| Case Report | ||
Open Vet. J.. 2025; 15(10): 5400-5407 Open Veterinary Journal, (2025), Vol. 15(10): 5400-5407 Case Report Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomographyMason White* and Michelle LauSmall Animal Specialist Hospital, North Ryde, Australia *Corresponding Author: Mason White. Small Animal Specialist Hospital, North Ryde, Australia. Email: mwhite [at] sashvets.com Submitted: 13/05/2025 Revised: 27/08/2025 Accepted: 10/09/2025 Published: 31/10/2025 © 2025 Open Veterinary Journal
AbstractBackground: Gastroesophageal intussusception is a rare condition in dogs, most commonly presenting as an acute, life-threatening disease. In some dogs with chronic disease, intermittent intussusception is suspected to underlie clinical signs, although its relationship with other oesophageal disorders remains poorly understood. Transient intussusception has only been reported in one endoscopic study, where concurrent assessment of the oesophageal hiatus was not possible. To the best of the authors’ knowledge, spontaneous reduction has never been definitively demonstrated tomographically. Case Description: A 10-year-old neutered male Australian Terrier presented to his local veterinarian after an episode of retching and collapse. He was referred to an internal medicine specialist and underwent computed tomography to look for possible underlying causes, as well as to investigate chronic, pre-existing respiratory disease. The gastric cardia and gastroesophageal junction herniated into the caudal thorax after contrast administration, before returning to a normal position on subsequent acquisitions, consistent with a sliding hiatal hernia. The patient was discharged for medical management and presented to a different referral hospital the following year for the evaluation of a sublumbar mass identified on abdominal ultrasound. Computed tomography revealed a mass arising from the right iliopsoas muscle, and a cytological diagnosis of hemangiosarcoma was made. The stomach was in its normal position on pre-contrast and delayed post-contrast images. However, on venous-phase images acquired between these time points, the gastric fundus was cranially herniated through the oesophageal hiatus into the caudal oesophageal lumen, with the gastroesophageal junction remaining appropriately positioned. These findings were consistent with self-resolving gastroesophageal intussusception. No treatment was pursued for the intussusception, and treatment for haemangiosarcoma was declined. A poor prognosis was given, and the patient died 5 days later without a postmortem examination. Conclusion: This is the first reported case of gastroesophageal intussusception in a dog that was tomographically determined to be self-resolving and the first case of gastroesophageal intussusception in association with an untreated hiatal hernia. Keywords: Chronic, Gastroesophageal, Intermittent, Intussusception, Transient. IntroductionGastroesophageal intussusception (GEI) is a rare condition that typically affects young, large-breed dogs and presents as an acute, life-threatening condition that requires rapid recognition and treatment. It has also been thought to occur intermittently, resulting in chronic, sporadic gastrointestinal signs, though definitive tomographic identification of a spontaneously resolving intussusception has not been reported. Therefore, it was previously impossible to attribute clinical signs to intermittent intussusception rather than intermittent hiatal herniation or other oesophageal diseases. To the best of the authors’ knowledge, this is the first case of true, spontaneously resolving GEI to be definitively documented tomographically, thereby demonstrating that a transient or intermittent form of the disease can occur. The pathophysiology of GEI is incompletely understood, and GEI may occur secondary to the gradual worsening of a pre-existing hiatal hernia over time. This case is the first report of GEI in a dog with a previously diagnosed, untreated hiatal hernia, demonstrating this theory to be plausible. Together, the findings in this case report demonstrate the feasibility of these theories that have previously been speculated but not definitively proven, providing insight into the pathophysiology of this poorly understood condition. Case DetailsA 10-year-old male neutered Australian Terrier presented to his regular