| Research Article | ||
Open Vet. J.. 2025; 15(9): 4024-4031 Open Veterinary Journal, (2025), Vol. 15(9): 4024-4031 Research Article Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climateGrace Tabitha Tenggi Olihta Simatupang1,2, Irkham Widiyono3*, Yanuartono Yanuartono3 and Ridha Avicena Ila Salsabila41Master of Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2The Directorate of Animal Police, Korsabhara Baharkam Polri, Depok, Indonesia 3Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Veterinary Science Doctoral Program, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Irkham Widiyono. Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: irkhamwidiyono [at] ugm.ac.id Submitted: 12/06/2025 Revised: 14/08/2025 Accepted: 22/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
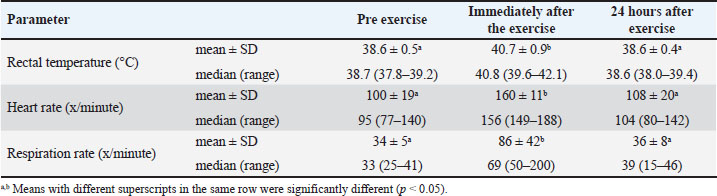
ABSTRACTBackground: During exercise, dogs may experience heat stress and metabolic disorders, which can lead to physical health problems and impair their ability to work optimally. Furthermore, working at high ambient temperatures could result in blood chemistry parameter changes that differ from those at low ambient temperatures. It may even result in metabolic disorders in dogs. The biological responses of Belgian Malinois dogs to training in tropical areas with high temperatures and humidity, such as those found in Indonesia, have not been reported. Aim: This study aimed to investigate the biological response of police dogs to search and rescue exercises in tropical areas with high ambient temperatures. Methods: This study included 14 healthy Belgian Malinois police dogs (seven females and seven males) aged between 5 and 9 years. The exercise consisted of a 60-minute regular session of search and rescue training, conducted in West Java with ambient temperatures ranging from 27°C to 33°C. Physical, hematological, and blood biochemical parameters were measured before, immediately, and 24 hours after the exercise. Results: During the study, the animals did not show signs of fatigue or intolerance to the training. The respiratory rate, heart rate, and rectal temperature increased with exercise and returned to baseline after 24 hours of rest. Meanwhile, exercise did not affect erythrocyte, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), granulocyte, lymphocyte, platelet counts, mean platelet volume (MPV), and platelet crit (PCT); however, leukocyte and mid-sized leucocyte (MID) counts increased during exercise. Exercise did not affect aspartate aminotransferase (AST), creatine kinase (CK), glucose, and calcium concentrations. Serum concentrations of lactate dehydrogenase, natrium, kalium, and phosphorus decreased with exercise. Serum chloride concentration increased with exercise. There was no evidence of muscle disorders; however, there is a potential for metabolic disturbances. All research parameters returned to baseline values 24 hours after exercise. Conclusion: Changes in physical, hematological, and blood chemistry parameters were induced by the search and rescue exercise, and all parameters returned to their initial values after a 24-hour recovery. Belgian Malinois dogs have a good biological response in tropical climates. Keywords: Biological response, Dog, Exercise, Tropical climate. IntroductionPolice often use dogs as pets and assistants in crowd control, detecting explosives and narcotics, and searching for natural disaster victims. Dogs trained as tracker dogs include Belgian Malinois, German Shepherd, Dutch Shepherd, and Beagle. Dogs that perform this heavy work often experience stress, heat stress, and metabolic disorders during training, which can result in physical health problems and prevent them from working optimally. Health problems in working dogs require attention. Maintaining dog health is a form of responsibility for owners (Hens, 2009). Physiological, hematological, and blood chemistry parameters changes are some indicators that determine health condition (Rovira et al., 2008; Alves and Santos, 2016). Various analyses of hematological changes, physiological changes, and blood chemistry in dogs can be used to determine the biological response of dogs to environmental factors. Changes in hematological values, physiological changes, and blood chemistry are observed when the body experiences disturbance, stress, or physical activity (Alves and Santos, 2016; Radisavljević et al., 2017; Milena et al., 2023; Ivasovic et al., 2023). In this regard, hematological and blood chemistry parameters, along with physiological value parameters, can be used to assess stress status in dogs (Bergeron et al., 2002; Alves and Santos, 2016; Radisavljević et al., 2017; Jahr et al., 2019). Environmental conditions also greatly influence physiological, hematological, and blood chemistry parameters. According to Robbins et al. (2017), ecological conditions greatly influence physiological changes, hematological values, and blood chemistry. Bartolomé et al. (2023) noted that these and ecological conditions greatly influence these parameters. Dogs participating in field tests exhibited respiratory alkalosis and hypocapnia under high (>21°C) ambient temperature conditions (Steiss and Wright, 2008). To date, no changes in physiological parameters related to routine training activities