| Research Article | ||
Open Vet. J.. 2025; 15(9): 4044-4059 Open Veterinary Journal, (2025), Vol. 15(9): 4044-4059 Research Article Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1Mohammad Sukmanadi1*, Sri Agus Sudjarwo1, Mustofa Helmi Effendi2, Aswin Rafif Khairullah3, Pudji Srianto4, Sri Pantja Madyawati4, Mirni Lamid5, Hani Plumeriastuti6, Imam Mustofa4, Adeyinka Oye Akintunde7, Bantari Wisynu Kusuma Wardhani8, Riza Zainuddin Ahmad3, Irma Melati9 and Bima Putra Pratama101Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor Indonesia 4Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 7Department of Agriculture and Industrial Technology, Babcock University, Ikenne, Nigeria 8Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 9Research Center for Limnology and Water Resources, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia *Corresponding Author: Mohammad Sukmanadi. Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: moh-s [at] fkh.unair.ac.id Submitted: 11/06/2025 Revised: 10/08/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: The metabolism of toxins after ingestion by animals involves transformation into various metabolites with different levels of toxicity. Aflatoxin B1 undergoes biotransformation into various compounds in the mitochondria that are closely related to its toxic effects. Capsicum annuum L. or chili is a plant that produces a spicy taste and sharp aroma produced by capsaicinoid compounds. Capsaicin in vitro has been shown to have effects on various cell types, including prostate cells, cells that undergo proliferation in the stomach, and hepatocytes, without causing significant side effects on normal cells. Aim: This study aimed to determine whether capsaicin inhibits the expression of alpha serine/threonine-protein kinase 1(AKT1) and mitogen-activated protein kinase 1 (MAPK1) target proteins in vivo by scoring using immunohistochemistry (IHC) and histopathological hepatic staining hematoxylin-eosin (HE). Methods: IHC was performed by counting the number of transformed cells using monoclonal antibodies and liver pathogenicity was assessed by scoring hepatic lesions (congestion, degeneration, and necrosis) using HE staining preparations. Results: Capsaicin treatment significantly reduced liver damage and aflatoxin B1-induced protein expression. Histopathological scores for degeneration, congestion, and necrosis were significantly lower in the aflatoxin B1 (AFB1) + capsaicin group (P3: 15.42 ± 0.65, 15.50 ± 0.50, 15.50 ± 0.58) than in the AFB1-only group (P2: 21.50 ± 0.57 for all variables; p < 0.05). Immunohistochemical analysis showed that capsaicin co-treatment decreased AKT1 (P3: 16.33 ± 0.69 vs. P2: 19.75 ± 0.56) and MAPK1 expression (P3: 13.83 ± 0.61 vs. P2: 20.00 ± 0.34), with a statistically significant reduction in MAPK1 expression (p < 0.05). Conclusion: Capsaicin demonstrated a protective effect by reducing liver damage and downregulating AKT1 and MAPK1 expression in mice with aflatoxin B1-induced hepatotoxicity. Keywords: Capsaicin, Aflatoxin B1, AKT1, MAPK1, Good health and well being. IntroductionToxins undergo metabolic processes that transform them into multiple metabolites with varying toxicity levels after ingestion by animals. While some of these metabolites are eliminated through excretory pathways, others may persist and accumulate in the body. Among the metabolites of aflatoxin B1 (AFB1), the most toxic is 8,9-epoxide-AFB1, which can form covalent bonds with deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) (Thongararm et al., 2025). Within mitochondria, AFB1 is metabolized into different toxic compounds (Chu et al., 2024). This biotransformation includes hydroxylation and oxidation, resulting in the formation of the highly reactive 8,9-epoxide form of AFB1. The hydroxylated products typically conjugate with glucuronic acid and sulfate groups to produce water-soluble metabolites that are excreted via urine (Eaton et al., 2025). Moreover, the reactive epoxide form can bind covalently to DNA at the N7 position of guanine or form conjugates with reduced glutathione and may also be hydrolyzed into AFB1-dihydrodiol (Johnson and Guengerich, 1997). Aflatoxicosis can result in acute, subacute, or chronic effects, primarily targeting the liver, which is the main organ affected by AFB1 toxicity (Hamali et al., 2021). Biological responses to AFB1 exposure include alterations in protein and RNA gene expression. Biomarkers, such as specific genes, proteins, and RNA molecules, can serve as indicators of AFB1-induced damage (Wang et al., 2022; Al-Ayoubi et al., 2024; Wu et al., 2024). Computational analyses using predictive interaction networks have been employed to identify potential gene and protein targets for further exploration, especially in the context of AFB1-induced hepatic injury. Several proteins have been implicated in liver tumor initiation, including glycogen synthase kinase 3 beta (Guo et al., 2024), the mammalian target of rapamycin (mTOR) (Bhat et al., 2013), epidermal growth factor receptor (Berasain et al., 2011), alpha serine/threonine-protein kinase 1 (AKT1) (Ho et al., 2012), mitogen-activated protein kinase 1 (MAPK1) (Chen et al., 2019), mitogen-activated protein kinase 3 (MAPK3) (Sun et al., 2022), and RAF proto-oncogene serine/threonine-protein kinase (Ghousein et al., 2020). To clarify the role of AFB1 in modulating these proteins, further investigations are necessary to assess their binding affinities and activation energies when interacting with AFB1 in model organisms such as mice (Mus musculus). Previous studies have emphasized the crucial involvement of protein kinase families in hepatocarcinogenesis by mediating signal transduction from growth factors and regulating cell cycle progression, survival, migration, and proliferation (Black and Black, 2013; He et al., 2021). Proteins identified as probable targets of AFB1 are primarily members of the protein kinase group. Capsicum annuum L. or chili is a plant that produces a spicy taste and sharp aroma produced by the capsaicinoid compound. Some capsaicinoid compounds include nordihydrocapsaicin, capsaicin, dihydrocapsaicin, norcapsaicin, homodihydrocapsaicin, homocapsaicin, and nonivamide (Sganzerla et al., 2014). Capsaicin is a major secondary metabolite in chili plants and has various pharmaceutical applications. Capsaicin is used as an analgesic to relieve pain and inhibits the expression of certain genes and proteins (Fattori et al., 2016). Capsaicin has been shown to have effects on various cell types, including prostate cells, cells undergoing gastric proliferation, and hepatocytes, without causing significant side effects on normal cells (Maharjan et al., 2024). Capsaicin inhibits the proliferation of various cell lines via an apoptotic mechanism involving the production of reactive oxygen species (ROS), activation of the Jun N-terminal kinase pathway, decreased mitochondrial depolarization, and release of cytochrome c, thereby activating caspase-3 (Yu et al., 2024). Capsaicin-mediated ROS production activates the MAPK protein kinase pathway, particularly MAPK3, which is dependent on mitogen stimulation and plays a role in apoptosis morphology and function. Capsaicin modulates the phosphorylation of a series of proteins in the MAPK pathway. This effect is important because the MAPK pathway plays a major role in regulating cell survival and growth (Moriguchi et al., 2019). Capsaicin significantly decreased ERK 1/2 phosphorylation. The interaction between ligands, such as AFB1, and target proteins, which can be small molecules or macromolecules, such as AKT1 and MAPK1, can be studied using the ligand-protein approach (Antonius et al., 2022). Several in vivo studies have demonstrated the protective effects of capsaicin against liver damage. For example, Ghorbanpour et al. (2023) reported that capsaicin reduced carbon tetrachloride-induced liver injury in rats by modulating oxidative stress and inflammatory responses. Capsaicin also influences key signaling pathways, including PI3K/AKT, MAPK/ERK, and NF-κB, which are involved in regulating cell proliferation, apoptosis, and inflammation (Lin et al., 2018; Sailo et al., 2025). These findings support the rationale for evaluating the effect of capsaicin on AKT1 and MAPK1 expression as potential molecular targets in AFB1-induced hepatotoxicity. This in vivo study aimed to evaluate the effects of the bioactive components of C. annuum L. on target proteins MAPK1 and AKT1 using immunohistochemistry (IHC) and histopathological examination with Hematoxylin-Eosin (HE) staining to determine the interaction between the receptor-ligand complex (antigen-antibody). Materials and MethodsLocation and time of researchThis study was conducted from December 2019 to February 2020. This study was conducted in several different laboratories. Making animal models and keeping them in experimental animal cages, Biochemistry Laboratory, Faculty of Medicine, Airlangga University, Indonesia. Preparation of preparations and immunohistochemistry examinations were performed at the Pathology Laboratory, Faculty of Veterinary Medicine, Airlangga University, Indonesia. Sample preparation was performed in silico at the Veterinary Pharmacy Laboratory, Faculty of Veterinary Medicine, Airlangga University. Research designThe experimental unit used in the study was the liver cells of male Balb/c strain mice (Mus musculus), physically selected as healthy, aged 60–75 days, and weighing 30–35 g. Samples were obtained from the experimental animal unit of the Biochemistry Laboratory, Faculty of Medicine, Airlangga University, Surabaya, Indonesia. 