| Research Article | ||
Open Vet. J.. 2025; 15(9): 4060-4074 Open Veterinary Journal, (2025), Vol. 15(9): 4060-4074 Research Article Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysisJunxue Qiu1†, Mengke Si1†, Xiaoyu Chong1, Yiwei Wang1, Baolei Yang1, Mingfeng Chu1, Yuchen Liang1, Wei Cheng1, Yanping Qi1, Xuelong Chen1,2* and Yan Liang1,2*1Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University, Fengyang, China 2Anhui Engineering Technology Research Center of Pork Quality Control and Enhance, Fengyang, China *Corresponding Author: Xuelong Chen, Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University, Fengyang, China. Email: cxlandqyp [at] 163.com; Yan Liang, Anhui Engineering Technology Research Center of Pork Quality Control and Enhance, Fengyang, China. Email: liangy [at] ahstu.edu.cn Submitted: 10/06/2025 Revised: 02/08/2025 Accepted: 10/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: A comprehensive understanding of the distribution of Streptococcus agalactiae, a major pathogen responsible for bovine mastitis, is critical for guiding therapeutic and control strategies. Aim: Thus, the objective of this meta-analysis is to investigate the prevalence and antimicrobial resistance (AMR) of S. agalactiae associated with bovine mastitis in China between 2004 and 2024.” Methods: The relevant literature was retrieved from PubMed, Google Scholar, and the China National Knowledge Infrastructure database. Following the PRISMA guidelines, 33 studies were included in the final analysis, of which 14 reported AMR data. All analyses were performed using the STATA software. Results: The pooled S. agalactiae prevalence was 24%. Subgroup analyses revealed a higher prevalence in Central China, an upward trend over time (2019–2024 vs. before 2019), and a higher prevalence in clinical cases than in subclinical mastitis. The pooled AMR data indicated that sulfonamides had the highest resistance rates, followed by aminoglycosides, β-lactams, lincosamides, macrolides, tetracyclines, quinolones, and chloramphenicol. Conclusion: These findings highlight the need for continued surveillance and refinement of therapeutic protocols to effectively manage S. agalactiae-induced mastitis in Chinese dairy herds. Keywords: Antimicrobial resistance, Bovine mastitis, Epidemiology, Meta-analysis, Streptococcus agalactiae. IntroductionMastitis, a highly prevalent disease in dairy cattle (Bovidae), is a significant threat to livestock welfare globally. Reduced milk production, increased operational costs, and the requirement for therapeutic interventions such as culling collectively contribute to significant economic losses (Shaheen and Ha, 2015; Krömker and Leimbach, 2017; Ashraf and Imran, 2020). The etiological complexity of bovine mastitis includes more than 80 bacterial species, among which Streptococcus agalactiae is a significant opportunistic pathogen, primarily associated with subclinical infections and elevated somatic cell counts (SCC) in affected herds (Keefe, 1997; Ikiz et al., 2013). Mastitis caused by S. agalactiae is a major challenge, second only to S. aureus, and exerts a negative impact on both animal health and dairy farm productivity (Richards et al., 2011; Radtke et al., 2012; Jaiva et al., 2018). Its prevalence continues to increase in both developed countries and agriculturally intensive regions. Undiagnosed or insufficiently treated animals serve as reservoirs of infection, and delays in treatment, isolation, or culling contribute to S. agalactiae intra-herd dissemination, particularly during milking (Skarbye et al., 2021). Despite regional variability in the antimicrobial resistance (AMR) profiles of S. agalactiae, the extensive use of antimicrobials remains a major concern, with increasing resistance trends observed in this pathogen (Keefe, 2012). Inappropriate use of antimicrobials intensifies AMR, complicating the therapeutic management of bovine mastitis and posing a significant global public health threat (Kuppusamy et al., 2018). The emergence of drug-resistant strains has led to increased antimicrobial use, contributing to environmental contamination and human health risks. Since 2011, China has intensified regulatory oversight of AMR and actively participated in the Global Action Plan (Yin et al., 2021). The National Action Plan for the Reduction of the Use of Veterinary Antimicrobial Drugs (2021–2025; Ministry of Agriculture and Rural Development of the People’s Republic of China, 2021) delineates specific goals and implementation measures, including improved access management, risk assessment, monitoring and surveillance, and guidance on AMR. China has made significant progress in AMR control compared with previous years. For instance, resistance to florfenicol in Escherichia coli isolated from poultry decreased from 65.3% in 2017 to 41.2% in 2022, whereas resistance to tetracycline in swine Salmonella decreased from 78.9% to 54.6% (China Veterinary Drug Inspection Institute, 2023). These epidemiological improvements reflect the effectiveness of strengthened regulatory frameworks under the current National Action Plan (2021–2025). Given the current global concern regarding AMR, investigating the prevalence and resistance patterns of S. agalactiae is essential. Traditional single-study approaches often lack generalizability due to regional variability and limited sample sizes. Meta-analytic methodologies were employed to systematically analyze data from studies published between 2004 and 2024 (Tonin et al., 2019) to address these limitations and integrate fragmented findings. Subgroup analyses were performed to evaluate coinfection rates from multiple perspectives, and the overall drug resistance rate was statistically analyzed to elucidate the temporal and geographical distribution, as well as the differences in resistance patterns across various forms of mastitis. The primary objective of this study was to assess the prevalence of S. agalactiae and its AMR characteristics. Changes in the prevalence and antibiotic resistance of S. agalactiae, a key pathogen in mastitis, not only affect the sustainable development of the dairy industry but also pose potential public health risks through the food chain. Studies have shown that S. agalactiae isolated from clinical mastitis cases carry multiple virulence genes (such as lacIV, gapC, and dltA), and these genes are also present in human clinical cases. Milk may serve as a vehicle for the transmission of strains from cows to humans (Liu et al., 2024). A meta-analysis of a large number of related studies can enhance the understanding of the spatial and temporal trends of S. agalactiae infection, address existing methodological limitations, and support the development of effective prevention and control strategies. Materials and MethodsLiterature searchFigure 1 outlines the steps and results of the literature retrieval process. A comprehensive and systematic literature search was conducted to identify studies related to S. agalactiae-associated bovine mastitis. Three databases were used: PubMed (http://www.pubmed.gov), Google Scholar (https://scholar.google.com), and the China National Knowledge Infrastructure (CNKI: https://www.cnki.net/). The search strategy employed the Boolean operator “AND” to combine the MeSH terms “bovine mastitis” and “Group B Streptococcus,” targeting peer-reviewed articles published in English or Mandarin Chinese. The time frame of 2004 to 2024 was selected to ensure contemporary relevance and capture trends in mastitis prevalence and AMR over the past 2 decades.