primary care clinic after an episode of retching and collapse. He had reportedly been retching as though attempting to vomit, before slowly collapsing into dorsal recumbency and quickly returning to normal once picked up by his owner. The patient had a history of chronic lymphoplasmacytic rhinitis, keratoconjunctivitis sicca, and xeromycteria. The patient received several medications for the above-mentioned conditions, including antimicrobials, antihistamines, oral pilocarpine, topical ocular medications, and prednisolone or non-steroidal anti-inflammatory drugs at different time points. The patient had a persistent biochemical hepatopathy that had been sonographically investigated and was thought to be associated with administration of the aforementioned medications. The initial physical examination revealed only mild left-sided, mucoid ocular discharge and left-sided mucopurulent nasal discharge. Routine hematology and serum biochemistry showed only mild, incidental changes, including an elevated alkaline phosphatase (ALP) (280 U/l). No specific treatment was pursued, and the patient was referred to an internal medicine specialist for assessment 2 weeks later. Upon presentation to the referral hospital, the patient’s physical examination was unchanged; therefore, four days later, the patient underwent contrast-enhanced computed tomography (CECT) examination of the head, thorax, and abdomen under general anaesthesia to look for causes of collapse and assess his chronic, pre-existing nasal disease. The patient was premedicated with methadone (0.2 mg/kg IV) and medetomidine (5 µg/kg IV). General anaesthesia was induced with alfaxalone (2 mg/kg IV to effect) and maintained with isoflurane delivered in oxygen. CECT (GE Healthcare Lightspeed CT, 16-slice CT, Illinois, United States) was performed with the patient in sternal recumbency. Iohexol 350 mg I/ml (Omnipaque®, GE Healthcare Australia Pty Ltd, Australia) was administered at a dose of 2 ml/kg body weight over a 20-second injection period. Venous phase acquisitions of the abdomen and thorax were acquired 60 seconds after the injection. Venous-phase images of the abdomen revealed cranial displacement of the gastroesophageal junction and gastric cardia into the caudal mediastinum, while the stomach was positioned normally in the cranial abdomen on pre-contrast abdominal and post-contrast thoracic images acquired before and after this time point, respectively. These findings were consistent with a sliding hiatal hernia. A focal, strongly contrast-enhancing nodule was also seen arising from the bladder neck, consistent with a polyp or neoplasia. Other CT findings were incidental and consistent with bilateral non-destructive rhinitis, a dependent mineral-attenuating cholelith, and degenerative renal cysts and infarcts. Rhinoscopy immediately following computed tomography (CT) revealed bilateral mucus accumulation in the nasal cavities, and histopathological examination of mucosal biopsies was consistent with bilateral, chronic, proliferative neutrophilic and plasmacytic rhinitis. Oesophagoscopy was performed immediately after rhinoscopy and revealed an asymmetrical, ovoid (non-circular) oesophageal sphincter with a focal, puckered region and tendency to remain open. The patient had no history of chronic or intermittent gastrointestinal signs; however, a sliding hiatal hernia was considered a possible cause of retching and collapse. No specific treatment was pursued for the hiatal hernia, and the patient was discharged with an updated medical management plan for rhinitis. A fluoroscopic investigation to assess for gastroesophageal reflux was recommended but ultimately not performed. The patient presented with a history of inappropriate urination, hyperglycemia, and glycosuria approximately 3 months later and was ultimately diagnosed with diabetes mellitus. Insulin therapy was initiated, and the following year, he underwent bilateral phacoemulsification and pseudophakia at a different referral hospital for treatment of diabetic cataracts. Right pelvic limb pain was noticed on a routine postoperative recheck examination approximately 3 months later, and the patient was referred to his local veterinarian for further investigation. Physical examination and pelvic limb radiographs with his local veterinarian raised concerns for acute exacerbation of degenerative osteoarthritis; however, the