24 experimental mice were adapted for 10 days before treatment, and their weight was measured for the first time, from day 1 to day 10. During the adaptation period, daily weight was measured. Temperature was measured, and an evaluation was performed. The treatments carried out in the study were divided into 4 treatment groups as follows: P0: The control was given PEG solvent. P1: Capsaicin (0.3 mg/0.5 ml) orally administered once a day for 10 days. P2: AFB1 (0.1 mg/0.5 ml) orally administered once a day for 10 days. P3: AFB1 (0.1 mg/0.5 ml) given once a day, orally, after 3 hours. Capsaicin (0.3 mg/0.5 ml) was given once a day, orally, for 10 days. Meanwhile, for AFB1 treatment in experimental animals for target protein expression, capsaicin treatment as an anti-Aflatoxin B1, observation of liver organ harvesting, and organ observation were carried out on the 11th day. Furthermore, sample preparation, IHC and HE staining of histopathology preparations were performed. IHC proceduresOn day 11, the mice were euthanized through cervical dislocation and subsequently buried. Tissue samples were immediately fixed in buffered formalin, dehydrated, and embedded in paraffin using standard histological procedures. A glass slide coated with poly-L-lysine was prepared, and the paraffin-embedded tissue was sectioned using a microtome at a thickness of 5 μm. The slices were floated in a warm water bath and mounted onto the prepared slides. Once attached, the tissue sections were deparaffinized and prepared for immunohistochemical analysis. Tissue processing included multiple steps: dehydration, clearing, impregnation, and embedding. Immunohistochemical staining followed the biotin-streptavidin method. Deparaffinization was performed by twice immersing the liver tissue slides in xylene for 2 minutes. This was followed by sequential immersions in absolute ethanol (twice for 1 minute each), 95% ethanol (twice for 1 minute each), 80% ethanol (once for 1 minute), and 70% ethanol (once for 1 minute). The slides were then rinsed under running water for 10–15 minutes before being treated with 3% hydrogen peroxide (H2O2) for 30 minutes to block endogenous peroxidase activity. The slides were washed three times in phosphate-buffered saline (PBS) for 2 minutes each time. The samples were then reimmersed in 70% ethanol for 1 minute and rinsed again with running water for 10–15 minutes. A second treatment with 3% H2O2 for 30 minutes was followed by three additional PBS washes (2 minutes each). Proteolytic digestion was performed by incubating the sections in 0.025% trypsin at 37°C for 6 minutes and then rinsing them in PBS three times for 2 minutes each time. Subsequently, the tissue was incubated with primary antibodies against AKT1 and MAPK1 (mouse anti-rat, dilution 1:50) for 30 minutes, followed by PBS washes (3 times for 2 minutes each time. For signal detection, the sections were treated with a biotinylated secondary antibody (rabbit anti-mouse) for 30 minutes and washed again with PBS (3 times, 2 minutes each). A 30-minute incubation with streptavidin-HRP and additional PBS washes (3 times, 2 minutes each) followed this step. The chromogenic substrate was applied for 5 minutes, after which the slides were washed with PBS (3 times, 2 minutes each) and rinsed in distilled water. Counterstaining was performed using Mayer’s Hematoxylin for 6 minutes, followed by a water rinse, and the mounting process was performed. This histopathological procedure assessed the expression of AKT1 and ERK in mouse hepatocytes. The expression levels were quantified using a modified Remmele scoring method (Novak et al., 2007). The immuno reactive score (IRS) calculation involved multiplying the score for the percentage of positively stained cells by the staining intensity score (Table 1). The IRS value for each sample represents the mean of observations taken from five high-power fields (400x magnification). Table 1. The semi-quantitative IRS scale is the result of multiplying the percentage score of positive cells (A) by the color reaction intensity score (B), so IRS=(A × B).
Histopathology preparation with Hematoxylin eosin stainingThe mouse liver was cut to a thickness of ±3 mm, inserted into a tissue cassette, and then inserted into an automatic tissue processor for dehydration, clearing, and infiltration. The dehydration process involves successively soaking the organs in 70%, 80%, 90%, and 96% ethanol, absolute ethanol I, absolute ethanol II, and absolute ethanol III, respectively. Furthermore, the clearing process involves soaking the organs in xylene I and xylene II solutions, and the infiltration process involves soaking the organs in paraffin I and paraffin II at 58°C for 2 hours. The organs were inserted into molds containing liquid paraffin using a paraffin embedding console and allowed to harden to form paraffin blocks. The organs in the paraffin were then cut using a microtome with a thickness of 3 µm. The thin ribbon cut results are placed on water in a water bath at a temperature of 45°C to remove folds. Furthermore, the preparation was lifted from the surface of the water with an object glass that had been reviewed with albumin solution and then dried overnight in an incubator at a temperature of 60°C. Next, the preparation underwent a deparaffinization and rehydration process by soaking it in xylene twice for 2 minutes, then rehydrating it using graded ethanol (absolute III, absolute II, absolute I, 96%, 80%) for 2 minutes each, then washing it with running water for 1 minute and drying it. The preparation was soaked in Mayer’s hematoxylin dye for 8 minutes, rinsed with running water, washed with lithium carbonate for 15–30 seconds, and rinsed again with running water. Then, the preparation was dipped in eosin dye for 2 minutes, rinsed with water for 30–60 seconds, dipped in 90% ethanol 10 times, absolute ethanol I 10 times, absolute ethanol II for 2 min, xylene I for 1 minute, and xylene II for 2 minutes. Next, the preparation was dried, dripped with entella, and covered with a cover glass. The histopathology of the liver examined was liver cell damage (hepatocytes) due to toxins (aflatoxin B1), characterized by hepatic injury resulting in changes in function and structure that were seen in microscopic observations with HE staining using 400x magnification, namely degeneration, congestion, and necrosis. Microscopic hepatic injury data were used to obtain congestion, degeneration, and necrosis scores using the modified Brunt system method by Brunt in Merat et al. (2010). Degeneration: Liver damage by toxic or immunological disorders causes hepatocytes to swell; congestion: Capillary dilation due to vasodilator stimulation, so that the vascularization of the injured location widens and contains blocked blood; necrosis: Tissue death, characterized by the presence of a dead core, is an advanced level of degeneration (Table 2). Table 2. The Brunt method hepatic injury score.
Data analysisImmunohistochemical data for AKT1 and MAPK1 (ERK) expression were assessed using the modified Remmele scoring system (Novak et al., 2007). The Remmele Index, also known as the IRS, is calculated by multiplying the score representing the percentage of immunoreactive cells by the staining intensity score. The IRS value for each sample was determined as the average of scores observed across five microscopic fields at 400x magnification. In addition to protein expression analysis, changes in liver histopathology—including signs of congestion, cellular degeneration, and necrosis—were also evaluated. The quantified expression levels of AKT1 and MAPK1, along with histopathological observations, served as key indicators in this assessment. The resulting data were analyzed using the IBM SPSS Statistics software version 21.0. If the data showed a normal distribution, parametric statistical tests were applied. Non-parametric tests were used in cases where the data remained non-normally distributed even after transformation. Specifically, the Kruskal-Wallis test was used to assess differences among groups. If the analysis yielded a significance level of p ≤ 0.05, a post hoc Z test followed. Alternatively, when comparisons involved only two groups, the Mann–Whitney U test was used, and detailed results from this test were provided in the appendix. A p-value below 0.05 indicated a statistically significant difference, whereas values above this threshold were considered insignificant. Ethical approvalThe Research Ethics Committee of the Faculty of Veterinary Medicine, Airlangga University, Animal Care and Use Committee approved this research (approval number: 1.KE.199.12.2019). ResultsExamination of the protective effect of capsaicin on the liver of mice using immunohistochemistryThe immunohistochemical analysis aimed to evaluate the expression levels of AKT1 (Fig. 1) and MAPK1/ERK (Fig. 2) in hepatocytes of Mus musculus (mice). Protein expression was assessed using the modified Remmele method (Novak et al., 2007), which calculates the IRS by multiplying the staining intensity by the percentage of immunoreactive cells. Averaging observations from five high-power fields (400× magnification) determined the IRS for each sample.
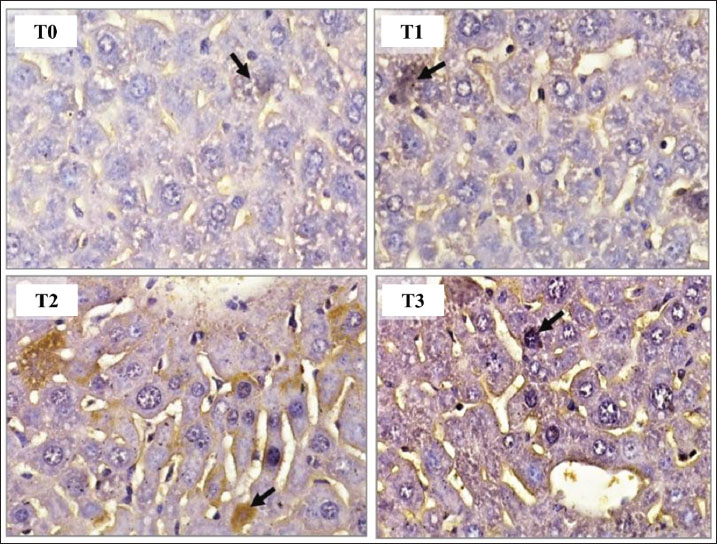
Fig. 1. AKT1 expression in hepatocytes of mice (Mus musculus) after capsaicin administration (0.3 mg/0.5 ml) and AFB1 induction (0.1 mg/0.5 ml). Immunohistochemistry staining with 400X magnification; Nikon H600L microscope; DS Fi2 300 megapixel camera.