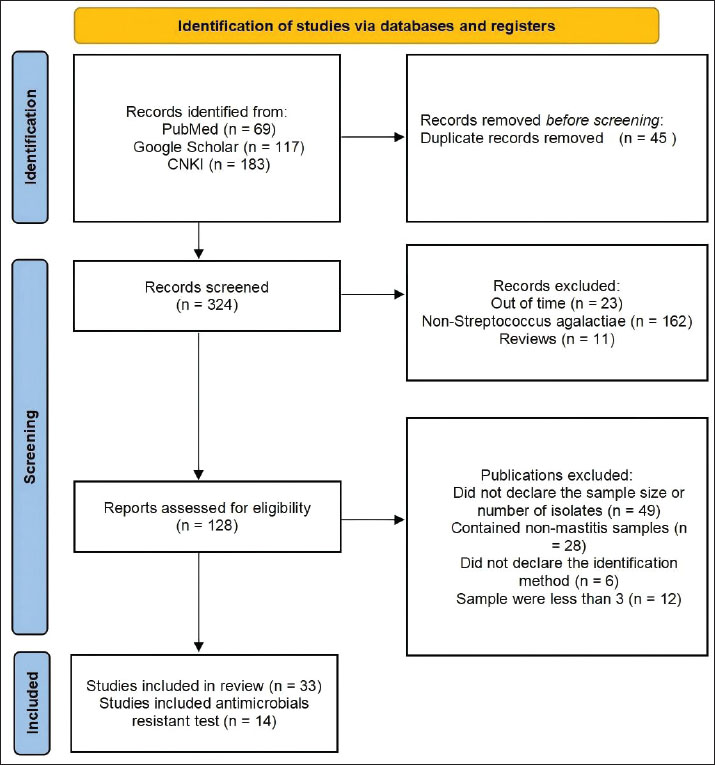
Fig. 1. Identification of studies using databases and registers. Inclusion and exclusion criteriaThe study followed the PRISMA guidelines, consistent with previous reports (Moher et al., 2015; Shamseer et al., 2015; Page et al., 2021). Two reviewers independently screened all titles and abstracts, with full-text assessment determining article eligibility. Reviewer agreement on study inclusion was strong (κ=0.86). Studies were excluded if they met any of the following criteria: (a) review articles, (b) duplicate publications, (c) irrelevant content or sample size <3, (d) absence of explicit bacterial identification methods, (e) samples involving non-mastitis conditions, (f) publication outside the target period (pre–2004 or post–2024), or (g) lack of stated sample size and number of bacterial isolates. Eligible studies were organized in Microsoft Excel (Table 1).
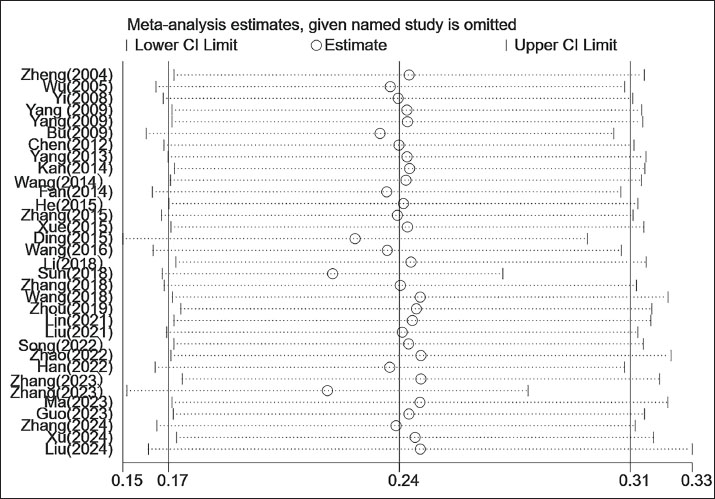
Table 1. Literature included in our study. Data extractionData were extracted using a standardized form capturing the following: author(s), year of publication, geographical location, sample size, number of S. agalactiae isolates, mastitis prevalence (as defined by clinical and subclinical criteria according to the National Mastitis Council’s Laboratory Handbook on Bovine Mastitis), bacterial identification methods, resistance values, and specific laboratory protocols used. Two reviewers independently performed the data extraction. Study quality was assessed using a modified Downs and Black checklist tailored to key quality indicators in observational research. Statistical analysisThe frequency and proportion of S. agalactiae isolates, antibiotic-resistant strains, and mastitis cases were determined using descriptive statistics. Resistance data were treated as binary outcomes consistent with definitions in the original publications. All analyses were performed using the STATA software (version 12.0; StataCorp, College Station, TX). The pooled prevalence estimates were calculated using a random-effects model. Forest plots illustrate pooled estimates with a 95% confidence interval (CI), which is an interval constructed using sample statistics in cases of repeated sampling. It has a 95% probability of containing the true population parameter value. Heterogeneity was assessed using Cochran’s Q test and the I2 statistic. Subgroup meta-analyses were conducted to evaluate potential sources of heterogeneity by isolation period, geographic region, mastitis type (clinical vs. subclinical), and identification method. For AMR analysis, eight major classes of antimicrobials commonly used in clinical veterinary practice were examined: β-lactams, quinolones, tetracyclines, chloramphenicol, lincosamides, sulfonamides, macrolides, and aminoglycosides. In addition, the resistance rates of clinical and subclinical cases isolated from S. agalactiae were calculated separately based on the total resistance rate calculation to provide a reference for subsequent treatment selection. All studies included in the analysis were re-evaluated for AMR data according to CLSI standards. Publication bias was assessed using Egger’s test and visualized via funnel plot analysis. ResultsInclusion of the publicationsAn initial search identified 369 articles from PubMed, Google Scholar, and CNKI. Based on the inclusion and exclusion criteria, 33 studies were ultimately included in the meta-analysis (Fig. 1). These studies were published between 2004 and 2024 and covered 11,780 milk samples from 7 regions in China, including 2,341 S. agalactiae isolates (Table 1). Prevalence of S. agalactiae infectionThe meta-analysis revealed a pooled prevalence of S. agalactiae at 24% (95% CI: 17%–31%), with significant heterogeneity among studies (I2=99.5%, p < 0.001). Sensitivity analyses demonstrated that the combined prevalence ranged from 17% to 31% following study-by-study exclusion, with no values falling outside the original confidence interval, thereby substantiating the robustness of the findings (Fig. S1, Sensitivity analysis). However, subgroup analysis was deemed necessary due to the presence of excessive heterogeneity. To investigate the sources of heterogeneity, subgroup analyses were conducted using the temporal period, geographic region, mastitis type, and identification method (Fig. 2).
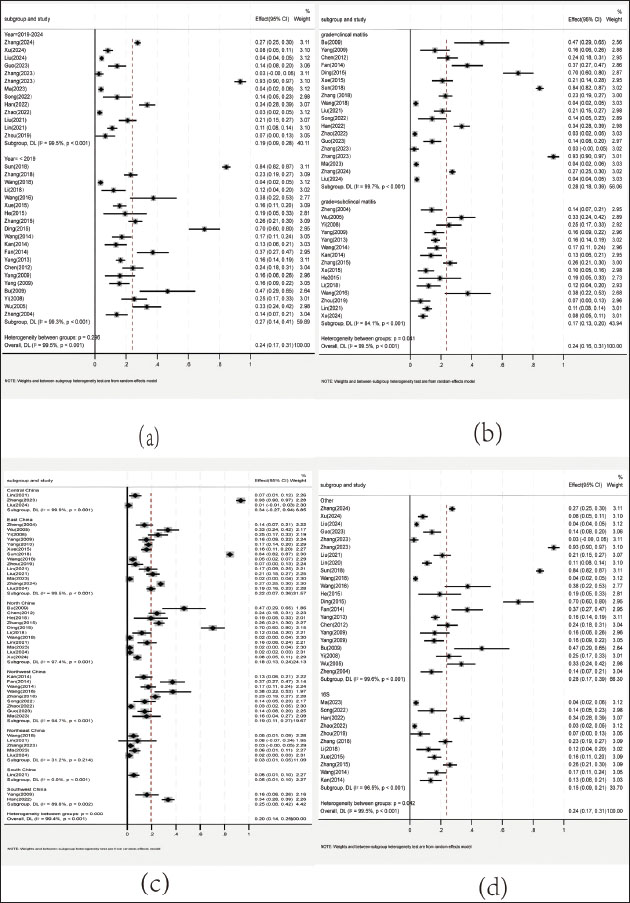
Fig. 2. S. agalactiae prevalence in 11,687 milk samples. Prevalence of S. agalactiae by region, time, mastitis type, and identification methodStudies were categorized into subgroups by collection period (pre-2019 vs. 2019–2024), region (East, West, North, South, Northwest, Northeast, Southwest, and Southeast China), and mastitis classification (clinical vs. subclinical). Data are shown in Figure 3. By mastitis type, the prevalence was 28% in clinical mastitis cases and 17% in subclinical mastitis cases. In terms of temporal trends, prevalence increased from 19% (pre-2019) to 27% (2019–2024). The highest pooled prevalence was observed in Central China (34%), whereas the lowest was in Northeast China (3%). The prevalence rate determined using other methods (28%) was higher than that determined using 16S rDNA (15%).