patient failed to improve with rest and pain relief. Severe muscle atrophy over the right hindquarters and pain on thoracolumbar and right pelvic limb palpation raised concerns for a neurogenic cause on follow-up examination. Routine haematology and biochemistry revealed mild leucocytosis (19.87 × 109/l) characterised by neutrophilia (17.25 × 109/l) and lymphopenia (0.98 × 109/l), as well as thrombocytopenia (93 × 109/l). Serum biochemistry revealed elevated ALT (921 U/l), elevated ALP (2210 U/l), mild hyperglycaemia (7.6 mmol/l), and hypokalaemia (3.5 mmol/l) with lipaemia and haemolysis. The next day, abdominal ultrasound was performed to investigate hepatic enzyme elevations. A large, irregular, heterogeneous, cystic mass was seen encircling the aorta at the level of the aortic trifurcation, with intraluminal echoes consistent with tumour thrombi extending cranially in the caudal vena cava from the mass to the level of the liver. The patient was referred to the Oncology Department at the Small Animal Specialist Hospital in North Ryde 4 days later. Physical examination revealed moderate right pelvic limb lameness and a small, ulcerated area in the middle of the tongue. Repeat haematology and biochemistry revealed mild anaemia and haemoconcentration (PCV/TPP=37/82), marginal hypocalcaemia (Ca=1.24 mmol/l), marginal hypochloraemia (Cl=107 mmol/l), and marked hyperglycaemia (Glucose=24.1 mmol/l). To further characterise the sonographically identified lesion and look for evidence of potential metastases, CECT examination of the head, thorax, and abdomen was performed under general anaesthesia. The patient was premedicated with methadone (0.3 mg/kg, IV), and general anaesthesia was induced with intravenous administration of propofol (2.9 mg/kg total) and maintained with isoflurane delivered in oxygen. Contrast-enhanced computed tomography (GE Healthcare Revolution EVO CT, 128-slice CT, Illinois, United States) was performed with the patient in sternal recumbency and an intravenous catheter placed in the left cephalic vein. Pre-contrast acquisitions of the head, thorax, and abdomen were acquired. Iohexol 300 mg I/ml (Omnipaque®, GE Healthcare Australia Pty Ltd, Australia) was then administered intravenously at a dose of 1.5 ml/kg body weight, with a fixed 20-second injection time. Bolus tracking was used to initiate scan acquisition, with the region of interest positioned over the caudal thoracic aorta and a trigger threshold of 100 Hounsfield units. Arterial-phase images were obtained 2 seconds after the trigger threshold was reached, followed by venous-phase images acquired 40 seconds later. A delayed-phase acquisition was performed 18 minutes after the initial trigger time based on a preliminary review of the earlier phases. Pre-contrast acquisitions demonstrated a normally positioned stomach; however, the gastric fundus was cranially herniated through the oesophageal hiatus into the caudal esophageal lumen on the venous-phase post-contrast scan (Figs. 1 and 2). The distal oesophageal wall encircled part of the gastric fundus, creating a target-like appearance on transverse-plane images (Fig. 3). The orad oesophagus contained a small-to-moderate amount of heterogeneous fluid-to-soft-tissue-attenuating material. Delayed post-contrast images showed the stomach had returned to its normal anatomical position.
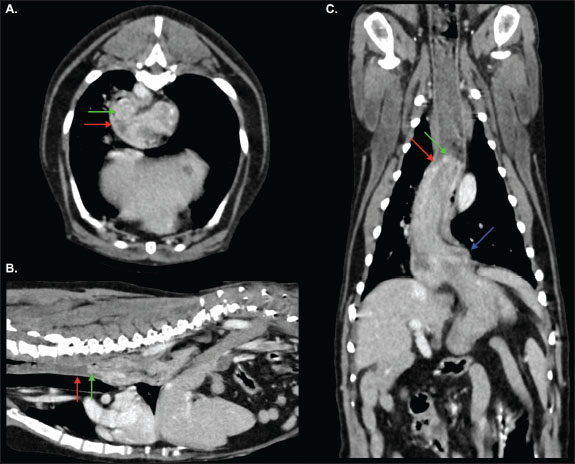
Fig. 1. Transverse (A), sagittal (B) and dorsal (C) images from the venous-phase acquisition. Part of the gastric fundus (green arrow) has herniated cranially through the oesophageal hiatus into the caudal thoracic oesophagus (red arrow). The gastroesophageal junction (blue arrow) remains in a normal position. The more proximal thoracic oesophagus is mildly distended by a small volume of heterogeneous, intraluminal, fluid-and-soft-tissue-attenuating material. Findings are consistent with gastroesophageal intussusception.
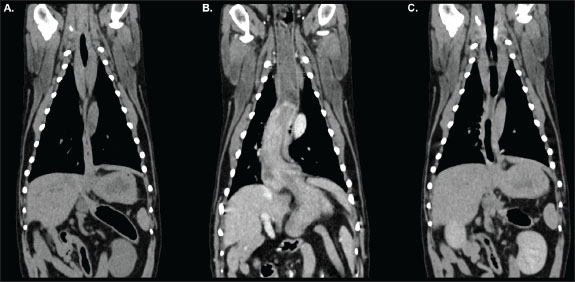
Fig. 2. Dorsal reconstructions of the thorax and cranial abdomen acquired during pre-contrast (A), venous-phase (B), and delayed post-contrast (C) phases of the study. The stomach is appropriately positioned with minimal distension of the oesophagus in the pre-contrast (A) and delayed (C) acquisitions. Gastroesophageal intussusception is identified during the venous-phase acquisition (B) as outlined in Figure 1.