Fig. 2. MAPK1 expression in hepatocytes of mice (Mus musculus) after capsaicin administration (0.3 mg/0.5 ml) and AFB1 induction (0.1 mg/0.5 ml). Immunohistochemistry staining with 400X magnification; Nikon H600L microscope; DS Fi2 300 megapixel camera. This study investigated how AFB1 exposure, with or without capsaicin co-treatment, affected AKT1 and MAPK1 protein expression. The mean IRS scores under each treatment condition are presented in Figure 3. Statistical analysis was conducted using the Kruskal-Wallis nonparametric test to compare differences among multiple groups, followed by post hoc Z-tests to determine pairwise differences. A p-value of 0.05 was considered statistically significant.
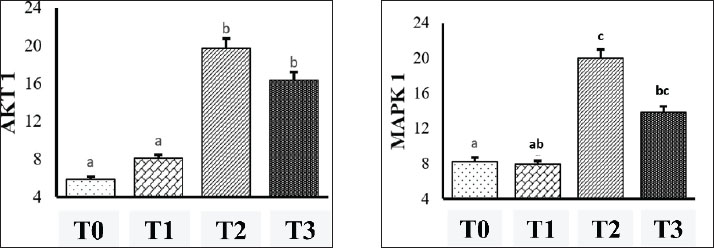
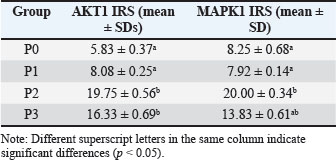
Fig. 3. AKT1 expression in hepatocytes of mice (Mus musculus) after capsaicin administration (0.3 mg/0.5 ml) and AFB1 induction (0.1 mg/0.5 ml). Immunohistochemistry staining with 400X magnification; Nikon H600L microscope; DS Fi2 300 megapixel camera. The mean IRS scores for AKT1 expression were as follows: P0 (5.83 ± 0.37), P1 (8.08 ± 0.25), P2 (19.75 ± 0.56), and P3 (16.33 ± 0.69). Statistical analysis using the Kruskal-Wallis test followed by post hoc Z-tests revealed significant differences: P2 versus P0 (p < 0.001), P2 versus P1 (p < 0.001), and P3 versus P1 (p < 0.01). Although the difference between P2 and P3 was not statistically significant (p=0.067), a significant reduction in AKT1 expression was observed in P3 (Table 3). The P1 group also exhibited significantly lower AKT1 expression than the P2 and P3 groups. However, AKT1 expression was not significantly different between P2 and P3, indicating that capsaicin was not statistically effective in suppressing AFB1-induced AKT1 expression. Nevertheless, a descriptive reduction in AKT1 levels was observed in the P3 group compared with the P2 group. Table 3. Mean IRS scores of AKT1 and MAPK1 expression (mean ± SD).
AKT1 scores were represented as mean ranks ± standard deviations and compared across groups using letter notation, where distinct letters indicate significant differences (p < 0.05) between the control and AFB1 groups. Immunostaining confirmed the localization of AKT1 in both the cytoplasmic and nuclear compartments of hepatocytes. Quantitatively, AKT1 expression was highest in P2 (19.75 ± 0.56) and P3 (16.33 ± 0.69), whereas much lower values were observed in the control (5.83 ± 0.37) and P1 (8.08 ± 0.25) groups. Similarly, MAPK1 expression followed a similar trend. The control (P0) and capsaicin-only (P1) groups did not differ significantly, but both had significantly lower MAPK1 levels than the AFB1-treated (P2) and combination (P3) groups. The P1 group also differed significantly from P2 but not from P3. Furthermore, P2 and P3 did not differ significantly, indicating that capsaicin partially attenuated MAPK1 upregulation. Histologically, MAPK1 was detected in both the cytoplasmic and nuclear regions. MAPK1 expression in P2 (20.00 ± 0.34) was markedly higher compared to P1 (7.92 ± 0.14), P0 (8.25 ± 0.68), and P3 (13.83 ± 0.61). However, no significant differences were observed among the P0, P1, and P3 groups. This indicates that while capsaicin may not statistically suppress AKT1 expression induced by AFB1, it can reduce MAPK1 expression both statistically and descriptively. Examination of capsaicin’s protective effect on mouse liver histopathologyHistopathological examination of the liver was performed to determine the pathogenesis of changes in the structure of hepatocyte cells in mice (Mus muscullus) (Fig. 4). The modified Brunt method was used to obtain data on degenerative, congestion, and necrosis scores of liver cells (Merat et al., 2010). The scale index is the result of grade 1 (mild), grade 2 (moderate), and grade 3 (severe). Data for each sample are the average values observed in 5 fields of view at 400x magnification. The difference test was performed using the Kruskal-Wallis nonparametric statistic, if p ≤ 0.05 was obtained, the post hoc test (Z test) was continued.
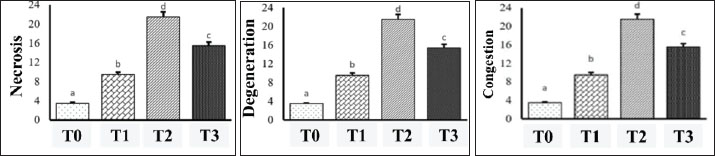
Fig. 4. Histopathology of mouse liver after capsaicin administration and AFB1 induction, hematoxylin–eosin staining; 400x magnification; scale 50 µm; Olympus® CX 41 Trinocular Microscope. Description: Green arrow: normal hepatocyte cells; Purple arrow: degeneration; Yellow arrow: necrosis; Blue arrow: congestion. a) Central vein; b) Sinusoid; c) Intravenous intima thickening. Research data on the effect of AFB1 induction with the protective effect of capsaicin on hepatic injury, degenerative conditions, congestion, and necrosis are shown in Figure 5.
Fig. 5. Mean ± rank error diagram of observations of necrosis, degeneration, and congestion of hepatocytes in mice (Mus musculus) with statistically significant differences between groups. Observation of hepatic injury values are expressed as ranked mean ± standard mean. Values are statistically represented as a, b, c, when compared with group P0; p < 0.05 compared with group P2 AFB1. Observation of hepatic injury values are expressed as ranked mean ± standard mean. Values are statistically represented a, b, c, when compared with group P0; p < 0.05 compared with group P2 (AFB1). In this study, congestive hepatic injury was assessed in mean rank. The values are statistically represented a, b, and c when compared between groups with p < 0.05. In the variables of necrosis, degeneration, and congestion, group P3 (CAP + AFB1=15.50; 15.42; 15.50) had a significant decrease in score compared with group P2 (AFB1=21.50; 21.50; 21.50) and was significantly different from group P3 (CAP + AFB1) and P1 (CAP) and P0 (control). This shows a significant decrease in number compared to groups P0 (control), P1 (CAP), and P3 (CAP + AFB1) (Fig. 5). The target of the reading is to change the histopathological picture of hepatocytes, namely, degeneration, congestion, and necrosis. The data results are processed with SPSS 21.0 assuming normal data distribution or non-homogeneous data variance, and then the Kruskal-Wallis nonparametric statistical test is carried out, which is identical to the one-way ANOVA test (if in the parametric test). After being transformed, if the data distribution is still not normal or not the same (assumption), then a different test is carried out using the Kruskal-Wallis nonparametric statistic, if p ≤ 0.05 is obtained, the post hoc test (Z test) is continued, while if using the Mann–Whitney test, the researcher presents it in the appendix. If p < 0.05, then there is a significant difference, and vice versa. DiscussionExamination of the protective effect of capsaicin on AKT1 and MAPK1 expression in mouse livers using the IHC methodThis study investigated the hepatoprotective potential of capsaicin against AFB1 toxicity, focusing on its effect on the expression of AKT1 and MAPK1 target proteins using IHC analysis. Aflatoxin, a mycotoxin widely found in food and feed, poses a significant food safety concern because of its high toxicity and widespread occurrence. It contributes substantially to the incidence of aflatoxicosis (Benkerroum, 2020). These toxicological concerns underscore the need for curative or preventive strategies and regulations regarding its presence in consumables. Although aflatoxins are primarily known for their carcinogenic potential, AFB1 has also been implicated in various acute and chronic health disorders. AFB1 undergoes biotransformation to AFB1-8,9-epoxide via cytochrome P450 enzymes upon entry into the liver (De-Sá-Júnior et al., 2013). This epoxide metabolite is considered the primary agent of AFB1’s genotoxicity (Marchese et al., 2018). Furthermore, AFB1-induced oxidative stress plays a critical role in its toxicological mechanisms (Benkerroum, 2020). Immunoreactive score evaluation revealed AKT1 and MAPK1 expression levels in each treatment group. Statistical analysis using the Kruskal-Wallis test indicated that capsaicin did not significantly reduce AKT1 expression in the AFB1-induced groups. However, descriptive analysis using the Mann–Whitney test showed a decrease in both AKT1 and MAPK1 expression following capsaicin treatment in AFB1-induced samples. Specifically, capsaicin treatment significantly inhibited MAPK1 expression, as MAPK1 levels in the AFB1+capsaicin group were comparable to those in the capsaicin-only group. Meanwhile, AKT1 expression showed a downward trend, though not statistically