Fig. 3. a: Forest plot of S. agalactiae prevalence in the period of pre–2019 and 2019–2024. b: Forest plot of S. agalactiae prevalence of S. agalactiae isolated in clinical and subclinical mastitis cases. c: Forest plot of S. agalactiae prevalence in different regions in China. d: Forest plot of the prevalence of S. agalactiae in different identification methods. Antimicrobial resistance of S. agalactiaeThe pooled AMR analysis indicated that S. agalactiae exhibited the most resistance to sulfonamides (63%; 95% CI: 31%–96%), followed by aminoglycosides (55%; 95% CI: 40%–70%), β-lactams (55%; 95% CI: 43%–66%), lincosamides (41%; 95% CI: 20%–61%), macrolides (40%; 95% CI: 18%–63%), tetracyclines (39%; 95% CI: 23%–56%), quinolones (19%; 95% CI: 10%–27%), and chloramphenicol (19%; 95% CI: 9%–30%) (Fig. 4).
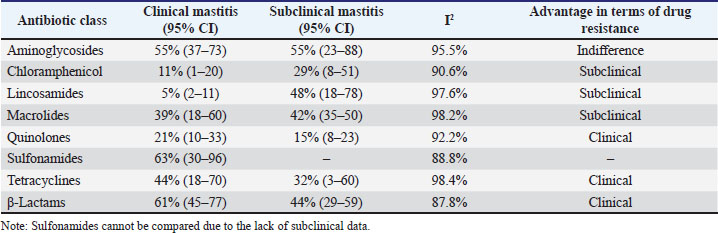
Fig. 4. Antimicrobial resistance of S. agalactiae. Comparison of AMR rates among clinical and subclinical typesA comparative analysis of antibiotic resistance rates in clinical and subclinical cases of S. agalactiae revealed no significant differences in aminoglycoside resistance. However, in subclinical cases, higher resistance rates were observed for chloramphenicol, lincosamides, and macrolides. Predominant resistance patterns for quinolones, β-lactams, and tetracyclines were observed in clinical cases. No comparison could be made for sulfonamides due to the lack of subclinical data (Table 2).
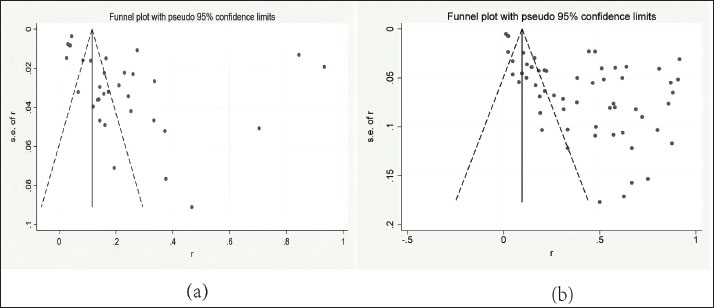
Table 2. Comparison of drug resistance rates among clinical and subclinical S. agalactiae types. Publication bias of S. agalactiae prevalence and AMR rateIn the Egger test, the P-values for prevalence and resistance rates were both less than 0.001, indicating statistically significant publication bias. Funnel plots for both prevalence and AMR analyses (Fig. 5) reveal asymmetric distributions of effect sizes, indicating potential publication bias. The degree of asymmetry is positively correlated with the extent of bias.
Fig. 5. a: Publication bias of S. agalactiae prevalence. b: Publication bias of S. agalactiae antimicrobial resistance rates. DiscussionBovine mastitis remains the most prevalent and economically harmful disease in the global dairy industry (Bradley, 2002; Ashraf and Imran, 2020). Controlling contagious mastitis, particularly caused by S. agalactiae, presents ongoing challenges in dairy-producing regions worldwide. Malinowski and Gajewski (2009) reported that this pathogen affects approximately 2%–25% of dairy cows annually. Accurate knowledge of the prevalence of S. agalactiae and its AMR patterns is essential for improving treatment protocols and developing targeted preventive strategies. This study identified a pooled prevalence of S. agalactiae in China at 24%, which exceeds that reported for several other countries, including Australia (16.9%) (Langhorne et al., 2024), Brazil (5.9%) (Tomazi et al., 2018), Ukraine (11%) (Elias et al., 2020), Northern Lebanon (15.1%) (Abboud et al., 2021), Northeastern Poland (15.6%) (Sztachańska et al., 2016), and Portugal (13.5%) (Rato et al., 2013). Although mastitis control programs in developed dairy-producing nations have historically reduced the incidence of S. agalactiae-associated mastitis, recent reports indicate a resurgence in some European countries, such as Denmark and Finland (Barkema et al., 2009). The enforcement of pasture management regulations, herd size expansion, and reduced cow-to-worker ratios are the contributing factors. Larger herds demand a corresponding increase in labor, and insufficient staffing may increase the risk of infection. Moreover, S. agalactiae can be transmitted from humans to cattle, introducing another route of infection (Zadoks and Schukken, 2006; Crestani et al., 2021). Based on evidence from German and U.S. studies, mastitis incidence was significantly positively correlated with antimicrobial dosage on the farm as a whole or in individual cows. The German study found a significant increase in antimicrobial dosage on farms with poor mammary health (high CMI) (Preine and Krömker, 2022), while USDA data showed that mammary disease, the most prominent indication for antimicrobial dosing (accounting for 22% of the dosing cases), saw a 25.7% increase in incidence along with a concomitant increase in antimicrobial dosage between 2002 and 2014 (García-Fernández, 2014). Thekey drivers of this phenomenon include non-selective dosing, such as the blanket dry cow therapy (DCT), also known as herd-wide dry lactation prophylaxis, that resulted in more than 50% of antimicrobials being used in uninfected cows (Scheer, 2013), and 35% of non-bacterial mastitis cases receiving unnecessary antimicrobials due to insufficient etiologic diagnosis (Lv et al., 2019), and flawed treatment protocols like unreasonably prolonged regimens and inappropriate drug selection problems. Reverse causality has also been verified, with a 63% decrease in total antimicrobials and a 25% decrease in mastitis incidence after the prohibition of prophylactic use in the Netherlands (Tennent, 2023), and a selective DCT trial showed that the use of drugs by infection status reduces dry stage dosage by 50% without increasing the risk of morbidity (Zhang et al., 2022). The prevalence of S. agalactiae increased from 19% (pre-2019) to 27% (2019–2024), reflecting a broader trend toward the increasing detection of mastitis pathogens in intensive farming systems (Barkema et al., 2009). This increase may be partly due to the adoption of molecular diagnostic methods, including PCR and MALDI-TOF MS, which offer better sensitivity than conventional culture-based techniques for detecting subclinical infections (Keefe, 2012). The extensive use of antibiotics for prophylaxis may disrupt commensal microbial communities and promote S. agalactiae colonization (Oliver and Murinda, 2012). Variability in management practices, such as hygiene protocols, housing systems, and feeding strategies, also complicates interregional comparisons of mammary health issues in dairy cows (Hill et al., 2009). A significant regional variation was observed within China. Central China exhibited the highest prevalence (34%), while Northeast China displayed the lowest (3%). These differences are associated with environmental and operational factors. For instance, the viability of S. agalactiae significantly decreases at temperatures below 10 °C, which may limit its survival in the colder northeast region (Liu et al., 2020). Central China is characterized by large-scale farming operations with high animal density, which may lead to the spread of mastitis-causing bacteria. Mastitis incidence rates are typically higher in intensive farming systems