in police working dogs, particularly Belgian Malinois, have been observed in Indonesia, where the ambient temperature exceeds 25°C. This study aims to determine changes in physiological parameters, hematology values, and blood chemistry of Belgian Malinois dogs at the Directorate of Animal Police of the Republic of Indonesia National Police, Indonesia, before, during, and after training. Materials and MethodsStudy duration and locationThis research was conducted in February and March 2025 within the Directorate of Animal Police of the Indonesian National Police in Depok, West Java, Indonesia. The temperature at the residential and training locations was approximately 27°C–33°C, and the air humidity was approximately 85%. Experimental designThis study involved 14 Belgian Malinois dogs, comprising seven males and seven females, aged 5–9 years, with a mean body weight of 34 ± 2.83 kg, who had been working as police dogs for more than 2 years. None of the animals was sterilized. All dogs were in good physical condition (BCS 4–5, according to the BCS 1–9 scoring system) and acclimated to the study site’s ambient temperature. The animals were fed up to 250 g per day (Maxi Adult, PT. Royal Canin Indonesia, Jakarta, Indonesia), which was administered in the morning, afternoon, and evening. Water was provided ad libitum. All animals were treated for endo- and ectoparasites for 2 weeks before being used in the study. During the study, the animals were in good clinical condition. The same officer accompanied, trained, and cared for/maintained each animal with the same method and materials before and during the study. Feeding was conducted 3 hours after treatment or training on the test day to minimize interference with the study results. Drinking water was provided freely before and after training. Each animal was given physical training (running) and skills for 60 minutes (7:00–8:00). During training, the animal ran toward the target, searched for the target, caught the target, and ran back to submit the results to the accompanying officer. Such activities were repeated continuously until the end of the training session. After the training session, each animal rested and was treated as usual. Data collectionPhysiological data collection (respiratory rate, heart rate, and rectal temperature measurement) and blood sampling for hematology and blood chemistry examinations were performed before training at 06:00–07:00 in each animal’s cage, immediately after training, and 24 hours after training ended in each cage. Blood was collected through the antibrachial anterior cephalic vein. Some blood was collected in EDTA tubes for hematology examination, and the other part was collected in tubes without anticoagulant for serum collection. Serum was separated by centrifugation and stored at −20°C until chemical analysis. Physical examination, physiological data collection (including respiratory rate, heart rate, and rectal temperature), and blood sampling were performed as described by Englar (2017). EvaluationHematology examinations (erythrocyte, hemoglobin/Hb, hematocrit, mean corpuscular volume/MCV, mean corpuscular hemoglobin/MCH, mid-sized leucocytes/MID, granulocyte, lymphocyte, leukocyte, platelet, mean platelet volume/MPV, plateletcrit/PCT) were performed using the RT-7600 Hematology Analyzer (Rayto, China). A blood chemistry examination [creatine kinase/(CK), glucose, lactate dehydrogenase/LDH, natrium/Na, kalium/K, calcium/Ca, phosphorus/P, chloride/Cl] was performed using the VetXpert Cube Veterinary Integrated Analyzer (Mindray, China). Data analysisData were analyzed using the Statistical Package for the Social Sciences version 26. The results of the study were presented as means and standard deviations. Physiological, hematological, and blood chemistry data were analyzed using the repeated measures analysis of variance (ANOVA) test method, provided that the standardized residual normality test results indicated the data were normally distributed. The Friedman test was performed if the results of the standardized residual normality test indicated that the data were not normally distributed. The repeated measures ANOVA test was then followed by the Bonferroni post hoc test, while the Friedman test was followed by the Dunn post hoc test to assess the differences in means between the time groups. p<0.05 was considered as the significance criterion. Ethical approvalThe experimental animal treatment approach has obtained ethical approval from the Faculty of Veterinary Medicine Ethics Commission of Universitas Gadjah Mada, Yogyakarta, Indonesia, with number 144/ECFKH/Int/2024, dated January 7, 2025. ResultsDuring the study, the animals did not show signs of fatigue or intolerance to the training. The physiological data examination results are presented in Table 1. Before morning exercise, the average respiratory rate, heart rate, and rectal temperature were 34 ± 5 x/minute, 100 ± 19 x/minute, and 38.6°C ± 0.5°C, respectively. After 60 minutes of exercise, a significant increase was observed in all three parameters (p < 0.05), and all three parameters returned to baseline values after a 24-hour rest (p > 0.05). Table 1. Physical parameters of police dogs at pre exercise, immediately after the exercise, and 24 hours after exercise.