significant, indicating a possible inhibitory effect of capsaicin requiring dose optimization. These observations support the concept of a dose-response relationship, as discussed by Sukmanadi et al. (2020), where the toxicological impact is proportional to the level of exposure to mycotoxins. Thus, capsaicin and AFB1 concentrations may influence the observed protein expression patterns. Capsaicin binds to 14 amino acid residues of AKT1, with 4 forming hydrogen bonds, whereas AFB1 interacts with 13 residues without hydrogen bonding. For MAPK1, capsaicin forms a hydrogen bond with 1 of the 15 involved residues, whereas AFB1 interacts with 7 residues, each forming a single hydrogen bond. According to Sukmanadi and Effendi (2021a), capsaicin and AFB1 overlapping binding residues indicate competition at the same binding sites, potentially leading to mutual inhibition effects. Capsaicin (8-methyl-N-vanillyl-6-nonenamide), a bioactive compound found in chili peppers, inhibits the metabolic activation of carcinogens, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, by microsomal enzymes and block α-hydroxylation (Anandakumar et al., 2012). These effects indicate that capsaicin may possess antimutagenic and anticarcinogenic properties by modulating xenobiotic-metabolizing enzymes (Singh et al., 1996; Fattori et al., 2016). AFB1 is predicted to form stable molecular complexes with AKT1 and MAPK1, triggering hepatotoxicity. Capsaicin may competitively inhibit the binding of AFB1 to these proteins, particularly AKT1, thereby preventing the activation of oncogenic signaling pathways (Marchese et al., 2018). MAPK1, also a member of the kinase family involved in liver cell function, was similarly affected, supporting the dual target hypothesis (Sukmanadi et al., 2020). Further analysis confirmed that AKT1 and MAPK1 are likely key capsaicin targets in inhibiting AFB1-induced hepatotoxicity. Amino acid residue similarity in capsaicin’s interactions with both proteins indicates overlapping or proximal binding sites, which may lead to similar receptor modulation (Sukmanadi et al., 2020). Additionally, potential activity scoring indicated high hepatoprotective potential for capsaicin, correlating with its ability to inhibit AKT1 and MAPK1/ERK signaling pathways. This inhibition may lead to decreased cell proliferation and enhanced apoptosis. The biological activity of capsaicin includes the modulation of gene expression related to cell proliferation, signal transduction, apoptosis, and autophagy (Zhang et al., 2017; Hacioglu, 2022). It induces G1 cell cycle arrest, autophagy, and caspase-3-mediated apoptosis through PI3K/Beclin-1/Bcl-2 and PI3K/Akt/mTOR signaling pathways (Lin et al., 2017). Capsaicin also downregulates phosphorylated ERK1/2 and p38 MAPK (Park et al., 2014) and alters cell signaling mechanisms to inhibit cancer development both in vitro and in vivo (Chow et al., 2007; Wang et al., 2016). Although we did not directly assess the phosphorylation status of AKT1 and MAPK1, capsaicin can suppress these pathways via inhibition of upstream kinases or TRPV1-mediated signaling (Zhang et al., 2007; Sánchez-Hernández et al., 2024). Therefore, we propose that the observed effects of capsaicin in our study may be mediated through similar mechanisms. Taken together, these findings indicate that capsaicin in C. annuum L. synergistically inhibits AKT- and MAPK1-mediated signaling pathways, promotes apoptotic responses, and has strong potential as a hepatoprotective agent against AFB1 toxicity. Examination of the protective effect of capsaicin on liver histopathology with HE staining in miceObservation of liver tissue revealed varying degrees of severity, with the highest damage scores corresponding to AFB1 induction. This indicates a direct correlation between AFB1 administration and hepatic damage extent. In contrast, capsaicin administration during the study did not cause significant hepatotoxicity, indicating its safety for liver tissue. The scoring system used to assess the severity of liver damage based on histopathological changes was adapted from Khan et al. (2024). Hepatic injuries can be classified through various approaches—some based on histopathological features, such as inflammation, necrosis, and cholestasis, and others based on the type of injury (cytotoxic, cholestatic, or mixed). Recent advances have refined this classification by including molecular-level insights (Kleiner, 2018). Reactive metabolites may form covalent bonds with cellular proteins or nucleic acids in cases of overdose. These metabolites bind to essential enzymes, leading to cellular dysfunction or death, especially under acute toxicity conditions (Gu and Manautou, 2012). Histopathological analysis showed that oral administration of AFB1 in mice resulted in hepatocyte structural damage characterized by degeneration and necrosis. The observed hepatic injury indicators included degeneration, congestion, and necrosis, which are considered sensitive markers of liver cell damage only after cellular injury has occurred. Degeneration features swollen hepatocytes and granular, inhomogeneous cytoplasm resulting from fluid accumulation within cells (Sui et al., 2019). The entry of fluid caused organelle swelling, making the cytoplasm appear granular. In addition, cytoplasmic vacuolization was observed, where large fat-containing vacuoles displaced the nucleus toward the cell periphery (Balla, 2013). Evidence of congestion, marked by venous lumen enlargement (as seen in group P2), further supported the AFB1-induced pathological progression. In contrast, mild hepatocyte degeneration in the control and capsaicin groups was considered a part of normal regenerative processes (Sukmanadi et al., 2021b). The observed degeneration was categorized as reversible; however, if left unregulated, it can progress to irreversible necrosis. Disruptions in cellular volume regulation, mainly involving sodium homeostasis regulated by the plasma membrane, sodium pumps, and adenosine triphosphate (ATP) production, cause swelling. Imperfect membrane barriers allow passive sodium influx, which must be actively expelled to maintain homeostasis (Gyimesi et al., 2020; Zamay et al., 2025). Necrosis, a continuation of degeneration or congestion, is exacerbated by deoxyhemoglobin accumulation and prolonged hypoxia, leading to cell death (Proskuryakov et al., 2002; Jin et al., 2024). The sodium-potassium pump (Na+/K+-ATPase), which is crucial for ion regulation, can be impaired by toxins, increasing membrane permeability, reducing ATP synthesis, or damaging the pump directly (Foulkes, 2000; Suhail, 2010; Delpire and Gagnon, 2018). Fatty degeneration, marked by triglyceride accumulation in hepatocytes, may be triggered by various factors, including toxins, protein malnutrition, diabetes, obesity, or anoxia (Kneeman et al., 2012). AFB1 exposure disrupts mitochondrial respiratory enzymes, resulting in decreased ATP production and hepatocyte death (Niknahad et al., 1995; Hua et al., 2021). Inflammatory infiltration, including neutrophils, lymphocytes, and plasma cells, was observed near the hepatic veins, indicating acute hepatitis. Inflammatory cells are normally present in lymphoid follicles of the liver portal area as part of its immune defense (Allameh et al., 2023). Capsaicin, the major pungent component of chili peppers, has antioxidant, anti-inflammatory, and analgesic effects (Basith et al., 2016; Maharjan et al., 2024). Gut microbiota may modulate the biological effects of capsaicin and aflatoxin B1. For instance, Zhang et al. (2024) demonstrated that specific microbial communities can influence capsaicin metabolism and its anti-inflammatory effects. Therefore, individual differences in gut microbiota composition may partially explain the variability in hepatotoxic or hepatoprotective responses. Although microbiota analysis was not conducted in the present study, this represents an important avenue for future research. Statistical analysis confirmed a significant difference in hepatocyte structural damage levels between treatment groups. AFB1 alone caused the most severe damage, whereas capsaicin co-treatment attenuated these effects. Severe hepatocellular damage in the AFB1-only group included extensive mitochondrial swelling, nuclear membrane lysis, cytoplasmic vacuolization, and rough endoplasmic reticulum disruption. AFB1 enters the body via contaminated food and is transported to the liver through the hepatic portal