with elevated stocking densities and suboptimal hygiene management (Usman et al., 2015). The higher prevalence of clinical mastitis (28%) compared to subclinical mastitis (17%) is due to the fact that S. agalactiae induces inflammatory responses (Keefe, 2012) and that clinical mastitis remains more frequently reported than its subclinical form (Song et al., 2020). However, the considerable prevalence of subclinical infections highlights the capacity of the pathogen for silent persistence and herd-level transmission. Subclinical cases often escape routine detection and serve as silent reservoirs for herd-level outbreaks (Reyher et al., 2011). The results demonstrated that S. agalactiae associated with subclinical mastitis exhibited higher resistance rates to chloramphenicol, lincosamides, and macrolides than strains associated with clinical mastitis, indicating their critical role in the latent spread of drug-resistant bacteria (Table 2). These findings emphasize the importance of implementing routine surveillance strategies and advocate for the inclusion of PCR screening and individual SCC assessment in national surveillance systems, with recommendations for initiating appropriate interventions if two consecutive tests show SCC above the threshold and a positive PCR result. 16S rDNA sequencing has insufficient resolution for closely related Streptococcus sp. (Hassan et al., 2001), and its sensitivity is limited in samples with low bacterial loads, such as subclinical mastitis (Keefe, 2012), resulting in a significantly lower detection rate (15%) than that of targeted PCR (28%). This underdiagnosis intensifies hidden transmission within the herd, resulting in an annual economic loss of up to $300 per cow (Rollin et al., 2015). Brazilian research has confirmed that the detection rate of sodA-PCR is 40% higher than that of 16S rRNA (Tomazi et al., 2018), while the species-level identification accuracy of MALDI-TOF MS can reach 97% (Nonnemann et al., 2019). Therefore, Chinese dairy farms should promote “enrichment culture + targeted PCR” or MALDI-TOF MS as the gold standard for diagnosis (Zanardi et al., 2014). Widespread AMR has significantly contributed to increasing resistance rates, posing serious risks to animal and public health (Collis et al., 2024). The resistance profile of S. agalactiae identified in this study showed high resistance to sulfonamides (63%), aminoglycosides (55%), and β-lactams (55%), with lower resistance to chloramphenicol (19%) and quinolones (19%). These results are consistent with those of previous studies, which reported elevated resistance to β-lactams, macrolides, and lincosamides and lower resistance to quinolones and tetracyclines. Pronounced regional differences in AMR patterns have been reported globally. For instance, sulfonamide resistance in Chinese S. agalactiae isolates (71%) considerably exceeds the 12% reported in Brazil (Tomazi et al., 2018), possibly reflecting the impact of regulatory restrictions on antimicrobial use in veterinary medicine. Such interregional variation highlights the need for regionally tailored AMR surveillance systems, as uniform AMR guidelines may not be universally applicable across distinct epidemiological contexts. The decline in tetracycline resistance (39% vs. 31.4% in Brazil) reflects China’s 2021–2025 objectives and supports stricter regulation of growth promoters (Tomazi et al., 2018). Sulfonamides displayed significantly higher resistance rates than other antimicrobial classes, whereas quinolones and chloramphenicol exhibited the lowest rates. Resistance to aminoglycosides, β-lactams, macrolides, lincosamides, and tetracyclines was intermediate relative to sulfonamides, quinolones, and chloramphenicol. Sulfonamides—widely favored for their broad-spectrum efficacy and low cost—have been extensively employed in Chinese dairy operations since the 1990s, leading to selective pressure that has facilitated the emergence and persistence of AMR (Han et al., 2022). Despite subsequent bans on their use in food-producing animals, the continued presence of resistance indicates well-established AMR mechanisms. Aminoglycoside resistance is frequently mediated by aminoglycoside-modifying enzymes (Munita and Arias, 2016), whereas β-lactam resistance commonly arises through mutations in genes encoding penicillin-binding proteins in streptococci. The comparable resistance rates of these two classes emphasize the need for careful antimicrobial regulation, particularly to avoid empirical combination therapy that could potentiate multidrug resistance. Similarly, the resistance rates of macrolides and lincosamides were closely aligned, likely reflecting cross-resistance mechanisms conferred by erm genes, which mediate resistance to macrolides, lincosamides, and streptogramin B (MLSB) antibiotics (DiPersio et al., 2011). Tetracyclines, historically misused for growth promotion in livestock production, continue to be used in some regions despite bans in many countries. Their use is positively correlated with increased clinical resistance rates (Manyi-Loh et al., 2018). However, S. agalactiae remains intrinsically sensitive to quinolones, and prescribing restrictions in several regions may have slowed the development of resistance (Redgrave et al., 2014). Chloramphenicol resistance remained low, likely due to its limited veterinary application, due to concerns about myelotoxicity, which reduces selective pressure (World Health Organization, 2019). A comparative analysis of antibiotic resistance rates in clinical and subclinical cases of S. agalactiae infection provides a basis for future treatment options. In the case of subclinical instances, the use of lincosamides and chloramphenicol should be prioritized to prevent the development of resistance (Yang et al., 2024). For clinical cases, the employment of β-lactams should be prioritized to avoid, given that clinical cases frequently receive high-dose β-lactam shock therapy, a practice that accelerates the evolution of resistance (Han et al., 2022). The use of macrolides is contraindicated in cases of mixed infections, and the development of targeted treatment regimens should be informed by culture characteristics (Zhang, 2023). The rate of quinolone resistance of S. agalactiae in subclinical mastitis is only 15%, justifying its continued use as a therapeutic option (Zhao et al., 2022). In this meta-analysis, the funnel plot shows more data points on the right side than on the left. The funnel plot for prevalence exhibits right skewness, indicating a lack of small-sample studies. Regions with high prevalence rates may be more willing to participate in studies by providing larger sample sizes, leading to data clustering. Studies