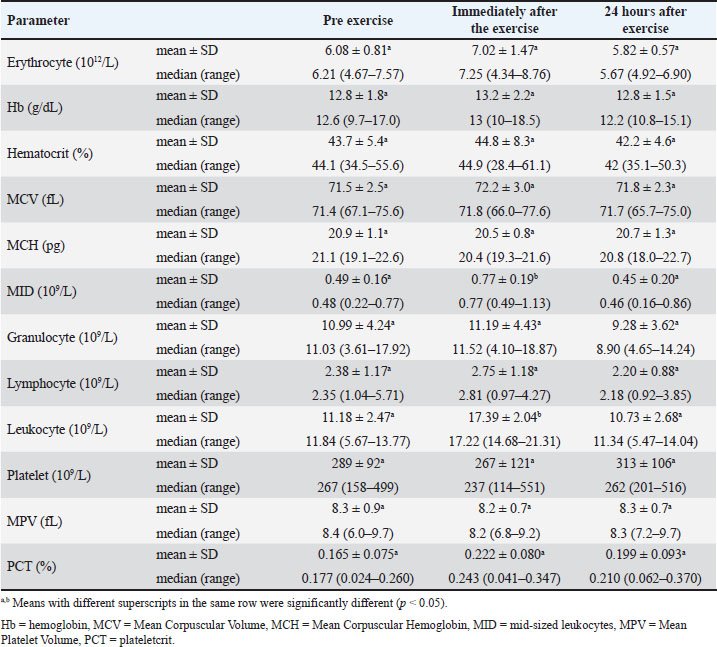
Table 2 presents the results of a hematological examination of dogs before, immediately after, and 24 hours after exercise in this study. The total erythrocyte count, Hb concentration, hematocrit, MCV, MCH, lymphocyte count, granulocyte count, platelet count, MPV, and PCT did not change significantly after exercise (p > 0.05). In this study, total leukocytes and MID significantly increased after exercise (p < 0.05) and returned to pre-exercise values after 24 hours of rest (p > 0.05). Table 2. Hematologic parameters of police dogs at pre exercise, immediately after the exercise, and 24 hours after exercise.
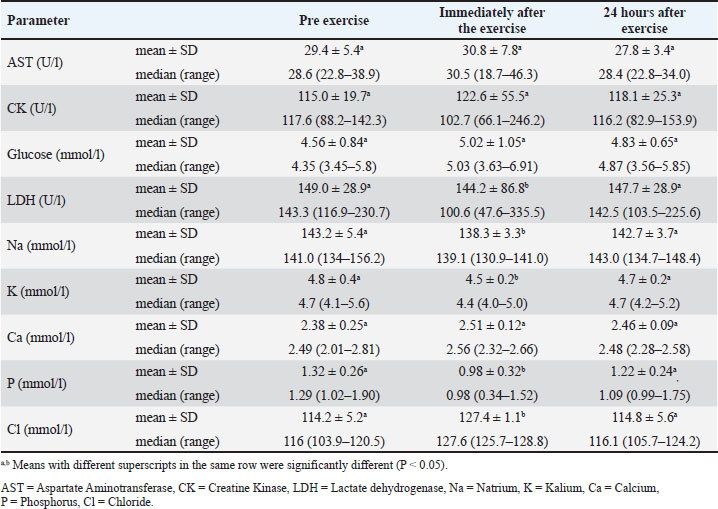
The biochemical examination results are presented in Table 3. Aspartate aminotransferase (AST), CK, and glucose concentrations did not change (p > 0.05), whereas LDH activity experienced a significant decrease (p < 0.05) but remained within normal reference ranges after training. The results of this study also showed a reduction in Na (p < 0.05), K (p < 0.05), and P (p < 0.05), as well as an increase in Cl (p < 0.05). Calcium concentration did not change after training (p > 0.05). Na, K, P, and Cl returned to pre-exercise concentrations after 24 hours of rest (p > 0.05). Table 3. Blood chemistry parameters of police dogs at pre exercise, immediately after the exercise, and 24 hours after exercise.