vein system (Ali et al., 2021; Chen et al., 2023), reaching the liver sinusoids and directly exposing hepatocytes to its toxic effects. Hepatotoxicity, as evidenced by higher damage scores, is associated with oxidative stress. Chili extracts have been shown to exert hepatoprotective effects by reducing liver weight and improving histological liver conditions in hepatotoxic models (Li et al., 2024). Chili contains n-hexane and piperine, both of which are known for their antioxidant properties, which prevent oxidant formation and repair oxidative damage (Batiha et al., 2020). As a primary detoxification organ, the liver contains microsomal and S9 enzymatic systems capable of metabolizing capsaicin (Chanda et al., 2008). This study further demonstrated that AFB1 inhibits key respiratory enzymes, including malate dehydrogenase and 2-oxoglutarate dehydrogenase, both of which are critical in the citric acid cycle. AFB1 also disrupts ATPase activity, reducing ATP production, compromising sodium pump function, and causing sodium accumulation in the cytoplasm (Kettritz and Loffing, 2023). Acute aflatoxicosis involves the formation of adducts between aflatoxins and macromolecules, such as proteins, lipids, and nucleic acids, disrupting their physiological functions (Benkerroum, 2020). Aflatoxins interfere with protein synthesis, DNA repair, and immune responses, whereas ROS-induced lipid peroxidation damages the membrane, mitochondria, and ER (Hua et al., 2021). This results in increased cytoplasmic osmolarity, cellular swelling, and eventual hepatocyte rupture—hallmarks of necrosis (Fathima et al., 2023). Aflatoxins also induce oxidative stress by triggering ROS formation through the mitochondrial redox cycle and NADPH oxidase activation via the PI3K/AKT pathway (Schieber and Chandel, 2014; Rascio et al., 2021). ROS-induced lipid oxidase peroxidation plays a critical role in the pathogenesis of hepatocellular carcinoma (Redza-Dutordoir and Averill-Bates, 2016). ROS-mediated DNA damage and decreased antioxidant enzyme activity, such as SOD, are commonly found in adjacent liver tissues (Juan et al., 2021; Jomova et al., 2023). AFB1 induces both cellular and subcellular structural damage in hepatocytes, with significant variation in damage severity among treatment groups. Capsaicin demonstrated a protective effect, potentially through AKT1 and MAPK1 expression modulation, supported by both in vivo and in vitro findings. Histological evaluations using hematoxylin and immunohistochemistry staining revealed notable differences in degeneration, congestion, and necrosis patterns among capsaicin, AFB1, or both treatment groups. Protective effect of capsaicin on in vivo AKT1 and MAPK1 expression in mouse liverSingle dose of capsaicin, drug solvent, scoring limitations, capsaicin dose used in the dosage regimen for mice in this experiment was determined based on previous research, namely 10 mg/kg body weight, dissolved in Polyethylene glycol (PEG) only one dose, the lack of variation in this dose makes the total number of samples used in this study less meaningful. PEG is a drug solvent used as an additional ingredient in formulations to increase drug dissolution. If the concentration is too high, it will initially act as a stabilizer and then change into a competitor (Maher and Brayden, 2021). The preparation procedure is less than ideal, resulting in less accurate scoring, further disrupting the average number of samples, often experiencing defects and damage to the preparation, causing errors in diagnosis. The differences in AKT1 and MAPK1 expression and hepatic injury that occurred in each sample were not significant enough to represent the differences in each group. Based on the statistical test, it can be concluded that the expression of AKT1 and MAPK1 and hepatic injury were not significantly different between the control group and the group given capsaicin induction, given aflatoxin B1 exposure, or with the group given both exposures. A larger number of samples and variations in capsaicin doses are needed to obtain significant differences in each treatment group. The dosage regimen for mice in this experiment was determined based on previous studies, namely 10 mg/kg body weight, dissolved in PEG by considering the treatment regimen, namely the composition that indicates the type and amount of drug given and the frequency of drug use in a study (Anandakumar et al., 2012). In previous in vitro studies, capsaicin treatment reduced the dose (Ahmed et al., 2017). Capsaicin is a novel epithelial growth factor receptor pathway modulator and a potent growth suppressor of both positive and negative estrogen receptors (Luján-Méndez et al., 2023). Capsaicin is also considered an antiandrogenic receptor drug (Zhao et al., 2025). Capsaicin was found to have different effects on cell growth, depending on the expression status of NADH oxidase (Chang et al., 2020). The efficacy of capsaicin as a chemopreventive agent has been proven through its ability to modulate the antioxidant defense system and mitochondrial enzyme system, in experiments induced by benzo(a)pyrene, the effect of capsaicin on lipid peroxidation, membrane-bound enzymes, and glycoprotein profiles (Anandakumar et al., 2012). Polyethylene glycol is a carrier material that is often used as an additional ingredient in formulations to increase the solubility of poorly soluble drugs (Sun et al., 2023). This material is a type of polymer that forms a polymer complex on organic molecules when added to the formulation to increase the speed of dissolution, which can form a complex with various drugs. Polyethylene glycol, also called macrogol, is a synthetic oxyethylene polymer. This polymer is easily soluble in various solvents, has a low melting point and toxicity, is semicrystalline, and is soluble in water, methanol, benzene, and dichloromethane (Ibrahim et al., 2022). The polyethylene glycol base is inert, not easily hydrolyzed, and does not promote fungi growth (Pirillo et al., 2021). Compound Structure: Polyethylene glycol is an acyclic polyether containing alcohol groups (OH) at both ends. Although the OH group is not a stable atom, it can form coordinate bonds with metal ions and produce stable complex compounds (Charlton et al., 2023). The OH group has a dual function, like water molecules, because it can stabilize by interacting with each other, namely, first with cations in a coordinated manner. Second, with anions through hydrogen bonds so that they are nucleophilic, the presence of this reaction prevents anions from interacting too strongly with metal ions, so that PEG is called an MFL (Lipfert et al., 2014). The optimum concentration of PEG is necessary. Determining the optimum concentration of PEG solvent functions to form a stable complex. The resulting complex is easily ionized if the PEG concentration is low. Conversely, if the PEG concentration is excessive, PEG, which was initially stabilizing, will turn into a competitor. Tissue observation at the tissue processing stage can form artifacts during the production process, including: Surgical biopsy procedures, fixation, tissue processing, embedding, microtomy, mounting, staining, and cover-slipping. Tissue observation under a microscope does not always yield normal histological or histopathological results. Long tissue processing from the start of organ removal to the glueing or mounting stage often experiences defects and damage to the preparation, which can cause errors in histopathological diagnosis. The new findings in this study are the effects of capsaicin as a hepatoprotection in preventing the occurrence of hepatic injury through the examination of the target proteins AKT1 and MAPK1 expressed in hepatocyte cells by computational examination with bioinformatics in silico and proven in vivo through IHC examination and microscopic examination of mouse liver histopathology using HE staining. This is based on the fact that key signaling molecules, target proteins, and certain genes, such as MAPK, AP-1, NF-kB, and p53, play important roles in chronic inflammation, deregulated cell proliferation, and cell survival during the development of hepatotoxicity (Zhang et al., 2021). Activation of receptor tyrosine kinases by ligands (CAP and AFB1) activates downstream signaling pathways with effects on angiogenesis, proliferation, migration, invasion, apoptosis, and cell survival (Zhang and Li, 2023). Conventional blocking of c-Met signaling by c-Met