in China tend to report positive results (such as high prevalence rates), while studies with negative results may not be published (Mohammadi, 2025). The funnel plot for resistance analysis shows a left skew, indicating a lack of studies with low resistance rates. To reach statistical significance, small-sample studies require larger effect sizes, making them more likely to report higher resistance rates/prevalence rates (Friese and Frankenbach, 2020). This study has several limitations. Potential confounders, such as pasture size, milking frequency, and detailed farm management practices, were not controlled across the included studies. Crucially, our meta-analysis lacked integrated data on environmental reservoirs (e.g., bedding contamination levels, water sources, and milking equipment biofilm) and specific farm-level risk factors (e.g., udder hygiene scoring, bedding management frequency/type, parlor sanitation protocols, and stocking density) known to be primary drivers of S. agalactiae transmission and persistence. The unusually broad CI for sulfonamide resistance may reflect sample size limitations or pronounced geographical variation in resistance rates and prescribing practices. The findings’ generalizability may also be affected by regional heterogeneity in farming systems and AMR pressures. Future research should prioritize undertaking prospective studies that combine environmental sampling, management audits, and bacterial isolation. The application of multivariate models to quantify the impact of specific reservoirs, such as bedding and equipment, as well as hygiene management and dry cow treatment measures, on the dynamics of S. agalactiae is also recommended. The development of targeted control strategies will thereby be facilitated. Additionally, the integration of environmental genomics (farm temperature and humidity monitoring), host immunomics (mammary tissue single-cell RNA sequencing), and pathogen functional genomics (CRISPR screening of virulence genes) can facilitate the construction of an “environment-host-pathogen” interaction model, thereby enabling a comprehensive analysis of the epidemiological driving mechanisms. Furthermore, whole-genome sequencing (WGS) should be incorporated to define clonal lineages via MLST and CRISPR typing and correlate antibiotic resistance genotypes, such as blaZ and ermB, with phenotypic characteristics to enable precise intervention against high-risk strains. ConclusionThe pooled prevalence of S. agalactiae was 24% (95% CI: 17%–31%), with higher rates in Central China, a temporal increase in prevalence, higher rates in clinical cases, alarmingly high resistance to sulfonamides, and lower resistance to quinolones and chloramphenicol. Future research should directly address existing limitations: conduct farm-level studies to investigate key management and environmental drivers; strengthening and standardizing national/regional AMR surveillance (combined with WGS for molecular characterization); analyzing pathogen reservoirs through comprehensive environmental sampling; exploring the impact of diagnostic methods on prevalence estimates; and conducting cost-effectiveness analyses of improved diagnostics and targeted treatment/ selective dry cow therapy (SDCT) in China. AcknowledgmentNone. FundingThis work was supported by the Anhui Education Department Key Projects (2023AH051884), Grass Feeding Livestock Resource Utilization and Health Science and Technology Innovation Team (2023AH010061), Veterinary Science Peak Discipline Project of Anhui Science and Technology University (XK-XJGF002), and Anhui Provincial Quality Engineering Project for the New Era of Educating People (Postgraduate Education) (2024cxcysj188). Author’s contributionAll authors contributed to the conception and design of the study. JQ: Conceptualization, protocol development, literature search, data curation, data analysis, and original manuscript drafting; MS: Literature search, data curation, manuscript drafting, and review; XC: Literature search and data curation; manuscript drafting and review; YW: Methodology, manuscript drafting, and review; BY: Conceptualization, manuscript drafting, and review; YL: Manuscript drafting and review; MC: Manuscript drafting and review; WC: Manuscript drafting and review; XC: Supervision, formal analysis, software, funding acquisition, and manuscript review; YQ: Supervision and manuscript review. YL: Supervision and manuscript review. All authors have approved the final version of the manuscript. Conflicts of interestThe authors declare that they have no conflicts of interest. Data availabilityThe original contributions presented in the study are included in the article/supplementary material. Further inquiries should be directed to the corresponding author. ReferencesAbboud, Z., Galuppo, L., Tolone, M., Vitale, M., Puleio, R., Osman, M., Loria, G.R. and Hamze, M. 2021. Molecular characterization of antimicrobial resistance and virulence genes of bacterial pathogens from Bovine and caprine mastitis in Northern Lebanon. Microorganisms 9, 148; doi:10.3390/microorganisms9061148 Ashraf, A. and Imran, M. 2020. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 21, 36–49; doi:10.1017/S1466252319000094 Barkema, H.W., Green, M.J., Bradley, A.J. and Zadoks, R.N. 2009. Invited review: the role of contagious disease in udder health. J. Dairy Sci. 92, 4717–4729; doi:10.3168/jds.2009-2347 Bradley, A. 2002. Bovine mastitis: an evolving disease. Vet. J. 164, 116–128; doi:10.1053/tvjl.2002.0724 Bu, R., Liu, D., Hua, Y., Kang, L., Gao, C. and Lu, W. 2009. Isolation and characterization of Streptococcus agalactiae from dairy cows with mastitis in the Tongliao area (In Chinese). Heilongjiang Anim. Sci. Vet. Med. 3, 76–77; doi:10.13881/j.cnki.hljxmsy.2009.05.038 Chen, S., Yang, J. and Li, T. 2012. Isolation, identification, and drug susceptibility test of dairy clinical mastitis pathogenic bacteria (In Chinese). China Dairy Cattle, 23–27. Collis, R.M., Biggs, P.J., Burgess, S.A., Midwinter, A.C., Liu, J., Brightwell, G. and Cookson, A.L. 2024. Assessing antimicrobial resistance in pasture-based dairy farms: a 15-month surveillance study in New Zealand. Appl. Environ. Microbiol. 90, e0139024; doi:10.1128/aem.01390-24 Crestani, C., Forde, T.L., Lycett, S.J., Holmes, M.A., Fasth, C., Persson-Waller, K. and Zadoks, R.N. 2021. The fall and rise of group B Streptococcus in dairy cattle: reintroduction due to human-to-cattle host jumps?. Microb. Genom. 