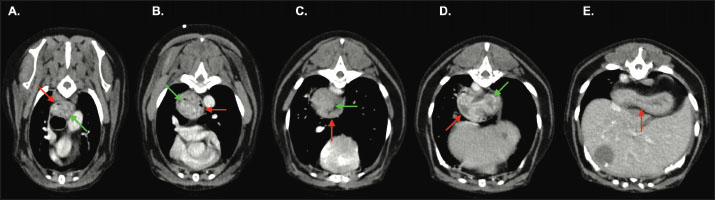
Fig. 3. Transverse-plane images of the thorax from the venous-phase acquisition at approximately the level of the T4 (A), T5 (B), T8 (C), T9 (D), and T10 (E) vertebrae. The gastric fundus (green arrow) has herniated cranially through the oesophageal hiatus into the caudal thoracic oesophagus (red arrow). Note that unlike in cases of hiatal herniation, the distal oesophageal walls are visualised surrounding part of the gastric fundus, creating a target-like appearance, indicative of intussusception. An osteoaggressive, markedly vascular, and locally invasive caudal retroperitoneal mass was identified arising from the right iliopsoas muscle and extending ventral to the L4–L7 vertebrae. The caudal vena cava contained an extensive intraluminal, soft-tissue attenuating filling defect, and marked proximal right pelvic limb muscular atrophy was identified, indicating tumour thrombi and neurogenic atrophy, respectively. Other incidental computed tomography findings included hepatic/biliary cysts, chronic renal infarcts, cystic left hepatic lymphadenopathy, spondylosis deformans, shoulder and stifle osteoarthrosis, a left thyroid nodule, a small right thyroid, bronchial plugging indicative of chronic inflammatory disease, and nasal changes consistent with chronic inflammatory rhinitis. Gastric changes were consistent with transient, self-resolving GEI. This may represent a one-time, spontaneous GEI, possibly triggered by iohexol-induced nausea (Sanderson, 2005), anaesthesia-associated lower oesophageal sphincter laxity (Van Geffen et al., 2006), or a combination thereof. Alternatively, the finding may reflect incidental capture of a chronic, intermittent GEI that is unrelated to intravenous contrast or anaesthetic administration. If this is the case, the intussusception could represent a progression or exacerbation of the previously documented hiatal hernia, or could represent a separate, concurrent, intermittently occurring disease entity. The sublumbar mass arising from the iliopsoas was suggestive of malignant neoplasia. Differential diagnoses included haemangiosarcoma or a neuroendocrine tumour. Ultrasound-guided fine-needle aspirates were obtained. Cytological review was performed by a specialist veterinary pathologist, revealing individual and clustered, hyperchromic, pleomorphic spindle cells with moderately basophilic cytoplasm, variable punctate vacuolation, intracellular haemosiderin, scant mitotic figures, and large nuclei containing stippled chromatin and prominent nucleoli, consistent with a right iliopsoas haemangiosarcoma. No treatments were considered necessary for the spontaneously resolved gastroesophageal intussusception because signs of gastrointestinal disease had not been reported. The sublumbar mass was deemed inappropriate for surgical resection due to its extent and invasiveness. A poor prognosis was given. Various chemotherapeutic options and palliative radiation therapy were discussed, but the patient’s owners declined further treatment. The patient was discharged the following morning to continue his ocular medications, insulin, appetite stimulants, antinausea medications, and ongoing pain relief. The patient’s owners reported a clinical deterioration in the form of whimpering, panting, hotness to the touch, and pelvic limb weakness when toileting 1 day after discharge. 3 days later, the patient presented to another 24-hour specialist facility having died at home. No postmortem investigations were conducted. DiscussionGastroesophageal intussusception is defined as the retrograde translocation of all or part of the stomach into the thoracic oesophageal lumen (Shibly et al., 2014). In some cases, other organs, including the duodenum, omentum, spleen, and pancreas, may invaginate alongside the stomach (Rowland and Robinson, 1978; Roach and Hecht, 2007; Emery et al., 2015; Brady et al., 2017; Grimes et al., 2020). Importantly, in cases of GEI, the gastroesophageal sphincter maintains a normal position; this distinguishes it from axial or sliding hiatal hernias, in which the gastroesophageal junction is cranially malpositioned in the thorax, as outlined by Leib and Blass (cited in Grimes et al., 2020). GEI has historically been considered to most commonly occur in young, large-breed dogs (von Werthern et al., 1996; Rowland and Robinson, 1978; Moore, 2008; Grimes et al., 2020). Male German Shepherds are particularly over-represented and may be predisposed; A 2020 retrospective study found that dogs suffering from GEI had a median age of 13.2 months, were male in 72% of cases, and were German Shepherds in one-third (33%) of cases (Grimes et al., 2020). There are, however, reports of GEI in older dogs (Pietra et