DiscussionAfter 60 minutes of exercise, the rectal temperature increased by 2.1°C. This increase is comparable to the rise in rectal temperature, ranging from 0.1°C to 3.0°C, found in dogs that completed a 4.5 km canicross run at an average speed of 15 km/hour (Erjavec et al., 2022). This increase in temperature occurs due to the increased energy demand for exercise and the need to dissipate heat produced as a by-product (Alves and Santos, 2016). According to Rovira et al. (2008), the increase in temperature occurs because muscle work produces large amounts of body heat. Additionally, the body of the dog responds to this increase in temperature by releasing heat through thermoregulation, specifically by increasing its heart and respiratory rate. In their research, Shull et al. (2021) and Lopedote et al. (2020) explained that after exercise, dogs exhibit a high heart rate, and the increase in heart rate during exercise in dogs is influenced by the dog’s body condition, which helps balance its body temperature. The respiratory rate increases during and immediately after exercise to compensate for the increased oxygen demand required to release energy (Lopedote et al., 2020). This finding is in line with the research results of Steiss et al. (2004) on Labrador Retriever dogs, Alves and Santos (2016) on Guarda Nacional Republicana (Portuguese Gendarmerie Unit) dogs, Erjavec et al. (2022) on Canicross dogs, and Ivasovic et al. (2023) on European Hounds, which showed that physiological parameters during exercise tended to increase compared to before or without exercise. The occurrence of a decrease after rest agrees with the findings of previous research. Studies on Labrador Retrievers, German Shepherds, and Belgian Malinois have shown that body temperature and heart rate decrease again after exercise and can be clearly observed once the animals have rested for 20 minutes (Ferasin and Marcora, 2009; Alves et al., 2020). The data on the dogs’ respiratory rate, heart rate, and rectal temperature in this study indicated that the animals were in a normal physiological condition during the study period. Healthy dogs that are not performing heavy activity have an average respiratory rate of 16–106 x/minute (Steiss et al., 2004; Alves and Santos, 2016), heart rate of 60–120 x/minute (Rijnberk and Stokhof, 2009; Alves and Santos, 2016), and temperature of 37.4°C–39.1°C (Alves and Santos, 2016; Hall and Carter, 2017). The total erythrocyte count, hemoglobin concentration, hematocrit, MCV, MCH, granulocyte, platelet, MPV, and PCT did not significantly change after exercise. The term “granulocyte” generally refers to white blood cells that contain cytoplasmic granules, encompassing not only neutrophils but also basophils, eosinophils, and mast cells (Cohen et al., 2021; Vorobjeva et al., 2023). No changes in erythrocyte count, Hb concentration, hematocrit, MCV, MCH, granulocyte, and platelet count were observed in police working dogs conducting crowd control exercises in Portugal (Portuguese Gendarmerie Unit) (Alves and Santos, 2016). This is likely related to police work training activities, which are not classified as long-term and heavy activities. In endurance training activities, it was reported that dogs that ran 600 km experienced a decrease in total erythrocytes, Hb, and hematocrit (Jahr et al., 2019). This decrease is likely related to exercise-induced plasma volume expansion (Davis et al., 2008; Dennis et al., 2008; Ermon et al., 2014). These dogs did not experience an increase in platelets, possibly because it is likely that exercise does not trigger the release of epinephrine and induce hemoconcentration or the release of platelets from the liver, lungs, and spleen. According to Heber and Volf (2015), intensive training induces hemoconcentration or triggers the release of platelets from the liver, lungs, and spleen. According to Kong et al. (2021), the MID includes monocytes, eosinophils, basophils, blasts, and other precursor leucocytes. The increase in