7, 7; doi:10.1099/mgen.0.000648 Ding, Y., Zhao, J., He, X., Li, M., Guan, H., Zhang, Z. and Li, P. 2016. Antimicrobial resistance and virulence-related genes of Streptococcus obtained from dairy cows with mastitis in Inner Mongolia, China. Pharm. Biol. 54, 162–167; doi:10.3109/13880209.2015.1025290 Dipersio, L.P., Dipersio, J.R., Beach, J.A., Loudon, A.M. and Fuchs, A.M. 2011. Identification and characterization of plasmid-borne erm(T) macrolide resistance in group B and group A Streptococcus. Diagn. Microbiol. Infect. Dis. 71, 217–223; doi:10.1016/j.diagmicrobio.2011.07.010 Elias, L., Balasubramanyam, A.S., Ayshpur, O.Y., Mushtuk, I.U., Sheremet, N.O., Gumeniuk, V.V., Musser, J.M.B. and Rogovskyy, A.S. 2020. Antimicrobial susceptibility of Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli isolated from mastitic dairy cattle in Ukraine. Antibiotics 9, 469; doi:10.3390/antibiotics9080469 Fan, J. 2014. The research on pathogens, antibiotic resistance, and the antigenic genes of Streptococcus of bovine mastitis (In Chinese). CVS Dissertation, Gansu: Gansu Agricultural University. Friese, M. and Frankenbach, J. 2020. P-Hacking and publication bias interact to distort meta-analytic effect size estimates. Psychol. Methods 25, 456–471; doi:10.1037/met0000246 García-Fernández, N. 2014. Mastitis treatment in dairy farms [Online]. Available via https://dellait.com/mastitis-treatment-in-dairy-farms/. Guo, C. 2023. Isolation and identification of bacterial pathogens and drug sensitivity test of dairy cow mastitis—Take Tianshui of Gansu as an example (In Chinese). China Dairy, 89–93. Han, G., Zhang, B., Luo, Z., Lu, B., Luo, Z., Zhang, J., Wang, Y., Luo, Y., Yang, Z., Shen, L., Yu, S., Cao, S. and Yao, X. 2022. Molecular typing and prevalence of antibiotic resistance and virulence genes in Streptococcus agalactiae isolated from Chinese dairy cows with clinical mastitis. PLoS One 17, e0268262. Hassan, A.A., Khan, I.U., Abdulmawjood, A. and Lämmler, C. 2001. Evaluation of PCR methods for rapid identification and differentiation of Streptococcus uberis and Streptococcus parauberis. J. Clin. Microbiol. 39, 1618–1621; doi:10.1128/jcm.39.4.1618-1621.2001 He, H. 2015. Isolation and identification of pathogenic bacteria of mastitis in dairy cows in the Qixian area (In Chinese). Livestock Poultry Ind. 6, 12–13; doi:10.19567/j.cnki.1008-0414.2015.06.010 Hill, A.E., Green, A.L., Wagner, B.A. and Dargatz, D.A. 2009. Relationship between herd size and annual prevalence of and primary antimicrobial treatments for common diseases on dairy operations in the United States. Prev. Vet. Med. 88, 264–277; doi:10.1016/j.prevetmed.2008.12.001 Ikiz, S., Başaran, B., Bingöl, E.B., Çetin, O., Kaşikçi, G., Özgür, N.Y., Uçmak, M., Yilmaz, O., Gündüz, M.C. and Sabuncu, A. 2013. Presence and antibiotic susceptibility patterns of contagious mastitis agents (Staphylococcus aureus and Streptococcus agalactiae) isolated from milks of dairy cows with subclinical mastitis. Turkish J. Vet. Anim. Sci. 37, 569–574. Javia, B.B., Purohit, J.H., Mathapati, B.S., Barad, D.B., Savsani, H.H., Ghodasara, S.N., Kalariya, V.A., Patel, U.D. and Nimavat, V.R. 2018. Molecular detection and antimicrobial resistance pattern of Staphylococci isolated from clinical and subclinical bovine mastitis. INDIAN J. Vet. Sci. Biotechnol. 14, 13–16. Kan, W. 2014. Isolation, characterization, and virulence gene analysis of Streptococcus and Enterococcus collected from bovine mastitis (In Chinese). CVS Dissertation, Gansu: Gansu Agricultural University. Keefe, G. 2012. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. North Am. Food Anim. Pract. 28, 203–216; doi:10.1016/j.cvfa.2012.03.010 Keefe, G.P. 1997. Streptococcus agalactiae mastitis: a review. Can. Vet. J. 38, 429–437. Krömker, V. and Leimbach, S. 2017. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domestic Animals 52(Suppl 3), 21–29; doi:10.1111/rda.13032 Kuppusamy, S., Kakarla, D., Venkateswarlu, K., Megharaj, M., Yoon, Y.E. and Lee, Y.B. 2018. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: a critical view. Agric. Ecosystems Environ. 257, 47–59. Langhorne, C., Horsman, S., Wood, C., Clark, R., Price, R., Henning, J., Grewar, J.D., Wood, B.J., Ranjbar, S., Mcgowan, M.R. and Gibson, J.S. 2024. Bacterial culture and susceptibility test results for clinical mastitis samples from Australia’s subtropical dairy region. J. Dairy Sci. 107, 1151–1163; doi:10.3168/jds.2023-23838 Li, G. 2018. Isolation and identification of the main pathogenic bacteria of mastitis in dairy cows in the Ulaanchabu region and a study on drug resistance (In Chinese). Heilongjiang Anim. Sci. Vet. Med. 11, 122–124; doi:10.13881/j.cnki.hljxmsy.2016.11.0383 Lin, L., Huang, X., Yang, H., He, Y., He, X., Huang, J., Li, S., Wang, X., Tang, S., Liu, G. and Pan, Z. 2021. Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J. Dairy Sci. 104, 4893–4903; doi:10.3168/jds.2020-19139 Liu, K., Gu, X., Zhang, L. and Qu, W. 2021. Drug and virulence gene test of Streptococcus agalactiae in the cow breast (In Chinese). Chin. J. Vet. Med. 41, 999–1003; doi:10.16303/j.cnki.1005-4545.2021.05.27 Liu, K., Liu, X., Yang, J., Gu, X., Zhang, L. and Qu, W. 2024. Streptococcus agalactiae isolated from clinical mastitis cases on large dairy farms in north China: phenotype, genotype of antimicrobial resistance and virulence genes. Front. Cell. Infect Microbiol. 14, 1417299; doi:10.3389/fcimb.2024.1417299 Liu, K., Sun, W., Zhang, L., Gu, X., Zhang, M., Huo, W., Zhou, M. and Qu, W. 2020. Geographical distribution and temporal changes of pathogenic bacteria causing mastitis in dairy cows in China (In Chinese). Chin. J. Vet. Med. 56, 22–26. Lv, T., Li, S. and Hao, Y. 2019. Application of a new on-farm differential chromogenic medium for fast identification of pathogens associated with mastitis in milk of dairy cows (In Chinese). Acta Vet. Zootech. Sin. 50, 851–860. Ma, X. 2023. Study on drug resistance and whole genome sequencing analysis of bovine Streptococcus agalactiae (In Chinese). MSc Thesis, Xinjiang: Xinjiang Agricultural University. Malinowski, E. and Gajewski, Z. 2009. Characteristics of cows mastitis caused by human foodborne pathogens [Online]. Available via: https://www.cabidigitallibrary.org Manyi-Loh, C., Mamphweli, S., Meyer, E. and Okoh, A. 2018. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23, 795. Mohammadi, M. 2025. Publication bias in prevalence studies should not be ignored. Syst. Rev. 14, 85; doi:10.1186/s13643-025-02845-9 Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P. and Stewart, L.A. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1; doi:10.1186/2046-4053-4-1 Munita, J.M. and Arias, C.A. 2016. Mechanisms of antibiotic resistance. Microbiol. Spectr. 4, 4; doi:10.1128/microbiolspec.VMBF-0016-2015 Nonnemann, B., Lyhs, U., Svennesen, L., Kristensen, K.A., Klaas, I.C. and Pedersen, K. 2019. Bovine mastitis bacteria resolved by MALDI-TOF mass spectrometry. J. Dairy Sci. 102, 2515–2524; doi:10.3168/jds.2018-15424 Oliver, S.P. and Murinda, S.E. 2012. Antimicrobial resistance of mastitis pathogens. Vet. Clin. North Am. Food Anim. Pract. 