clinical signs is essential. Moreover, risk assessment is pathologically determined. ConclusionThe present perspective is the first attempt in Iraq to prove the harmful influence of the virulent A. hydrophila on the occurrence of mortalities, markable clinical manifestations, and fluctuating relative antioxidant gene expression of SOD, GPx, and CAT in both C. carpio and O. niloticus. Cyprinus carpio demonstrated higher mortalities and major clinical picture in comparison to O. niloticus. Additionally, there were prominent histopathological changes in the hepato-renal tissues and spleen particularly in C. carpio related to O. niloticus. The dignified antioxidants genes displayed variations in reaction to the bacterial challenge which was more prominent in O. niloticus compared to C. carpio, and the gene expression for CAT was the most expressed among the genes. Nile tilapia has their gene expression augmented especially in the spleen, and the genes were more expressed compared to C. carpio. AcknowledgmentsThe authors are thankful to the Deanship of Graduate Studies and Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program. Conflict of interestThe authors declare no competing interests. Authors’ contributionsAll authors contributed equally to this study Consent for publicationAll authors review and approve the manuscript for publication. Institutional review board statementAll animal-handling protocols were performed based on the regulations of Institutional Animal Care and Use Committee (IACUC) with oversight of the Basrah University (Approval Number: BU-IACUC-2024-1-20). All experimental procedures were directed in obedience to the ethical guidelines approved by the National Institutes of Health for Use and Treatment of Laboratory Animals and following ARRIVE guidelines. Data availabilityAll data regarding this study are presented in the paper. ReferencesAbdelazim, A., Khater, S., Ali, H., Shalaby, S., Afifi, M., Saddick, S. and Almaghrabi, O.A. 2019. Panax ginseng improves glucose metabolism in streptozotocin-induced diabetic rats through 5′ adenosine monophosphate kinase up-regulation. Saudi J. Biol. Sci. 26(7), 1436–1441. Abdel Rahman, A.N., Doan, V.H., Elsheshtawy, H.M., Dawood, A., Salem, S.M.R., Sheraiba, N.I., Masoud, S.R., Abdelnaeim, N.S., Khamis, T., Alkafafy, M. and Mahboub, H.H. 2022a. Dietary Salvia officinalis leaves enhances antioxidantimmune-capacity, resistance to Aeromonas sobria challenge, and growth of Cyprinus carpio. Fish Shellfish Immunol. 127(2022), 340–348. Abdel Rahman, A.N., Mansour, D.A., Abd El-Rahman, G.I., Elseddawy, N.M., Zaglool, A.W., Khamis, T., Mahmoud, S.F. and Mahboub, H.H. 2022b. Imidacloprid toxicity in Clarias gariepinus: protective role of dietary Hyphaene thebaica against biochemical and histopathological disruption, oxidative stress, immune genes expressions, and Aeromonas sobria infection. Aquaculture 555, 738170. Aboelgalagel, W.H. 2015. Bacteriological and molecular studies of some pathogenic bacteria isolated from Oreochromis niloticus in fish farms. MV Sc. Thesis, Microbiology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr el-Sheikh, Egypt. Ahmad, H.I., Jabbar, A., Mushtaq, N., Javed, Z., Hayyat, M.U., Bashir, J., Naseeb, I., Abideen, Z.U., Ahmad, N. and Chen, J. 2022. Immune tolerance vs. immune resistance: the interaction between host and pathogens in infectious diseases. Front. Vet. Sci. 9, 827407. Al Shammari, N.A., AL-Niaeem, K.S. and Al-Hawash, A.B. 2023. Molecular identification of some zoonotic bacteria isolated from fishes Cyprinus carpio L. and Oreochromis niloticus (L.). J. Asqu. Bio. Fish. 27(4), 1047– 062. Alzahrani, O.M., Elumalai, P., Nada, H.S., Ahmed, S.A., Zaglool, A.W., Shawky, S.M., Alkafafy, M. and