tyrosine kinase inhibitors or by PI3K/AKT and MAPK agonists can lead to resistance or side effects. The binding of c-Met ligands can trigger signaling pathways, including conventional PI3K/AKT and MAPK (Hu et al., 2017). The efficacy of capsaicin as a chemopreventive agent has been proven through its ability to modulate the antioxidant defense system and mitochondrial enzyme system. In experiments induced by benzo(a)pyrene, the effect of capsaicin on lipid peroxidation, membrane-bound enzymes, and glycoprotein profiles has been demonstrated. Chili compounds have been reported as hepatoprotectors. The ethanol extract of chili reduces liver mass and volume and improves the histopathological picture of the liver of hepatotoxic rat models. Chili contains n-hexane extract, which has been shown to have potent antioxidants, and chili also contains piperine, which has been shown to reduce liver damage scores (Anandakumar et al., 2009). Drug discovery research using herbal medicine on c-Met-dependent signaling, related to AKT1 and MAPK1, is increasingly being conducted, and it is hoped that it will be more effective and safe. The immunohistochemical upregulation of AKT1 and MAPK1 observed in the AFB1-treated groups was consistent with the histological evidence of hepatocellular degeneration, congestion, and necrosis. These molecular changes indicate the activation of pro-survival and stress response pathways in response to toxic insult (Ali et al., 2021). Capsaicin co-treatment reduced MAPK1 expression significantly and AKT1 expression, correlating with milder histopathological alterations, indicating a protective modulation of these pathways (Hu et al., 2024). Mechanistically, capsaicin may inhibit AKT1 and MAPK1 phosphorylation by acting upstream at PI3K or through direct antioxidant activity that limits ROS-mediated kinase activation. In silico docking also supports the potential of capsaicin to bind these proteins competitively, possibly preventing AFB1-induced signaling. These findings highlight capsaicin as a potential dietary hepatoprotective agent against exposure to mycotoxin. In veterinary contexts, this may be relevant for improving livestock health and food safety. From a translational perspective, the effects of capsaicin could inform future preventive strategies in regions where aflatoxin contamination of food is endemic. ConclusionIn conclusion, although capsaicin has been reported to exhibit both pro- and anti-inflammatory effects depending on dose and context, our findings support its hepatoprotective role in reducing aflatoxin B1-induced liver damage, as demonstrated by decreased lesion severity and reduced AKT1/MAPK1 expression. AcknowledgmentsThe authors would like to thank Dr. Nove Hidajati, DVM, M.Health., for managerial support, Prof. Dr. Lilik Maslachah, DVM, M.Health., for expertise support, and Dr. Rahmi Sugihartuti, DVM, M.Health., for technical support. Conflict of interestThe authors declare no conflict of interest. FundingThe authors funded this study. Author’s contributionsMS, SAS, ARK, HP, and IM: conceived the idea, designed the mainframe of this manuscript, and analyzed and interpreted data. BWKW, IM, BPP, RZA, and ML: manuscript drafting. MHE, PS, SPM, and AOA: critically read and revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript. Data availabilityAll data are available in the revised manuscript. ReferencesAhmed, S.S., Faten, Z.M. and Al-Shimaa, M.A. 2017. In vitro studies on anticancer activity of capsaicin a component of hot chili pepper against human hepatocellular carcinoma cells. Cell Sci. Mol. Biol. 2(4), 555–591. Al-Ayoubi, C., Rocher, O., Naylies, C., Lippi, Y., Vignard, J., Puel, S., Puel, O., Oswald, I.P. and Soler, L. 2024. More than a mutagenic Aflatoxin B1 precursor: the multiple cellular targets of Versicolorin A revealed by global gene expression analysis. Environ. Pollut. 363(Pt 1), 125138; doi:10.1016/j.enp.2008.07.013 Ali, F.A.Z., Abdel-Maksoud, F.M., Abd Elaziz, H.O., Al-Brakati, A. and Elmahallawy, E.K. 2021. Descriptive histopathological and ultrastructural study of rats with hepatocellular alterations induced by aflatoxin B1. Animals (Basel) 11(2), 509. Allameh, A., Niayesh-Mehr, R., Aliarab, A., Sebastiani, G. and Pantopoulos, K. 2023. Oxidative stress in liver pathophysiology and disease. Antioxidants (Basel) 12(9), 1653. Anandakumar, P., Jagan, S., Kamaraj, S., Ramakrishnan, G., Clara, J.B., Pathitha, D., Kavitha, T. and Devaki, T. 2009. Reducing effect of capsaicin on alterations in lipid metabolism during mouse lung carcinoma. Arch. Pharm. Res. 32, 229–234; doi:10.1016/j.archpharmres.2012.09.010 Anandakumar, P., Kamaraj, S., Jagan, S., Ramakrishnan, G., Asokkumar, S., Naveenkumar, C., Raghunandhakumar, S. and Devaki, T. 2012. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm Res. 61(11), 1169–1175. Antonius, Y., Kharisma, V.D., Widyananda, M.H., Ansori, A.N.M., Trinugroho, J.P., Ullah, M.E., Naw, S.W., Jakhmola, V. and Wahjudi, M. 2022. Prediction of aflatoxin-B1 (AFB1) molecular mechanism network and interaction to oncoproteins growth factor in hepatocellular carcinoma. Pure Appl. Microbiol. 16(3), 1844–1854. Balla, T. 2013. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93(3), 1019–1137; doi:10.1152/physrev.00028.2012 Basith, S., Cui, M., Hong, S. and Choi, S. 2016. Harnessing the therapeutic potential of capsaicin and its analogs in patients with pain and other diseases. Molecules 21(8), 966. Batiha, G.E.S., Alqahtani, A., Ojo, O.A., Shaheen, H.M., Wasef, L., Elzeiny, M., Ismail, M., Shalaby, M., Murata, T., Zaragoza-Bastida, A., Rivero-Perez, N., Magdy Beshbishy, A., Kasozi, K.I., Jeandet, P. and Hetta, H.F. 2020. Biological properties, bioactive constituents, and pharmacokinetics of some capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 21(15), 5179; doi:10.1016/j.ijms.2015.01.013 Benkerroum, N. 2020. Chronic and acute toxicities of flatoxins: mechanisms of action. Int. J. Environ. Res. Public Health 17(2), 423. Berasain, C., Latasa, M.U., Urtasun, R., Goñi, S., Elizalde, M., Garcia-Irigoyen, O., Azcona, M., Prieto, J. and Avila, M.A. 2011. Epidermal growth factor receptor (EGFR) crosstalk in liver cancer. Cancers (Basel) 3, 2444–2461. Bhat, M., Sonenberg, N. and Gores, G.J. 2013. MTOR pathway in hepatic malignancies. Hepatology 58(2), 810–818. Black, A.R. and Black, J.D. 2012. Protein kinase C signaling and cell cycle regulation. Front. Immunol. 3(1), 423. Chanda, S., Bashir, M., Babbar, S., Koganti, A. and Bley, K. 2008. Hepatic and skin metabolism of capsaicin in vitro. Drug Metab. Dispos. 36(4), 670–675. Chang, C.F., Islam, A., Liu, P.F., Zhan, J.H. and Chueh, P.J. 2010. Capsaicin acts through tNOX (ENOX2) to induce autophagic apoptosis in p53-mutated HSC-3 cells but autophagy in p53-functional SAS oral cancer cells. Cancer Res. 10, 3230–3247. Charlton, N.C., Mastyugin, M., Török, B. and Török, M. 2023. Structural features of small-molecule antioxidants and their strategic modifications to improve their potential bioactivity. Molecules 28(3), 1057. Chen, C., Nelson, L.J., Ávila, M.A. and Cubero, F.J. 2019. Mitogen-activated protein kinases and cholangiocarcinoma: the missing link. Cells 8(10), 1172. Chen, Y., Liu, H., An, T., Wu, Q., Zhang, H., Loor, J.J., Cheng, J., Wang, J. and Sun, J. 2023. L. intestinalis/L. rhamnosus protects against AFB1-induced liver damage: involvement of the intestinal mucosal barrier. One Health Adv. 1, 24. Chow, J., Norng, M., Zhang, J. and Chai, J. 2007. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells—Mechanisms behind a possible new ‘hot’ cancer treatment. Biochim. Biophys. Acta. 1773(4), 565–576. Chu, Y., Yu, A., Wang, H., Rajput, S.A., Yu, Q. and Qi, D. 2024. Biological mechanisms of aflatoxin B1-induced abnormalities in bile metabolism in ducklings. Animals (Basel). 14(20), 2996. Delpire, E. and Gagnon, K.B. 2018. Water homeostasis and cell volume maintenance and regulation. Curr. Membr. 81(1), 3–52. De-Sá-Júnior, P.L., Pasqualoto, K.F.M., Ferreira, A.K., Tavares, M.T., Damião, M.C.F.C.B., De Azevedo, R.A., Câmara, D.A.D., Pereira, A., De Souza, D.M. and Parise Filho, R. 2013. RPF101, a new capsaicin-like analogue, disrupts the microtubule network accompanied by arrest in the G2/M phase, inducing apoptosis and mitotic catastrophe in the MCF-7 breast cancer cells. Toxicol. Appl. Pharmacol. 