28, 165–185; doi:10.1016/j.cvfa.2012.03.005 Page, M.J., Mckenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., Mcdonald, S., Mcguinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P. and Moher, D. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189; doi:10.1016/j.jclinepi.2021.03.001 Preine, F. and Krömker, V. 2022. Associations between udder health, udder health management and antimicrobial consumption: insights into the mechanisms influencing antibiotic usage in German dairy farms. Milk. Sci. Int. Milchwissenschaft. 75, 75. Radtke, A., Bruheim, T., Afset, J.E. and Bergh, K. 2012. Multiple-locus variant-repeat assay (MLVA) is a useful tool for molecular epidemiologic analysis of Streptococcus agalactiae strains causing bovine mastitis. Vet. Microbiol. 157, 398–404; doi:10.1016/j.vetmic.2011.12.034 Rato, M.G., Bexiga, R., Florindo, C., Cavaco, L.M., Vilela, C.L. and Santos-Sanches, I. 2013. Antimicrobial resistance and molecular epidemiology of streptococci from bovine mastitis. Vet. Microbiol. 161, 286–294; doi:10.1016/j.vetmic.2012.07.043 Redgrave, L.S., Sutton, S.B., Webber, M.A. and Piddock, L.J. 2014. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 22, 438–445. Reyher, K.K., Dufour, S., Barkema, H.W., Des Côteaux, L., Devries, T.J., Dohoo, I.R., Keefe, G.P., Roy, J.P. and Scholl, D.T. 2011. The National Cohort of Dairy Farms--a data collection platform for mastitis research in Canada. J. Dairy Sci. 94, 1616–1626. Richards, V.P., Lang, P., Pavinski Bitar, P.D., Lefébure, T., Schukken, Y.H., Zadoks, R.N. and Stanhope, M.J. 2011. Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae. Infection. Genet. Evol. 11, 1263–1275; doi:10.1016/j.meegid.2011.04.019 Rollin, E., Dhuyvetter, K.C. and Overton, M.W. 2015. The cost of clinical mastitis in the first 30 days of lactation: an economic modeling tool. Prev. Vet. Med. 122, 257–264; doi:10.1016/j.prevetmed.2015.11.006 Scheer, A. 2013. Het effect van selectief droogzetten op het ontstaan van mastitis. MSc Thesis, Utrecht: Utrecht University. Shaheen, M. and Ha, T. 2015. A treatise on bovine mastitis: disease and disease economics, etiological basis, risk factors, impact on human health, therapeutic management, prevention and control strategy. Adv. Dairy Res. 4, 1–10. Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P. and Stewart, L.A. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349, g7647; doi:10.1136/bmj.g7647 Skarbye, A.P., Krogh, M.A., Denwood, M., Bjerring, M. and Østergaard, S. 2021. Effect of enhanced hygiene on transmission of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in dairy herds with automatic milking systems. J. Dairy Sci. 104, 7195–7209; doi:10.3168/jds.2020-19635 Song, Q., Da, J., Li, Y., Shi, J., Li, Z., Zhao, X. and Zhang, Y. 2022. Isolation, identification, and drug resistance analysis of main pathogens of dairy cows clinical mastitis in the Wuzhong Area of Ningxia (In Chinese). Prog. Vet. Med. 43, 70–75; doi:10.16437/j.cnki.1007-5038.2022.02.009 Song, X., Huang, X., Xu, H., Zhang, C., Chen, S., Liu, F., Guan, S., Zhang, S., Zhu, K. and Wu, C. 2020. The prevalence of pathogens causing bovine mastitis and their associated risk factors in 15 large dairy farms in China: an observational study. Vet. Microbiol. 247, 108757; doi:10.1016/j.vetmic.2020.108757 Sun, J., Sun, X., An, P. and Liu, G. 2018. Study on pathogenic bacteria gene diagnosis of cow mastitis in the Shanghai Area (In Chinese). China Dairy Cattle 2, 28–31; doi:10.19305/j.cnki.11-3009/s.2018.02.008 Sztachańska, M., Barański, W., Janowski, T., Pogorzelska, J. and Zduńczyk, S. 2016. Prevalence and etiological agents of subclinical mastitis at the end of lactation in nine dairy herds in North-East Poland. Pol. J. Vet. Sci. 19, 119–124; doi:10.1515/pjvs-2016-0015 Tennent, S. 2023. Targeted antibiotic use in dairy cows offers wide range of benefits [Online]. Available via https://www.farmersweekly.co.nz/technology/targeted-antibiotic-use-in-dairy-cows-offer-wide-range-of-benefits/. Tomazi, T., Ferreira, G.C., Orsi, A.M., Gonçalves, J.L., Ospina, P.A., Nydam, D.V., Moroni, P. and Dos Santos, M.V. 2018. Association of herd-level risk factors and incidence rate of clinical mastitis in 20 Brazilian dairy herds. Prev. Vet. Med. 161, 9–18; doi:10.1016/j.prevetmed.2018.10.007 Tonin, F.S., Wiecek, E., Torres-Robles, A., Pontarolo, R., Benrimoj, S.I., Fernandez-Llimos, F. and Garcia-Cardenas, V. 2019. An innovative and comprehensive technique to evaluate different measures of medication adherence: the network meta-analysis. Res. Soc. Administ. Pharm. 15, 358–365; doi:10.1016/j.sapharm.2018.05.010 Usman, T., Wang, Y., Liu, C., Wang, X., Zhang, Y. and Yu, Y. 2015. Association study of single nucleotide polymorphisms inJAK2andSTAT5Bgenes and their differential mRNA expression with mastitis susceptibility in Chinese Holstein cattle. Anim. Genet. 46, 371–380; doi:10.1111/age.12306 Wang, K. and Li, L. 2016. Isolation and identification of pathogenic bacteria of mastitis in dairy cows (In Chinese). Livestock Poultry Ind. 8, 14–15; doi:10.19567/j.cnki.1008-0414.2016.08.011 Wang, S., Chen, H., Zhao, Y., Wang, F., Cai, J., Cao, S., Cai, Y. and Xu, M. 2018. Study on isolation and antibiotic resistance of Streptococcus agalactiae from bovine mastitis in some regions of China (In Chinese). China Dairy Cattle 11, 36–39. doi:10.19305/j.cnki.11-3009/s.2018.11.009 Wang, S., Yang, X., Li, J., Ran, D. and Wang, Z. 2014. Isolation and Identification of Streptococcus agalactiae from Bovine Mastitis in Xinjiang (In Chinese). Xinjiang Agric. Sci. 51, 1328–1334. World Health Organization, W. H. 2019. Critically important antimicrobials for human medicine. Geneva: World Health Organization. Wu, Y. and Xu, Y. 2005. Isolation and identification of the main causative organisms of cryptogenic mastitis in dairy cows from a farm in Jinhua and drug susceptibility test (In Chinese). Heilongjiang Anim. Sci. Vet. Med. 42, 42–43; doi:10.13881/j.cnki.hljxmsy.2005.09.032 Xu, J., Cai, Y., Ma, Y., Fu, Y., Yuan, L. and Xu, M. 2024. Isolation, identification, and drug resistance of Streptococcus agalactiae from dairy cattle with mastitis in Urumqi and its surrounding areas (In Chinese). Farm. feed 23, 53–56; doi:10.13300/j.cnki.cn42-1648/s.2024.08.013 Xue, M. and Zhu, J. 2015. Isolation and identification of pathogenic bacteria in cows with mastitis in Weifang and the drug resistance test (In Chinese). Chin. J. Anim. Quar. 32, 19–23. Yang, R., Li, Y., Li, J., Li, X., Deng, B. and Dang, X. 2009a. Isolation and identification of clinical mastitis pathogens from Ya’an, Sichuan