al., 2003; Kaewamatawong et al., 2010; Brady et al., 2017; Rohwedder & Hellmuth, 2021), female dogs (Greenfield et al., 1997; McGill et al., 2009; Kaewamatawong et al., 2010), cats (Van Camp et al., 1998; Van Geffen et al., 2006; Abbaszadeh Hasiri et al., 2013; Martinez et al., 2001; Tayler et al., 2021) and a variety of other breeds, including Australian Shepherd (Shibly et al., 2014), Siberian Husky (Greenfield et al., 1997; McGill et al., 2009; Takeuchi et al., 2021), Briard (Walker and Ryan, 2017), Pug (Pietra et al., 2003), Labrador (Graham et al., 1998), Golden Retriever (Kaewamatawong et al., 2010), Boxer (Brincin et al., 2022), Doberman (Sharma and A, 2005), Dalmatian (Järvinen et al., 1995) and mixed breeds (Rohwedder and Hellmuth, 2021), etc. This case report is the first to document the condition in an Australian Terrier. Dogs with GEI most commonly present with gastrointestinal signs such as vomiting and regurgitation, and may suffer haematemesis, dyspnoea, and abdominal discomfort (Shibly et al., 2014; Grimes et al., 2020). Acute-persistent and chronic-recurrent disease patterns have been described (von Werthern et al., 1996; Shibly et al., 2014; Grimes et al., 2020). Acute, persistent GEI is the most commonly reported form in dogs (Shibly et al., 2014; Grimes et al., 2020) and is caused by herniation and entrapment of the stomach into the caudal oesophageal lumen, with or without other organs. The presenting clinical signs are caused by oesophageal obstruction and intra-thoracic mass effect, with or without secondary aspiration pneumonia (von Werthern et al., 1996; McGill et al., 2009; Lockwood et al., 2010; Shibly et al., 2014). Displacement of the stomach into the caudal oesophagus impairs expansion of the caudal lung lobes and can result in respiratory distress (von Werthern et al., 1996; Lockwood et al., 2010). Compression of the pulmonary parenchyma, pulmonary vasculature, and caudal vena cava impedes venous return and leads to hypovolaemic shock, which is often the cause of rapid decline and death in these patients (Lockwood et al., 2010; Torad and Hassan, 2015). Additionally, compromised blood supply to the stomach and other prolapsed organs may lead to ischaemic necrosis and endotoxic shock (von Werthern et al., 1996; Lockwood et al., 2010). The prognosis has traditionally been considered poor (Lockwood et al., 2010); rapid deterioration and death can occur without prompt diagnosis and aggressive treatment, and mortality rates of up to 95% have been reported by Leib and Blass, as cited by Martinez et al. (2001). This might be attributed, at least in part, to a failure to recognise the condition and intervene before deterioration. A more recent study demonstrated a far better prognosis: 88% of dogs undergoing surgical intervention surviving to discharge, and 65% achieved long-term survival, though some chronic-form cases were included in this cohort (Grimes et al., 2020). Postoperative sequelae and complications, particularly megaoesophagus and secondary aspiration pneumonia, were common following GEI correction (Grimes et al., 2020); the latter of which may contribute to respiratory signs (Lockwood et al., 2010). Chronic or episodic forms of GEI have also been reported, typically resulting in intermittent gastrointestinal signs (von Werthern et al., 1996; Greenfield et al., 1997; Grimes et al., 2020; Shibly et al., 2014). This is the predominant form reported in cats (Van Camp et al., 1998; Van Geffen et al., 2006; Martinez et al., 2001; Shibly et al., 2014), although chronic disease has been associated with GEI in dogs, albeit less commonly (Pietra et al., 2003; Grimes et al., 2020). One retrospective study of canine gastroesophageal intussusception noted that dogs with a chronic history were less likely to have previously been diagnosed with megaoesophagus than those with an acute history of clinical signs (Grimes et al., 2020). The authors of these cases proposed that GEI could have resulted from one of the following: a hiatal hernia that resulted in gastrointestinal signs until the stomach eventually invaginated into the oesophagus; undiagnosed megaoesophagus; or a lax lower oesophageal sphincter that allowed intermittent, self-correcting GEI. To the best of the authors knowledge, there is only one report of spontaneous resolution of a GEI in a dog. This was identified endoscopically in an 8-week-old Husky with a 6-week history of regurgitation (Greenfield et al., 1997). In this case, the stomach was seen to intermittently intussuscept into the oesophagus, and although GEI had not been documented previously, the duration of clinical signs led the authors to consider chronically recurrent intussusception to be the likely cause of