Mahboub, H.H. 2023. Pseudomonas putida: sensitivity to various antibiotics, genetic diversity, virulence, and role of formic acid to modulate the immune-antioxidant status of the challenged Nile tilapia compared to carvacrol oil. J. Fish. 8(1), 6. Ammar, A.M., Abd El-Galil, S.Y., Mohamed, B.E. and Gharib, A.A. 2023. Motile aeromonads as a Nile tilapia bacterial infection: a review on prevalence, molecular characterization, effect on immune response and alternatives control measures. Zagazig Vet. J. 51(1), 112–128. Baldissera, M.D., Souza, C.F., Parmeggiani, B., Leipnitz, G., Verdi, C.M., Santos, R.V., Stefani, L.M. and Baldisserotto, B. 2018. The disturbance of antioxidant/oxidant balance in fish experimentally infected by Aeromonas caviae: relationship with disease pathophysiology. Microb. Pathog. 122, 53–57. Bayır, M., Çapan, E.C. and Keşan, S. 2022. Bioinformatics and mRNA expression of catalase gene and determination of catalase enzyme activity in zebrafish (Danio rerio) exposed to the herbicide paraquat Iranian J. Fish. Sci. 21(5), 1278–1297. Cai, H., Zhu, X.X., Li, Z.F., Zhu, Y.P. and Lang, J.H. 2018. MicroRNA dysregulation and steroid hormone receptor expression in uterine tissues of rats with endometriosis during the implantation window. Chin. Med. J. (Engl). 131, 2193–2204. Chowdhury, S. and Saikia, K. 2020. Oxidative stress in fish: a review. J. Sci. Res. 12(1), 145–160. Eissa, M.E., Alaryani, F.S., Elbahnaswy, S., Khattab, M.S., Elfeky, A., AbouelFadl, K.Y., Eissa, E.S.H., Ahmed, R.A., Van Doan, H. and El-Haroun, E. 2023. Dietary inclusion of Pediococcus acidilactici probiotic promoted the growth indices, hematobiochemical indices, enzymatic profile, intestinal and liver histomorphology, and resistance of Nile Tilapia against Aspergillus flavus. Anim. Feed Sci. Tech. 306, 115814. El Asely, A.M., Abbass, A. and Austin, B. 2020. Ziziphus mauritiana supplementation of Nile tilapia (Oreochromis niloticus) diet for improvement of immune response to Aeromonas hydrophila infection. J. Fish Physiol. Bio. 46(4), 1561. El Gamal, S.A., Adawy, R.S., Zaki, V.H. and Zahran, E. 2023. Host–pathogen interaction unveiled by immune, oxidative stress, and cytokine expression analysis to experimental Saprolegnia parasitica infection in Nile tilapia. J. Smart Agr. Tech. 3, 100083. Faheemn, M., Nusrat Jahan, N. and Lone, K. 2016. Histopathological effects of bisphenol-a on liver, kidneys and gills of Indian major carp, Catla catla (Hamilton, 1822). J. Anim. Plant Sci. 26(2), 514–522. Hendam, B.M., Munir, M.B., Eissa, M.E., El-Haroun, E., van Doan, H., Chung, T.H. and Eissa, E.S.H. 2023. Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim. Feed Sci. Technol. 303, 115696. Hong, Y., Yin, H., Huang, Y., Huang, Q. and Yang, X. 2020. Immune response to abamectin-induced oxidative stress in Chinese mitten crab, Eriocheir sinensis. Ecotoxicol. Environ. Saf. 18, 109881–109889. Hoseini, S.M., Rajabiesterabadi, H., Abbasi, M., Khosraviani, K., Hoseinifar, S.H. and Van Doan, H. 2022. Modulation of humoral immunological and antioxidant responses and gut bacterial community and gene expression in rainbow trout, Oncorhynchus mykiss, by dietary lactic acid supplementation. Fish shellfish immunol. 125, 26–34. Hoseinifar, S.H., Jahazi, M.A., Mohseni, R., Yousefi, M., Bayani, M., Mazandarani, M., Van Doan, H. and El-Haroun, E.R. 2021. Dietary apple peel-derived pectin improved growth performance, antioxidant enzymes and immune response in common carp, Cyprinus carpio (Linnaeus, 1758). Aquaculture 535, 736311. Jiang, B., Li, Q., Zhang, Z., Huang, Y., Wu, Y., Li, X. and Jian, J. 2023. Selection and evaluation of stable reference genes for quantitative real-time PCR in the head kidney leukocyte of Oreochromis niloticus. Aquacul. Rep. 31, 101660. Li, C., Li, M., Li, S., Xing, Y., Yang, C.Y., Li, A., Borok, Z., Langhe, S. and Minoo, P. 2015. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 33, 999–1012. Liang, H., Liang, R., Wang, H., Zhang, X., Yan, X. and Shen, W. 2022. Apelin participates in host defense against bacterial infection and promotes bacterial clearance in large yellow croaker (Larimichthys crocea). Aquaculture 549, 737803. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(8), 402–408. Mahboub, H.H., Faggio, C., Hendam, B.M., Algharib, S.A., Alkafafy, M., Hashem, M.A., Mahmoud, Y.K., Khamis, T., Abdel-Ghany, H.M., Masoud, S.R. and Rahman, A.N.A. 2022a. Immune-antioxidant trait, Aeromonas veronii resistance, growth, intestinal architecture, and splenic cytokines expression of Cyprinus carpio fed Prunus armeniaca kernel-enriched diets. Fish Shellfish Immunol. 124, 182–191. Mahboub, H.H., Nada, H.S., Abdel-Ghany, H.M., Ghanem, R., Ahmed Ismail, T. and Abdel Rahman, A.N. 2022b. Detection, diagnosis, Koch’s postulate, hepatorenal and antioxidant indicators for some systemic pathogenic fungi invading the liver and kidneys of African catfish (Clarias gariepinus) in Egypt with a histopathological approach. J. Aquac. Res. 53, 2670–2685. Mohammadi, G., Rafiee, G., El Basuini, M.F., Abdel-Latif, H.M. and Dawood, M.A. (2020). The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish Shellfish Immunol. 106, 36–43. Mu, X., Chai, T., Wang, K., Zhang, J., Zhu, L., Li, X. and Wang, C. 2015. Occurrence and origin of sensitivity toward difenoconazole in zebrafish (Danio reio) during different life stages. Aquat. Toxicol. 160, 57–68. Okamoto, T. and Okabe, S. 2000. Ultraviolet absorbance at 260 and 280 nm in RNA measurement is dependent on measurement solution. Int. J. Mol. Med. 5(6), 657–659. Pal, S., Roy, D., Ray, S.D. and Homechaudhuri, S. 2019. Aeromonas hydrophila induced mitochondrial dysfunction and apoptosis in liver and spleen of Labeo rohita mediated by calcium and reactive oxygen species. Turk. J. Fish. Aquat. Sci. 20(4), 255–266. Pamplona, R. and Costantini, D. 2011. Molecular and structural antioxidant defenses against oxidative stress in animals. American J. Physiol. Reg. Integ. Comp. Physi. 301(4), R843–R863. Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D. and Bitto, A. 2017. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017(1), 8416763. Rashidian G, Mahboub HH, Hefny AA, Fahim A, Prokić MD, Rainis S, Faggio C. 2022. Mooseer (Allium hirtifolium) boosts growth, general health status, and resistance of rainbow trout (Oncorhynchus mykiss) against Streptococcus. Fish Shellfish Immunol. 120, 360–368. Sidorczuk, M.G., Brzóska, M.M., Jurczuk, M. and Jakoniuk, J. 2009. Oxidative damage to proteins and DNA in rats exposed to cadmium and/or ethanol. Chem. Biol. Interact 180(1), 31–38. Sielska, A., Cembrowska-Lech, D., Kowalska-Góralska, M., Czerniawski, R., Krepski, T. and Skuza, L. 2024. Effects of copper nanoparticles on oxidative stress genes and their enzyme activities in common carp (Cyprinus carpio). Eur. Zool. J. 91(1), 354–365. Sun, Y., Xiang, Y., He, M., Zhang, X., Wang, S., Guo, W., Liu, C., Cao, Z. and Zhou, Y. 2019a. Evaluation of Lactococcus lactis HNL12 combined with Schizochytrium limacinum algal meal in diets for humpback grouper (Cromileptes altivelis). Fish Shellfish Immunol. 94, 880–888. Sun, Y., Zhuang, Z., Wang, X., Huang, H., Fu, Q. and Yan, Q. 2019b. Dual RNA-seq reveals the effect of flgM gene of Pseudomonas plecoglossicida on immune response of Epinephelus coioides. Fish Shellfish Immunol. 87, 515–523. Torrecillas, S., Makol, A., Caballero, M. J., Montero, D., Robaina, L., Real, F. and Izquierdo, M.S. 2007. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 23(5), 969–981. Ünver, B. and Bakıcı, M.Z. 2021. Motile aeromonad septicemia (MAS) at Cyprinus carpio L., 1758 (Actinopterygii:Cyprinidae) in Lake Tödürge (Sivas/Turkey). Arq. Bras. Med. Vet. Zootec. 73(2), 320–326. World Health Organization (WHO). 2021. Zoonotic disease: emerging public health threats in the region. Available via http://www.emro.who.int/fr/about-who/rc61/zoonotic-diseases Yu, H., Wang, C., Deng, W., Liu, G., Liu, S. and Ji, H. 2021. Characterization and expression profiling of glutathione peroxidase 1 gene (GPX1) and activity of GPX in Onychostoma macrolepis suffered from thermal stress. Turk. J. Fish. Aqua. Sci. 21(11), 541–551. | ||
| How to Cite this Article |
| Pubmed Style Shammari NAHA, Al-niaeem KS, Al-hawash AB, Abomughaid MM, Abdelazim AM, Elumalai P, Eissa EH, Mahboub HH, Elbaghdady HAM. Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Vet. J.. 2025; 15(7): 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 Web Style Shammari NAHA, Al-niaeem KS, Al-hawash AB, Abomughaid MM, Abdelazim AM, Elumalai P, Eissa EH, Mahboub HH, Elbaghdady HAM. Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. https://www.openveterinaryjournal.com/?mno=255558 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.12 AMA (American Medical Association) Style Shammari NAHA, Al-niaeem KS, Al-hawash AB, Abomughaid MM, Abdelazim AM, Elumalai P, Eissa EH, Mahboub HH, Elbaghdady HAM. Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Vet. J.. 2025; 15(7): 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 Vancouver/ICMJE Style Shammari NAHA, Al-niaeem KS, Al-hawash AB, Abomughaid MM, Abdelazim AM, Elumalai P, Eissa EH, Mahboub HH, Elbaghdady HAM. Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 Harvard Style Shammari, N. A. H. A., Al-niaeem, . K. S., Al-hawash, . A. B., Abomughaid, . M. M., Abdelazim, . A. M., Elumalai, . P., Eissa, . E. H., Mahboub, . H. H. & Elbaghdady, . H. A. M. (2025) Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Vet. J., 15 (7), 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 Turabian Style Shammari, Nadia A. H. Al-, Khalidah S. Al-niaeem, Adnan B. Al-hawash, Mosleh Mohammad Abomughaid, Aaser M. Abdelazim, Preetham Elumalai, El-sayed Hemdan Eissa, Heba H. Mahboub, and Heba Allah M. Elbaghdady. 2025. Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Veterinary Journal, 15 (7), 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 Chicago Style Shammari, Nadia A. H. Al-, Khalidah S. Al-niaeem, Adnan B. Al-hawash, Mosleh Mohammad Abomughaid, Aaser M. Abdelazim, Preetham Elumalai, El-sayed Hemdan Eissa, Heba H. Mahboub, and Heba Allah M. Elbaghdady. "Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus." Open Veterinary Journal 15 (2025), 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 MLA (The Modern Language Association) Style Shammari, Nadia A. H. Al-, Khalidah S. Al-niaeem, Adnan B. Al-hawash, Mosleh Mohammad Abomughaid, Aaser M. Abdelazim, Preetham Elumalai, El-sayed Hemdan Eissa, Heba H. Mahboub, and Heba Allah M. Elbaghdady. "Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus." Open Veterinary Journal 15.7 (2025), 3012-3023. Print. doi:10.5455/OVJ.2025.v15.i7.12 APA (American Psychological Association) Style Shammari, N. A. H. A., Al-niaeem, . K. S., Al-hawash, . A. B., Abomughaid, . M. M., Abdelazim, . A. M., Elumalai, . P., Eissa, . E. H., Mahboub, . H. H. & Elbaghdady, . H. A. M. (2025) Comparative study of mortalities, clinical manifestations, antioxidant-associated genes, and histopathological degeneration upon experiment infection by Aeromonas hydrophila in Cyprinus carpio and Oreochromis niloticus. Open Veterinary Journal, 15 (7), 3012-3023. doi:10.5455/OVJ.2025.v15.i7.12 |