MID observed in this study is consistent with that reported in previous studies. Increased monocyte numbers were observed in dogs after participating in a 600 km sled race in Norway (Jahr et al., 2019). The precise mechanisms underlying this response are not yet fully understood. However, according to Zaid et al. (2024), physical exercise induces the release of certain hormones, promoting the proliferation and mobilization of monocytes from the bone marrow into the circulatory system. Furthermore, the physical strain and tissue damage caused by exercise may activate the immune system, encouraging the migration of monocytes to the affected areas to support tissue repair and recovery. In addition to the increase in MID, these dogs had increased leukocyte counts. The leucocyte increase in this study was also still within the normal range. Normal dog leukocytes are 6.6–18.4 × 109/l (Lumsden et al., 1979). An increase in leukocytes can be observed after a short period of exercise. Dogs undergoing an agility test of 360–400 m with 40 obstacles experienced increased leukocytes (Rovira et al., 2007). Dogs undergoing more strenuous activity and long duration (sled racing competitions of 600 km or more) or transport showed increased leukocytes, neutrophils, and cortisol, whereas lymphocytes decreased (Bergeron et al., 2002; Davis et al., 2008). In our study, lymphocytes did not change after exercise because the dogs did not undergo heavy exercise that would have caused severe stress. Creatine kinase, AST, and LDH are indicators of (sub)clinical muscle damage during exercise in human athletes (Baird et al., 2012) and animals, including dogs (Aktas et al., 1995; Chanoit et al., 2001; Rovira et al., 2008; Millard et al., 2014; Klein et al., 2020). In this study, dogs did not experience an increase in CK, AST, and LDH activity because the exercise was not sufficiently strenuous. Excessive increases in AST and CK activity could indicate rhabdomyolysis and performance failure (Jahr et al., 2019). Increased CK may occur due to musculoskeletal damage associated with training and racing (McKenzie et al., 2007), followed by temporary changes in muscle cell permeability (Erjavec et al., 2022). Another study found that dogs undergoing intense exercise exhibited increased CK and AST activity. Alaskan sled dogs with five consecutive days of endurance exercise (McKenzie et al., 2007) and dogs participating in long-distance sled racing (Hinchcliff et al., 1993; Burr et al., 1997; Hinchcliff et al., 2004; Ermon et al., 2014) had elevated CK and AST. A study on police dogs during riot control training showed that LDH did not change after training (Alves and Santos, 2016). However, LDH increased after an agility test covering a distance of 360–400 m with 40 obstacles (Rovira et al., 2007). The unchanged enzymatic activity in our recent study indicates that no muscle damage occurred during exercise. During the study, the dogs’ blood glucose concentrations remained unchanged and within the reference range. Similar findings have also been reported in dogs undergoing agility exercise (Baltzer et al., 2012). This may indicate a balance between glucose production in the liver and glucose uptake by the muscles during and after exercise (Rizza et al., 1980; Jensen and Richter, 2012; Mennitti et al., 2024). This study observed a decrease in blood Na, K, and P concentrations, followed by an increase in Cl concentration after exercise. The results of this study are consistent with those of previous studies. Similar studies have shown that after activity, dogs will experience a decrease in K (Hinchcliff et al., 1997a; Hinchcliff et al., 1997b; Jahr et al., 2019; Steiss et al., 2004; McKenzie et al., 2007), a decrease in Na (Angle et al., 2009; Alves et al., 2020), and an increase in Cl (Steiss et al., 2004). Na, K, and P concentrations decrease due to increased cellular uptake (Steiss et al., 2004). Post-exercise hyponatremia could be associated with increased renal losses (Rovira et al., 