disease. However, the inability to assess concurrent hiatal abnormalities is a limitation of endoscopic evaluation of the oesophagus (Walker and Ryan, 2017). A hiatal hernia is a key differential for GEI and may be a predisposing condition (Grimes et al., 2020). Computed tomography allows for differentiation between hiatal hernias and GEI (Rohwedder and Hellmuth, 2021). To the best of the authors’ knowledge, only one other report has described the computed tomographic features of GEI in dogs; however, that case involved acute-onset disease with immediate surgical intervention (Rohwedder and Hellmuth, 2021), and spontaneous resolution was not observed. Our case provides the first tomographic evidence that spontaneous resolution of a confirmed GEI can occur in dogs, lending plausibility to the theory that transient GEI can occur in dogs and that intermittent GEI may therefore underlie chronic clinical signs in some patients. Oesophageal abnormalities, such as megaoesophagus, dysmotility, or oesophageal hiatus laxity, were speculated by Applewhite et al. (2002) to predispose affected animals to GEI, as cited by Grimes et al. (2020). Van Geffen et al. (2006) also cited a 1984 review by Leib and Blass that found that the majority dogs with GEI had pre-existing oesophageal disease, most commonly megaoesophagus. Another retrospective study found that 36% of dogs with GEI were previously diagnosed with megaoesophagus (Grimes et al., 2020), and pre-existing or concurrent megaoesophagus has also been reported in a number of cats with GEI (Van Camp et al., 1998; Martinez et al., 2001; Van Geffen et al., 2006). The link between megaoesophagus and GEI is poorly understood. It is thought that megaoesophagus might lead to dilatation of the lower oesophageal sphincter and subsequent intussusception of the stomach into the oesophagus during episodes of regurgitation or vomiting (Van Camp et al., 1998; Grimes et al., 2020). Unlike the previous case describing CT features of acute-onset GEI (Rohwedder and Hellmuth, 2021), concurrent or pre-existing megaoesophagus was never documented in our patient. Grimes et al. (2020) also reported that dogs with chronic clinical signs were less likely to have a prior diagnosis of megaoesophagus than those with acute presentations. This might imply different pathophysiologies between the acute persistent and chronic intermittent forms of the disease. An alternative theory suggests that lower oesophageal sphincter dilation could occur with reflux oesophagitis secondary to recurrent regurgitation and vomiting caused by pre-existing gastrointestinal disease (Rohwedder and Hellmuth, 2021). Hiatal hernias in particular have been proposed to cause chronic, intermittent gastrointestinal signs until the stomach eventually invaginates into the oesophagus (Grimes et al., 2020). A pre-existing hiatal hernia has been reported in a pug that subsequently developed GEI (Pietra et al., 2003); however, this dog had undergone surgical treatment of the hernia prior to the development of GEI, confounding the association. Our report is the first documented case of GEI in a patient with a history of untreated hiatal hernia. At the time of hiatal hernia diagnosis, oesophagoscopy revealed a subjectively puckered, dilated lower oesophageal sphincter, which might support the theory that gradual dilation can lead to eventual invagination of the stomach into the oesophagus. However, our patient did not suffer from chronic or recurrent gastrointestinal signs between the diagnosis of hiatal hernia and GEI. Although clinically-silent oesophageal reflux cannot be excluded, the oesophageal sphincter changes in this patient may have been incidental and unrelated to the tomographically identified sliding hernia or GEI. Therefore, although lower oesophageal sphincter laxity might be speculated in this single-animal case report, the associations between GEI and concurrent or pre-existing gastrointestinal disease must be interpreted with caution and should be the subject of future research. This case report definitively demonstrates that GEI can exist as a transient, spontaneously resolving entity and is not synonymous with entrapment and strangulation. This lends plausibility to the notion that chronic-intermittent or transient forms of the disease might occur in dogs. However, it must be noted that the patient outlined in this case report did not have the typical history of chronic, intermittent gastrointestinal signs that have been attributed to supposedly intermittent GEI in the past, and that GEI was only definitively documented on one occasion. Although recurrent, intermittent, and clinically-silent episodes of GEI might occur, recurrent or intermittent GEI was not definitively identified