266, 385–398; doi:10.1016/j.toxicol.2012.03.017 Eaton, D.L., Williams, D.E. and Coulombe, R.A. 2025. Species differences in aflatoxin B1 biotransformation: primary determinants of relative carcinogenic potency in different animal species. Toxins 17, 30. Fathima, S.D., Gururaj, N., Sivapathasundharam, B., Vennila, A.A., Lavanya, M.K.K. and Sarayushivani, U. 2023. Histopathological significance of necrosis in oral lesions: a review. Oral Maxillofac. Pathol. 27(2), 340–347. Fattori, V., Hohmann, M., Rossaneis, A., Pinho-Ribeiro, F. and Verri, W. 2016. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 21(7), 844. Foulkes, E.C. 2000. Transport of toxic heavy metals across cell membranes. Proc. Soc. Exp. Biol. Med. 223(3), 234–240. Ghorbanpour, A., Salari, S., Baluchnejadmojarad, T. and Roghani, M. 2023. Capsaicin protects against acute septic liver injury by attenuating apoptosis and mitochondrial dysfunction. Heliyon 9(3), 14205. Ghousein, A., Mosca, N., Cartier, F., Charpentier, J., Dupuy, J.W., Raymond, A.A., Bioulac-Sage, P. and Grosset, C.F. 2020. MiR-4510 blocks the development of hepatocellular carcinoma through RAF1 targeting and RAS/RAF/MEK/ERK signaling inactivation. Liver Int. 40(1), 240–251. Gu, X. and Manautou, J.E. 2014. Molecular mechanisms underlying liver injury caused by chemicals. Expert Rev. Mol. Med. 14(1), 4. Guo, J., Jiang, X., Lian, J., Li, H., Zhang, F., Xie, J., Deng, J., Hou, X., Du, Z. and Hao, E. 2024. Evaluation of the effect of GSK-3β on liver cancer based on the PI3K/AKT pathway. Front. Cell Dev. Biol. 12, 1431423. Gyimesi, G., Pujol-Giménez, J., Kanai, Y. and Hediger, M.A. 2020. Sodium-coupled glucose transport, SLC5 family, and therapeutically relevant inhibitors: molecular discovery to clinical application. Pflugers. Arch. 472(9), 1177–1206. Hacioglu, C. 2013. Capsaicin inhibits cell proliferation by enhancing oxidative stress and apoptosis through SIRT1/NOX4 signaling pathways in HepG2 and HL-7702 cells. J. Biochem. Mol. Toxicol. 36(3), 22974; doi:10.1016/j.jbmt.2013.09.014 Hamali, H., Ashrafi-Helan, J. and Khordadmehr, M. 2021. Natural aflatoxicosis in neonatal calves in a dairy herd – pathological diagnosis. Bulg. J. Vet. Med. 24(2), 291–296. He, Y., Sun, M.M., Zhang, G.G., Yang, J., Chen, K.S., Xu, W.W. and Li, B. 2021. Targeting PI3K/Akt signal transduction in cancer therapy. Signal Transduct. Target Th. 6(1), 425. Ho, C., Wang, C., Mattu, S., Destefanis, G., Ladu, S., Delogu, S., Armbruster, J., Fan, L., Lee, S.A., Jiang, L., Dombrowski, F., Evert, M., Chen, X. and Calvisi, D.F. 2012. Coactivation of AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homogene) in the mouse liver promotes rapid carcinogenesis via the mammalian target of rapamycin complex 1 (mTOR), forkhead box M1 (FOXM1)/SKP2, and c-Myc pathways. Hepatology 55(3), 833–845. Hu, C.T., Wu, J.R., Cheng, C.C. and Wu, W.S. 2017. Therapeutic targeting of HGF/c-Met signaling in hepatocellular carcinoma: alternative approaches. Cancers (Basel). 9(6), 58. Hu, Q., Liu, H., Wang, R., Yao, L., Chen, S., Wang, Y. and Lv, C. 2024. Capsaicin attenuates LPS-induced acute lung injury by inhibiting inflammation and autophagy through regulation of the TRPV1/AKT pathway. J. Inflamm. Res. 17, 153–170. Hua, Z., Liu, R., Chen, Y., Liu, G., Li, C., Song, Y., Cao, Z., Li, W., Li, W., Lu, C. and Liu, Y. 2021. Aflatoxins contamination induces severe hepatotoxicity through multiple mechanisms. Front. Pharmacol. 11, 605823. Ibrahim, M., Ramadan, E., Elsadek, N.E., Emam, S.E., Shimizu, T., Ando, H., Ishima, Y., Elgarhy, O.H., Sarhan, H.A., Hussein, A.K. and Ishida, T. 2022. Polyethylene glycol (PEG): the nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Pharmacol. 201, e01503. Jin, X., Zhang, Y., Wang, D., Zhang, X., Li, Y., Wang, D., Liang, Y., Wang, J., Zheng, L., Song, H., Zhu, X., Liang, J., Ma, J., Gao, J., Tong, J. and Shi, L. 2024. Metabolite and protein shifts in mature erythrocytes under hypoxia. iScience 27(4), 109315. Johnson, W.W. and Guengerich, F.P. 1997. Reaction of Aflatoxin B1 exo-8,9-epoxide with DNA: kinetic analysis of covalent binding and DNA-induced hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 94(12), 6121–6125. Jomova, K., Raptova, R., Alomar, S.Y., Alwasel, S.H., Nepovimova, E., Kuca, K. and Valko, M. 2010. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97(10), 2499–2574; doi:10.1016/j.archtoxicol.2010.08.010 Juan, C.A., Pérez De La Lastra, J.M., Plou, F.J. and Pérez-Lebeña, E. 2021. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22(9), 4642. Kettritz, R. and Loffing, J. 2023. Physiology and pharmacology in a clinical context. Pharmacol. Ther. 249(1), 108489. Khan, R., Anwar, F. and Ghazali, F.M. 2024. A comprehensive review of mycotoxins: toxicology, detection, and effective mitigation approaches. Heliyon 10(8), e28361. Kleiner, D.E. 2018. Recent advances in the histopathology of drug-induced liver injury. Surg. Pathol. Clin. 11, 297–311. Kneeman, J.M., Misdraji, J. and Corey, K.E. 2012. Secondary causes of NAFLD. Therap. Adv. Gastroenterol. 5(3), 199–207. Li, S., Hao, L., Yu, F., Li, N., Deng, J., Zhang, J., Xiong, S. and Hu, X. 2024. Capsaicin: a spicy way to treat liver disease. Front. Pharmacol. 15, 1451084. Lin, R.J., Wu, I.J., Hong, J.Y., Liu, B.H., Liang, R.Y., The Yuan, T.M. and Chuang, S.M. 2010. Capsaicin-induced TRIB3 upregulation promotes cancer cell apoptosis. Cancer Manag. Res. 10, 4237–4248. Lin, Y.T., Wang, H.C., Hsu, Y.C., Cho, C.L., Yang, M.Y. and Chien, C.Y. 2017. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int. J. Mol. Sci. 18(7), 1343; doi:10.1016/j.ijms.2013.07.016 Lipfert, J., Doniach, S., Das, R. and Herschlag, D. 2014. Understanding nucleic acid-ion interactions. Annu. Rev. Biochem. 83(1), 813–841. Luján-Méndez, F., Roldán-Padrón, O., Castro-Ruíz, J.E., López-Martínez, J. and García-Gasca, T. 2023. Capsaicinoids and their effects on cancer: the “Double-Edged Sword” from the molecular scale. Cells 12(21), 2573. Maharjan, A., Vasamsetti, B.M.K. and Park, J.H. 2024. A comprehensive review of capsaicin: biosynthesis, industrial production, processing, applications, and clinical uses. Heliyon 10(21), 39721. Maher, S. and Brayden, D.J. 2021. Formulation strategies to improve intestinal permeation enhancer efficacy. Adv. Drug Deliv. Rev. 177, 113925. Marchese, S., Polo, A., Ariano, A., Velotto, S., Costantini, S. and Severino, L. 2018. Aflatoxin B1 and M1: biological properties and their involvement in the development of cancer. Toxins 10(6), 214. Merat, S., Farzaneh, K.S., Nouraie, M., Derakhshan, M.H., Tavanga, S.M., Mossaffa, S., Malekzadeh, R. and Sotoudeh, M. 2010. A modification of the brunt system for scoring liver histology in patients with non-alcoholic fatty liver disease. Arch. Iran. Med. 13(1), 38–44. Moriguchi, M., Watanabe, T., Kadota, A. and Fujimuro, M. 2019. Capsaicin induces apoptosis in KSHV-positive primary effusion lymphoma by suppressing ERK and p38 MAPK signaling and IL-6 expression. Front. Oncol. 9(1), 83. Niknahad, H., Khan, S. and O’Brien, P.J. 1995. Hepatocyte injury resulting from the inhibition of mitochondrial respiration at low oxygen concentrations involves reductive stress and oxygen activation. Chem. Biol. Interact. 98(1), 27–44; doi:10.1016/j.cheminteract.2012.09.016 Novak, M., Madej, J.A. and Dziegeil, P. 2007. Intensity of Cox 2 expression in soft tissue fibrosarcoma cells in dogs as related to grade of tumor malignancy. Bull. Vet. Inst. Pulawy 51(2), 275–279. Park, S.Y., Kim, J.Y., Lee, S.M., Jun, C.H., Cho, S.B., Park, C.H., Joo, Y.E., Kim, H.S., Choi, S.K. and Rew, J.S. 2014. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 9, 499–502. Pirillo, V., Pollegioni, L. and Molla, G. 2018. Analytical methods for investigating enzyme-catalyzed degradation of polyethylene terephthalate. FEBS. J. 288(16), 4730–4745. Proskuryakov, S.Y., Gabai, V.L. and Konoplyannikov, A.G. 2002. Necrosis is an active and controlled form of PCD. Biochemistry (Mosc) 67, 387–408. Rascio, F., Spadaccino, F., Rocchetti, M.T., Castellano, G., Stallone, G.S., Netti, G.S. and Ranieri, E. 2021. The pathogenic role of the PI3K/AKT pathway in cancer onset and drug resistance: an updated review. Cancers (Basel) 13(16), 3949. Redza-Dutordoir, M. and Averill-Bates, D.A. 2016. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA). -. Mol. Cell. Biol. Lipids. 1863(12), 2977–2992. Sailo, B.L., Garhwal, A., Mishra, A., Hegde, M., Vishwa, R., Girisa, S., Abbas, M., Alqahtani, M.S., Abdulhammed, A., Sethi, G., Kempson, I. and Kunnumakkara, A.B. 2025. Potential of capsaicin as a combinatorial agent to overcome chemoresistance and to improve outcomes of cancer therapy. Biochem. Pharmacol. 