Province, China, and analysis of drug resistance (In Chinese). Chin. J. Vet. Med. 45, 41–42. Yang, Y., Cheng, G., Zhao, R., Chen, S. and Zhao, H. 2009b. Isolation, identification, and drug sensitivity test of the primary pathogens of dairy cow mastitis in Anhui Province (In Chinese). Prog. Vet. Med. 30, 113–115; doi:10.16437/j.cnki.1007-5038.2009.06.022 Yang, Y., Liu, Y., Ding, Y., Yi, L., Ma, Z., Fan, H. and Lu, C. 2013. Molecular Characterization of Streptococcus agalactiae Isolated from Bovine Mastitis in Eastern China. PLoS One 8, e67755; doi:10.1371/journal.pone.0067755 Yang, Y., Xie, S., He, F., Xu, Y., Wang, Z., Ihsan, A. and Wang, X. 2024. Recent development and fighting strategies for lincosamide antibiotic resistance (In Chinese). Clin. Microbiol. Rev. 37, 16123; doi:10.1128/cmr.00161-23 Yi, M. 2008. Research on main pathogenic bacteria and genic treatment of dairy cow mastitis in the shanghai area (In Chinese). PVM Thesis, Shanghai: Shanghai Jiao Tong University. Yin, J., Wang, Y., Xu, X., Liu, Y., Yao, L. and Sun, Q. 2021. The progress of global antimicrobial resistance governance and its implication to china: a review. Antibiotics 10, 356; doi:10.3390/antibiotics10111356 Zadoks, R.N. and Schukken, Y.H. 2006. Use of molecular epidemiology in veterinary practice. Vet. Clin. North Am. Food Anim. Pract. 22, 229–261; doi:10.1016/j.cvfa.2005.11.005 Zanardi, G., Caminiti, A., Delle Donne, G., Moroni, P., Santi, A., Galletti, G., Tamba, M., Bolzoni, G. and Bertocchi, L. 2014. Short communication: comparing real-time PCR and bacteriological cultures for Streptococcus agalactiae and Staphylococcus aureus in bulk-tank milk samples. J. Dairy Sci. 97, 5592–5598; doi:10.3168/jds.2014-7947 Zhang, J., Gao, M., Wang, W., Han, X., Wu, J. and Ye, G. 2024. Genetic diagnosis and drug sensitivity analysis of mastitis-causing bacteria in dairy cows in the Yangtze River Delta region (In Chinese). China Dairy Cattle 1, 34–40; doi:10.19305/j.cnki.11-3009/s.2024.01.007 Zhang, L. 2018. Drug susceptibility and molecular characterization of the main pathogens of mastitis in dairy cows (In Chinese). FSH Thesis, Lanzhou: Lanzhou University. Zhang, T., Zhu, X., Zhang, C. and Lu, C. 2022. Research on using internal teat sealant combining different drugs for dry-off prevention of mastitis in dairy cows (In Chinese). China Dairy Cattle 22, 24–28. Zhang, X., Li, Z., Liu, H., Li, N., Yan, G. and Lei, L. 2023a. Isolation, identification, and drug resistance analysis of mastitis pathogens in a dairy farm in Changchun (In Chinese). Chin. J. Vet. Med. 59, 52–58. Zhang, Y. 2015. Study on the main isolated and identified pathogenic bacteria of dairy cow mastitis and research the resistance in different areas in Hebei Province (In Chinese). Vet Thesis, Hebei: Hebei Agricultural University. Zhang, Y. 2023. Epidemiological analysis of drug resistance, serotype, and virulence genes in S. agalactiae (In Chinese). Vet Thesis, Anhui: Anhui Medical University. Zhang, Y., Hu, Y., Li, B., Li, H., Wang, Z., Duan, L., Li, S. and Shi, H. 2023b. Analysis of drug resistance and virulence genes of Streptococcus agalactiae from Henan Cattle (In Chinese). Chin. J. Anim. Infect. Dis. 31, 71–78; doi:10.19958/j.cnki.cn31-2031/s.20210817.005 Zhao, Y., Shao, W., Wang, F., Ma, J., Chen, H., Wang, S., Wu, Y., Wang, C., Zheng, N., Wang, J. and Liu, H. 2022. Antimicrobial resistance and virulence genes of Streptococcus agalactiae isolated from mastitis milk samples in China. J. Vet. Res. 66, 581–590; doi:10.2478/jvetres-2022-0069 Zheng, H. 2004. Epidemiological investigation of subclinical mastitis in Nanjing, study of pathogens and detection of multiplex PCR (In Chinese). Vet Thesis, Nanjing: Nanjing Agricultural University. Zhou, M., Zuo, X., Xue, Y., Bao, X., Zhang, J., Yang, Z., Zhu, G. and Lu, Y. 2019. Isolation, antibiotic resistance, and conservative antigen analysis of cow mastitis-related pathogens from one dairy farm in Jiangsu province (In Chinese). J. Yangzhou Univ. Agric. Life Sci. Ed. 40, 54–60; doi:10.16872/j.cnki.1671-4652.2019.06.009 Supplementary
Fig. S1. Sensitivity analysis. | ||
| How to Cite this Article |
| Pubmed Style Qiu J, Si M, Chong X, Wang Y, Yang B, Chu M, Liang Y, Cheng W, Qi Y, Chen X, Liang Y. Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Vet. J.. 2025; 15(9): 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 Web Style Qiu J, Si M, Chong X, Wang Y, Yang B, Chu M, Liang Y, Cheng W, Qi Y, Chen X, Liang Y. Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. https://www.openveterinaryjournal.com/?mno=263713 [Access: January 11, 2026]. doi:10.5455/OVJ.2025.v15.i9.10 AMA (American Medical Association) Style Qiu J, Si M, Chong X, Wang Y, Yang B, Chu M, Liang Y, Cheng W, Qi Y, Chen X, Liang Y. Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Vet. J.. 2025; 15(9): 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 Vancouver/ICMJE Style Qiu J, Si M, Chong X, Wang Y, Yang B, Chu M, Liang Y, Cheng W, Qi Y, Chen X, Liang Y. Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Vet. J.. (2025), [cited January 11, 2026]; 15(9): 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 Harvard Style Qiu, J., Si, . M., Chong, . X., Wang, . Y., Yang, . B., Chu, . M., Liang, . Y., Cheng, . W., Qi, . Y., Chen, . X. & Liang, . Y. (2025) Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Vet. J., 15 (9), 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 Turabian Style Qiu, Junxue, Mengke Si, Xiaoyu Chong, Yiwei Wang, Baolei Yang, Mingfeng Chu, Yuchen Liang, Wei Cheng, Yanping Qi, Xuelong Chen, and Yan Liang. 2025. Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Veterinary Journal, 15 (9), 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 Chicago Style Qiu, Junxue, Mengke Si, Xiaoyu Chong, Yiwei Wang, Baolei Yang, Mingfeng Chu, Yuchen Liang, Wei Cheng, Yanping Qi, Xuelong Chen, and Yan Liang. "Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis." Open Veterinary Journal 15 (2025), 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 MLA (The Modern Language Association) Style Qiu, Junxue, Mengke Si, Xiaoyu Chong, Yiwei Wang, Baolei Yang, Mingfeng Chu, Yuchen Liang, Wei Cheng, Yanping Qi, Xuelong Chen, and Yan Liang. "Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis." Open Veterinary Journal 15.9 (2025), 4060-4074. Print. doi:10.5455/OVJ.2025.v15.i9.10 APA (American Psychological Association) Style Qiu, J., Si, . M., Chong, . X., Wang, . Y., Yang, . B., Chu, . M., Liang, . Y., Cheng, . W., Qi, . Y., Chen, . X. & Liang, . Y. (2025) Prevalence and antimicrobial resistance of Streptococcus agalactiae associated with bovine mastitis in China: A meta-analysis. Open Veterinary Journal, 15 (9), 4060-4074. doi:10.5455/OVJ.2025.v15.i9.10 |