2008). Increased Cl in the blood occurs due to a decrease in plasma HCO3 due to decreased renal acid excretion during respiratory alkalosis (Stephen and DiBartola, 2012). The dogs in this study appeared to have an electrolyte imbalance, which could indicate an acute respiratory alkalosis. Steiss et al. (2004) stated that acute respiratory alkalosis is associated with minor changes in electrolyte concentrations, with decreasing Na, K, ionized Ca, and P concentrations and increasing Cl concentrations. In our recent study, the reported calcium concentration was total calcium (not ionized calcium), which remained unchanged after exercise. Respiratory alkalosis has also been reported in dogs participating in field exercise tests under high (> 21°C) ambient temperature conditions (Steiss and Wreight, 2008). Moreover, exercise and heat stroke could be causes of respiratory alkalosis in dogs (Wagner 1977; Johnson et al., 2000). Respiratory alkalosis observed in our recent study may be associated with increased respiratory rate, from 34 ± 5 x/minute to 86 ± 42 x/minute, during exercise (hyperventilation). At high ambient temperatures, dogs (Labrador Retrievers) respond to exercise with hyperventilation, which then results in hypocapnia, and respiratory alkalosis (Steiss and Wright, 2008). An increase in blood pH was also observed in Labrador Retrievers that underwent 10 minutes of training by repeatedly retrieving a dummy thrown approximately 40 to 50 yards (Matwichuk et al., 1999). In the study reported here, all electrolytes had returned to their resting concentrations (prior to exercise) 24 hours after exercise, indicating that alkalosis and electrolyte disorders had been resolved in the dogs. ConclusionTo the best of the authors’ knowledge, this is the first report on changes in physiological, hematological, and biochemical parameters in police dogs (Belgian Malinois) during and after search and rescue training in tropical areas with high ambient temperatures. The training did not cause muscle damage, but it can cause metabolic disorders. Changes in physical, hematological, and blood chemistry parameters returned to baseline after a 24-hour rest. AcknowledgmentsThe authors express their immense gratitude to the Directorate of Animal Police Korsabhara Baharkam Polri Indonesia and the Indonesian National Police for the staff support and facilities provided for this research. Conflict of interestThe authors have no conflicts of interest to declare. FundingThe authors would like to thank the Indonesian National Police for providing laboratory support. Authors’ contributionsIW, Y, and GTTOS: Creating the concept and research design. GTTOS and RAIS: Conducting sample collection, laboratory analysis, data interpretation, and drafting the manuscript. GTTOS, IW, Y, and RAIS are involved in manuscript revision, editing, and approving the final version of the manuscript for publication. Data AvailabilityAll data are available in the manuscript. ReferencesAktas, M., Lefebvre, H.P., Toutain, P.L. and Braun, J.P. 1995. Disposition of creatine kinase activity in dog plasma following intravenous and intramuscular injection of skeletal muscle homogenates. J. Vet. Pharmacol. Ther. 18(1), 1–6. Alves, J.C. and Santos, A. 2016. Physiological, haematological and biochemical shifts in police working dogs during a riot control exercise. Comp. Exerc. Physiol. 12(4), 193–198. Alves, J.C., Santos, A., Jorge, P. and Lafuente, M.P. 2020. Changes in physiological, haematological, and biochemical parameters in police working dogs during a treadmill incremental exercise test. Comp. Exerc. Physiol. 16(5), 387–394. Angle, C.T., Wakshlag, J.J., Gillette, R.L., Stokol, T., Geske, S., Adkins, T.O. and Gregor, C. 2009. Hematologic, serum biochemical, and cortisol changes associated with anticipation of exercise and short duration high-intensity exercise in sled dogs. Vet. Clin. Pathol. 38(3), 370–374. Baird, M.F., Graham, S.M., Baker, J.S. and Bickerstaff, G.F. 2012. Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 1, 960363. Baltzer, W.I., Firshman, A.M., Stang, B., Warnock, J.J., Gorman, E. and Mckenzie, E.C. 2012. The effect of agility exercise on eicosanoid excretion, oxidant status, and plasma lactate in dogs. BMC Vet. Res. 8, 1–11. Bartolomé, E., Sánchez-Guerrero, M.J., Perdomo-González, D.I. and Valera, M. 2023. Stress and behavior assessment in police dogs due to challenging situations: differences due to training objectives. Dog Behav. 9(3), 1. Bergeron, R., Scott, S.L., Émond, J.P., Mercier, F., Cook, N.J. and Schaefer, A.L. 2002. Physiology and behavior of dogs during air transport. Can. J. Vet. Res. 66(3), 211. Burr, J.R., Reinhart, G.A., Swenson, R.A., Swaim, S.E., Vaughn, D.M. and Bradley, D.M. 1997. Serum biochemical values in sled dogs before and after competing in long-distance races. JAVMA 211, 175–179. Chanoit, G.P., Lefebvre, H.P., Orcel, K., Laroute, V., Toutain, P.L. and Braun, J.P. 2001. Use of plasma creatine kinase pharmacokinetics to estimate the amount of exercise-induced muscle damage in Beagles. Am. J. Vet. Res. 62(9), 1375–1380. Cohen, T., Simmons, S.C., Pham, H.P. and Staley, E.M. 2021. Granulocyte transfusion: clinical updates and a practical approach to transfusion. Clin. Lab. Med. 41(4), 647–657. Davis, M.S., Davis, W.C., Ensign, W.Y., Hinchcliff, K.W., Holbrook, T.C. and Williamson, K.K. 2008. Effects of training and strenuous exercise on hematologic values and peripheral blood leukocyte subsets in racing sled dogs. J. Am. Vet. Med. Assoc. 232(6), 873–878. Dennis, M.M., Nelson, S.N., Cantor, G.H., Mosier, D.A., Blake, J.E. and Basaraba, R.J. 2008. Assessment of necropsy findings in sled dogs that died during Iditarod Trail sled dog races: 23 cases (1994–2006). J. Am. Vet. Med. Assoc. 232(4), 564–573. Englar, R.E. 2017. Part two performing the canine physical examination. In Performing the small animal physical examination, 1st edition. Ed., R.E. Englar. New Jersey, NJ: John Wiley & Sons, Inc, pp: 544–1115. Erjavec, V., Vovk, T. and Nemec Svete, A. 2022. The effect of two acute bouts of exercise on oxidative stress, hematological, and biochemical parameters, and rectal temperature in trained canicross dogs. Front. Vet. Sci. 9, 767482. Ermon, V., Yazwinski, M., Milizio, J.G. and Wakshlag, J.J. 2014. Serum chemistry and electrolyte alterations in sled dogs before and after a 1600 km race: dietary sodium and hyponatraemia. J. Nutr. Sci. 3, 26. Ferasin, L. and Marcora, S. 2009. Reliability of an incremental exercise test to evaluate acute blood lactate, heart rate and body temperature responses in Labrador retrievers. Comp. Exerc. Physiol. 179, 839–845. Hall, E.J. and Carter, A.J. 2017. Comparison of rectal and tympanic membrane temperature in healthy exercising dogs. Comp. Exerc. Physiol. 13(1), 37–44. Heber, S. and Volf, I. 2015. Effects of physical (in)activity on platelet function. Biomed. Res. Int. 2015(1), 165078. Hens, K. 2009. Ethical responsibilities towards dogs: an inquiry into the dog–human relationship. J. Agric. Environ. Ethics 22, 3–14. Hinchcliff, K., Constable, P. and Disilvestro, R. 2004. Muscle injury and antioxidant status in sled dogs competing in a long-distance sled dog race. Comp. Exerc. Physiol. 1(1), 81–85. Hinchcliff, K.W., Olson, J., Crusberg, C., Kenyon, J., Long, R., Royle, W. and Burr, J. 1993. Serum biochemical changes in dogs competing in a long-distance sled race. J. Am. Vet. Med. Assoc. 202(3), 401–405. Hinchcliff, K.W., Reinhart, G.A., Burr, J.R. and Swenson, R.A. 1997b. Exercise-associated hyponatremia in Alaskan sled dogs: urinary and hormonal responses. J. Appl. Physiol. 83(3), 824–829. Hinchcliff, K.W., Reinhart, G.A., Burr, J.R., Schreier, C.J. and Swenson, R.A. 1997a. Effect of racing on serum sodium and potassium concentrations and acid-base status of Alaskan sled dogs. J. Am. Vet. Med. Assoc. 