in this case. Furthermore, the association between supposedly intermittent GEI and clinical signs has not been established; further research is required to determine whether intermittent GEI is the true cause of clinical signs when suspected. Both the hiatal hernia and self-resolving gastroesophageal intussusception occurred as transient abnormalities during contrast-enhanced CT studies. To the best of the authors’ knowledge, this is the first case of GEI to be documented immediately after the administration of iodinated contrast media, and it is possible that both conditions occurred secondary to nausea induced by iohexol administration, with or without any association with other disease processes (Sanderson, 2005). Therefore, this report lends credibility to multiple theories about the pathophysiology of GEI, and further research is required to better understand the potential associations with iodinated contrast administration. ConclusionIn conclusion, this case tomographically demonstrates for the first time that a self-resolving, non-entrapped, transient form of GEI can occur in dogs. Although recurrent, intermittent GEI was not directly observed in this case, these findings support the hypothesis that intermittent GEI may underlie chronic, episodic gastrointestinal signs in some patients, as has been previously proposed. Further research is required to definitively determine whether an association exists between intermittent intussusception and chronic or intermittent clinical signs. To the best of our knowledge, this is also the first reported case of GEI in a dog with a pre-existing, uncorrected hiatal hernia and the first to describe spontaneous onset of GEI following intravenous iodinated contrast administration. These observations support the possibility that such events could represent adverse reactions to contrast administration or anaesthesia. Further studies are needed to clarify the potential associations between transient GEI, general anaesthesia, iodinated contrast administration, hiatal herniation, and other disease processes. Conflicts of interestThe authors declare no conflicts of interest. FundingThe authors declare that no financial support was received for the research, authorship, or publication of this article. Author’s contributionDr. Mason White performed a literature review, drafted the manuscript, coordinated and included revisions, produced figures for publication, and approved the final manuscript. Dr. Michelle Lau interpreted diagnostic imaging studies during clinical workup, contributed to manuscript revisions, and approved the final manuscript. Data availabilityThe data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. ReferencesAbbaszadeh Hasiri, M., Shojaee Tabrizi, A. and Ahrari Khafi, M.S. 2013. Gastroesophageal intussusception in a domestic short-hair cat. IJVR 14, 358–361. Brady, R., Biskup, J. and Latimer, C. 2017. Gastro-oesophageal intussusception with splenic involvement in an adult dog. Vet. Rec. Case Rep. 4. Brincin, C., Ryan, T. and Harris, K. 2022. Gastroesophageal intussusception secondary to induction of emesis with subsequent development of septic pericardial effusion after corrective surgery. J. Small Anim. Pract. 63, 72–77. Emery, L., Biller, D., Nuth, E. and Haynes, A. 2015. Ultrasonographic Diagnosis of Gastroesophageal Intussusception in a 7 Week Old German Shepherd. Isr. J. Vet. Med. 70, 41–46. Graham, K.L., Buss, M.S., Dhein, C.R., Barbee, D.D. and Seitz, S.E. 1998. Gastroesophageal intussusception in a Labrador retriever. Can. Vet. J. 39, 709–711. Greenfield, C.L., Quinn, M.K. and Coolman, B.R. 1997. Bilateral incisional gastropexies for treatment of intermittent gastroesophageal intussusception in a puppy. J. Anim. Vet. Med. Assoc. 211, 728–730. Grimes, J.A., Fleming, J.T., Singh, A., Campbell, B.G., Hedlund, C.S., Tobias, K.M., Arai, S., Ham, K.M., Repellin, R., Schroeder, R., Sumner, J.P., Abrams, B., Boudreau, B., Lewis, B. and Wallace, M.L. 2020. Characteristics and long-term outcomes of dogs with gastroesophageal intussusception. J. Anim. Vet. Med. Assoc. 256, 914–920. Järvinen, A.K., Saario, E., Andresen, E., Happonen, I., Saari, S. and Rajamäki, M. 1995. Lung Injury Leading to Respiratory Distress Syndrome in Young Dalmatian Dogs. J. Vet. Intern. Med. 9, 162–168. Kaewamatawong, T., Banlunara, W., Wangrattanaparanee, V. and Kiertkrittikhoon, S. 2010. Clinical, Radiographic and Pathological Features of Persistent Gastroesophageal Intussusception in an Adult Dog: a Case Report. Thai J. Vet. Med. 40, 221–225. Lockwood, A., Radlinksy, M. and Crochik, S. 2010. Gastroesophageal intussusception in a German shepherd. Compend. Contin. Educ. Vet. 32, E1–E4. Martinez, N., Cook, W., Troy, G. and Waldron, D. 2001. Intermittent gastroesophageal intussusception in a cat with idiopathic megaesophagus. J. Am. Anim. Hosp. Assoc. 