236, 116828; doi:10.1016/j.biochempharmacol.2012.11.6828 Sánchez-Hernández, R., Benítez-Angeles, M., Hernández-Vega, A.M. and Rosenbaum, T. 2024. Recent advances in the structure and function relationships of the TRPV4 ion channel. Channels (Austin). 18, 2313323. Schieber, M. and Chandel, N.S. 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24(10), R453–R462. Sganzerla, M., Coutinho, J.P., De Melo, A.M.T. and Godoy, H.T. 2014. Fast method for the analysis of capsaicinoids from Capsicum chinense fruits. Food Res. Int. 64, 718–725. Singh, S., Natarajan, K. and Aggarwal, B.B. 1996. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a potent inhibitor of nuclear transcription factor-kappa B activation by diverse agents. J. Immunol. 157(10), 4412–4420; doi:10.1016/j.jimmunol.2015.09.010 Suhail, M. 2010. Na, K-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J. Clin. Med. Res. 2(1), 1–17. Sui, Y., Kong, X., Fan, R., Ye, Y., Mai, H., Zhuo, S., Lu, W., Ruan, P., Fang, S. and Yang, T. 2019. Long-term treatment with metformin in the prevention of fatty liver in Zucker diabetic fatty rats. Metab. Syndr. 11(1), 94. Sukmanadi, M. and Effendi, M.H. 2021. The protective effect of Capsaicin (Capsicum annum L) against the induction of Aflatoxin B1 in hepatocytes: a study of liver histopathology in mice (Mus musculus). Res. J. Pharm. 2018. Technol. 58(2), 813–816. Sukmanadi, M., Effendi, M.H., Fikri, F. and Purnama, M.T.E. 2021. Liver-histological improvement after capsaicin administration in mice with aflatoxin B1 toxication. Pharmacogn. J. 13(6), 1577–1581. Sukmanadi, M., Sudjarwo, S.A. and Effendi, M.H. 2020. molecular mechanism of capsaicin from (Capsicum annuum L.) on expression of MAPK1 and AKT1 protein as candidate of anticancer drugs: in silico study. Pharmacogn. J. 12(4), 916–919; doi:10.1016/j.pharmacogn.2012.09.010 Sun, H., Qian, X., Yang, W., Zhou, W., Zhou, C., Liu, S., Shi, H. and Tian, W. 2022. Novel prognostic signature based on HRAS, MAPK3 and TFRC identified to be associated with ferroptosis and the immune microenvironment in hepatocellular carcinoma. Am. J. Transl. Res. 14(10), 6924–6940. Sun, S., Cui, Y., Yuan, B., Dou, M., Wang, G., Xu, H., Wang, J., Yin, W., Wu, D. and Peng, C. 2023. Drug delivery systems based on polyethylene glycol hydrogels for bone regeneration enhancement. Bioeng. Biotechnol. 11(1), 1117647. Thongararm, P., Chancharoen, M., Suwanwong, N., Ruchirawat, S., Ruchirawat, M., Fedeles, B.I., Croy, R.G. and Essigmann, J.M. 2025. Structurally similar mycotoxins aflatoxin B1 and sterigmatocystin trigger different and distinctive high-resolution mutational spectra in mammalian cells. Toxins 17(3), 112. Wang, F., Zhao, J., Liu, D., Zhao, T., Lu, Z., Zhu, L., Cao, L., Yang, J., Jin, J. and Cai, Y. 2016. Capsaicin reactivates hMOF and inhibits cell growth in gastric cancer cells. Cancer Biol. Ther. 17(11), 1117–1125. Wang, Y., Liu, F., Zhou, X., Liu, M., Zang, H., Liu, X., Shan, A. and Feng, X. 2022. Curcumin alleviates oral exposure to aflatoxin b1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney. Antioxidants (Basel) 11(6), 1082. Wu, Y., Adeel, M.M., Xia, D., Sancar, A. and Li, W. 2024. Nucleotide excision repair of aflatoxin-induced DNA damage within 3D human genome organization. Nucleic Acids Res. 52(19), 11704–11719. Yu, Y., Liu, S., Yang, L., Song, P., Liu, Z., Liu, X., Yan, X. and Dong, Q. 2024. Roles of reactive oxygen species in inflammation and cancer. MedComm (2020). 5(4), e519. Zamay, T.N., Zamay, S.S., Zamay, G.S., Kolovskaya, O.S., Kichkailo, A.S. and Berezovski, M.V. 2025. Systemic mechanisms of ionic regulation in carcinogenesis. Cancers 17(2), 286–289. Zhang, L.L., Yan Liu, D., Ma, L.Q., Luo, Z.D., Cao, T.B., Zhong, J., Yan, Z.C., Wang, L.J., Zhao, Z.G., Zhu, S.J., Schrader, M., Thilo, F., Zhu, Z.M. and Tepel, M. 2007. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circulat. Res. 100, 1063–1070. Zhang, N. and Li, Y. 2023. Receptor tyrosine kinases: biological functions and anticancer targeted therapy. Med. Comm. 4,e446. Zhang, T., Ma, C., Zhang, Z., Zhang, H. and Hu, H. 2021. NF-κB signaling in inflammation and cancer. Med. Comm. 2(4), 618–653. Zhang, W., Zhang, Y., Fan, J., Feng, Z. and Song, X. 2024. Pharmacological activity of capsaicin: mechanisms and controversies. Mol. Med. Rep. 29(3), 38–39. Zhang, Y., Deng, X., Lei, T., Yu, C., Wang, Y., Zhao, G., Luo, X., Tang, K., Quan, Z. and Jiang, D. 2017. Capsaicin inhibits osteosarcoma cell proliferation and induces apoptosis via the mitogen-activated protein kinase pathway. Oncol. Rep. 38(5), 2685–2696. Zhao, J., Liu, X., Zhang, T., Luo, H., Zhang, J. and Shao, C. 2025. Capsaicin functions as an androgen receptor antagonist that inhibits prostate cancer growth in cellular and mouse models. Food Biosci. 69(1), 107000 | ||
| How to Cite this Article |
| Pubmed Style Sukmanadi M, Sudjarwo SA, Effendi MH, Khairullah AR, Srianto P, Madyawati SP, Lamid M, Plumeriastuti H, Mustofa I, Akintunde AO, Wardhani BWK, Ahmad RZ, Melati I, Pratama BP. Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Vet. J.. 2025; 15(9): 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 Web Style Sukmanadi M, Sudjarwo SA, Effendi MH, Khairullah AR, Srianto P, Madyawati SP, Lamid M, Plumeriastuti H, Mustofa I, Akintunde AO, Wardhani BWK, Ahmad RZ, Melati I, Pratama BP. Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. https://www.openveterinaryjournal.com/?mno=263939 [Access: November 22, 2025]. doi:10.5455/OVJ.2025.v15.i9.9 AMA (American Medical Association) Style Sukmanadi M, Sudjarwo SA, Effendi MH, Khairullah AR, Srianto P, Madyawati SP, Lamid M, Plumeriastuti H, Mustofa I, Akintunde AO, Wardhani BWK, Ahmad RZ, Melati I, Pratama BP. Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Vet. J.. 2025; 15(9): 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 Vancouver/ICMJE Style Sukmanadi M, Sudjarwo SA, Effendi MH, Khairullah AR, Srianto P, Madyawati SP, Lamid M, Plumeriastuti H, Mustofa I, Akintunde AO, Wardhani BWK, Ahmad RZ, Melati I, Pratama BP. Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Vet. J.. (2025), [cited November 22, 2025]; 15(9): 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 Harvard Style Sukmanadi, M., Sudjarwo, . S. A., Effendi, . M. H., Khairullah, . A. R., Srianto, . P., Madyawati, . S. P., Lamid, . M., Plumeriastuti, . H., Mustofa, . I., Akintunde, . A. O., Wardhani, . B. W. K., Ahmad, . R. Z., Melati, . I. & Pratama, . B. P. (2025) Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Vet. J., 15 (9), 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 Turabian Style Sukmanadi, Mohammad, Sri Agus Sudjarwo, Mustofa Helmi Effendi, Aswin Rafif Khairullah, Pudji Srianto, Sri Pantja Madyawati, Mirni Lamid, Hani Plumeriastuti, Imam Mustofa, Adeyinka Oye Akintunde, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Irma Melati, and Bima Putra Pratama. 2025. Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Veterinary Journal, 15 (9), 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 Chicago Style Sukmanadi, Mohammad, Sri Agus Sudjarwo, Mustofa Helmi Effendi, Aswin Rafif Khairullah, Pudji Srianto, Sri Pantja Madyawati, Mirni Lamid, Hani Plumeriastuti, Imam Mustofa, Adeyinka Oye Akintunde, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Irma Melati, and Bima Putra Pratama. "Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1." Open Veterinary Journal 15 (2025), 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 MLA (The Modern Language Association) Style Sukmanadi, Mohammad, Sri Agus Sudjarwo, Mustofa Helmi Effendi, Aswin Rafif Khairullah, Pudji Srianto, Sri Pantja Madyawati, Mirni Lamid, Hani Plumeriastuti, Imam Mustofa, Adeyinka Oye Akintunde, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Irma Melati, and Bima Putra Pratama. "Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1." Open Veterinary Journal 15.9 (2025), 4044-4059. Print. doi:10.5455/OVJ.2025.v15.i9.9 APA (American Psychological Association) Style Sukmanadi, M., Sudjarwo, . S. A., Effendi, . M. H., Khairullah, . A. R., Srianto, . P., Madyawati, . S. P., Lamid, . M., Plumeriastuti, . H., Mustofa, . I., Akintunde, . A. O., Wardhani, . B. W. K., Ahmad, . R. Z., Melati, . I. & Pratama, . B. P. (2025) Protective effect of capsaicin on AKT1 and MAPK1 expression in the liver of mice (Mus musculus) induced by aflatoxin B1. Open Veterinary Journal, 15 (9), 4044-4059. doi:10.5455/OVJ.2025.v15.i9.9 |