37, 234–237. McGill, S.E., Lenard, Z.M., See, A.M. and Irwin, P.J. 2009. Nonsurgical Treatment of Gastroesophageal Intussusception in a Puppy. J. Am. Anim. Hosp. Assoc. 45, 185–190. Moore, L.E. 2008. Gastroesophageal intussusception.In Small Animal Gastroenterology. Hannover, Germany: Jörg M Steiner Schlütersche, p: 148. Pietra, M., Gentilini, F., Pinna, S., Fracassi, F., Venturini, A. and Cipone, M. 2003. Intermittent Gastroesophageal Intussusception in a Dog: clinical Features, Radiographic and Endoscopic Findings, and Surgical Management. Vet. Res. Commun. 27, 783–786. Roach, W. and Hecht, S. 2007. What Is Your Diagnosis?. J. Anim. Vet. Med. Assoc. 231, 381–382. Rohwedder, T. and Hellmuth, V.C. 2021. Gastroesophageal intussusception with complete herniation of the spleen in a 12 months old dog with idiopathic megaoesophagus. Vet. Rec. Case Rep. 9, 15. Rowland, M.G. and Robinson, M. 1978. Gastro–oesophageal intussusception in an adult dog. J. Small Anim. Pract. 19, 121–125. Sanderson, S. 2005. Current Concepts for the Management of Chronic Renal Failure in the Dog and Cat--Early Diagnosis and Supportive Care. In Proceedings of the World Small Animal Veterinary Association World Congress. Sharma, A. 2005. Gastroesophageal Intussusception in a Doberman Dog: a Rare Condition. Indian J. Vet. Pathol. 29, 56–57. Shibly, S., Karl, S., Hittmair, K.M. and Hirt, R.A. 2014. Acute gastroesophageal intussusception in a juvenile Australian shepherd dog: endoscopic treatment and long-term follow-up. BMC Vet. Res. 10, 109. Takeuchi, R., Takagi, S., Hosoya, K., Ohta, H., Itami, T. and Okumura, M. 2021. Postoperative improvement of megaesophagus and esophageal motility in a 19-day-old puppy with gastroesophageal intussusception. J. Vet. Med. Sci. 83, 1740–1744. Tayler, S., Mullowney, D., Lataretu, A., Plested, M., Tuan, J. and Kathrani, A. 2021. Gastroesophageal intussusception and extreme esophageal dilatation secondary to bilateral laryngeal paralysis in a cat. J. Vet. Intern. Med. 35, 1088–1092. Torad, F.A. and Hassan, E.A. 2015. Gastroesophageal Intussusception in a 50-Day-Old German Shepherd Dog. Top. Companion Anim. Med. 30, 22–24. von Werthern, C.J., Montavon, P.M. and Flückiger, M.A. 1996. Gastroesophageal intussusception in a young German shepherd dog. J. Small Anim. Pract. 37, 491–494. Van Camp, S., Love, N.E. and Kumaresan, S. 1998. Radiographic Diagnosis—Gastroesophageal Intussusception in a Cat. Vet. Radiol. Ultrasound 39, 190–192. Van Geffen, C., Saunders, J.H., Vandevelde, B., Van Ham, L., Hoybergs, Y. and Daminet, S. 2006. Idiopathic megaoesophagus and intermittent gastro-oesophageal intussusception in a cat. J. Small Anim. Pract. 47, 471–475. Walker, J.J.A. and Ryan, T.M. 2017. Gastro-oesophageal intussusception in a 25-week-old Briard. Vet. Rec. Case Rep. 5. | ||
| How to Cite this Article |
| Pubmed Style White M, Lau M. Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Vet. J.. 2025; 15(10): 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 Web Style White M, Lau M. Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. https://www.openveterinaryjournal.com/?mno=258050 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i10.58 AMA (American Medical Association) Style White M, Lau M. Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Vet. J.. 2025; 15(10): 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 Vancouver/ICMJE Style White M, Lau M. Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Vet. J.. (2025), [cited January 25, 2026]; 15(10): 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 Harvard Style White, M. & Lau, . M. (2025) Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Vet. J., 15 (10), 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 Turabian Style White, Mason, and Michelle Lau. 2025. Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Veterinary Journal, 15 (10), 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 Chicago Style White, Mason, and Michelle Lau. "Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography." Open Veterinary Journal 15 (2025), 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 MLA (The Modern Language Association) Style White, Mason, and Michelle Lau. "Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography." Open Veterinary Journal 15.10 (2025), 5400-5407. Print. doi:10.5455/OVJ.2025.v15.i10.58 APA (American Psychological Association) Style White, M. & Lau, . M. (2025) Spontaneous onset and resolution of gastroesophageal intussusception during contrast-enhanced computed tomography. Open Veterinary Journal, 15 (10), 5400-5407. doi:10.5455/OVJ.2025.v15.i10.58 |