210(11), 1615–1618. Ivasovic, F., Matos, J.N., Wyler, M. and Glaus, T.M. 2023. Effects of breed, exercise, and a two-month training period on NT-proBNP-levels in athletic dogs. Anim 13(1), 16. Jahr, T.H., Fergestad, M.E., Brynildsrud, O., Brun-Hansen, H. and Skancke, E. 2019. Haematological and serum biochemical values in Norwegian sled dogs before and after competing in a 600 km race. Acta Vet. Scand. 61, 1–9. Jensen, T.E. and Richter, E.A. 2012. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 590(5), 1069–1076. Johnson, R.A. and Autran de Morais, H. 2006. Chapter 11—Respiratory acid-base disorders. In Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. Ed., Dibartola, S.P. Philadelphia, PA: W.B. Saunders, pp: 283–296. Klein, R., Nagy, O., Tóthová, C. and Chovanová, F. 2020. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet. Med. Int. 1(1), 5346483. Kong, N., Chen, G., Wang, H., Li, J., Yin, S., Cao, X., Wang, T., Li, X., Li, Y., Zhang, H., Yu, S., Tang, J., Sood, A., Zheng, Y. and Leng, S. 2021. Blood leukocyte count as a systemic inflammatory biomarker associated with a more rapid spirometric decline in a large cohort of iron and steel industry workers. Respir. Res. 22, 1–13. Lopedote, M., Valentini, S., Musella, V., Vilar, J.M. and Spinella, G. 2020. Changes in pulse rate, respiratory rate and rectal temperature in working dogs before and after three different field trials. Anim 10(4), 733. Lumsden, J.H., Mullen, K. and McSherry, B.J. 1979. Canine hematology and biochemistry reference values. Can. J. Comp. Med. 43(2), 125. Matwichuk, C.L., Taylor, S., Shmon, C.L., Kass, P.H. and Shelton, G.D. 1999. Changes in rectal temperature and hematologic, biochemical, blood gas, and acid-base values in healthy Labrador Retrievers before and after strenuous exercise. Am. J. Vet. Res. 60(1), 88–92. McKenzie, E.C., Jose-Cunilleras, E., Hinchcliff, K.W., Holbrook, T.C., Royer, C., Payton, M.E., Williamson, K., Nelson, S., Willard, M.D. and Davis, M.S. 2007. Serum chemistry alterations in Alaskan sled dogs during five successive days of prolonged endurance exercise. J. Am. Vet. Med. Assoc. 230(10), 1486–1492. Mennitti, C., Farina, G., Imperatore, A., De Fonzo, G., Gentile, A., La Civita, E., Carbone, G., De Simone, R.R., Di Iorio, M.R., Tinto, N., Frisso, G., D’Argenio, V., Lombardo, B., Terracciano, D., Crescioli, C. and Scudiero, O. 2024. How does physical activity modulate hormone responses?. Biomol 14(11), 1418. Milena, L., Widyastuti, S. and Kendran, A.A.S. 2023. Profil Eritrosit Anjing Pelacak di Kepolisian Negara Republik Indonesia Resor Kota Malang. Bul. Vet. Udayana 15(40), 540–544. Millard, R. 2014. Exercise physiology of the canine athlete. In Canine rehabilitation and physical therapy, 2nd ed. Eds., D. Millis and D. Levine. Philadelphia, PA: W.B. Saunders, pp: 162–179. Radisavljević, K., Vučinić, M., Becskei, Z., Stanojković, A. and Ostović, M. 2017. Comparison of stress level indicators in blood of free-roaming dogs after transportation and housing in the new environment. J. Appl. Anim. Res. 45(1), 52–55. Rijnberk, A. and Stokhof A.A. 2009. Chapter 08—General examination. In Medical history and physical examination in companion animals, 2nd ed. Eds., A. Rijnberk and F.J. van Sluijs. Philadelphia, PA: W.B. Saunders, pp: 47–62. Rizza, R.A., Cryer, P.E., Haymond, M.W. and Gerich, J.E. 1980. Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metab 29(11), 1155–1163. Robbins, P.J., Ramos, M.T., Zanghi, B.M. and Otto, C.M. 2017. Environmental and physiological factors associated with stamina in dogs exercising in high ambient temperatures. Front. Vet. Sci. 4, 144. Rovira, S., Muñoz, A. and Benito, M. 2007. Hematologic and biochemical changes during canine agility competitions. Vet. Clin. Pathol. 36(1), 30–35. Rovira, S., Munoz, A. and Benito, M. 2008. Effect of exercise on physiological, blood and endocrine parameters in search and rescue-trained dogs. Vet. med. (Praha). 53(6), 333. Shull, S.A., Rich, S.K., Gillette, R.L. and Manfredi, J.M. 2021. Heart rate changes before, during, and after treadmill walking exercise in normal dogs. Front. Vet. Sci. 8, 641871. Steiss, J., Ahmad, H.A., Cooper, P. and Ledford, C. 2004. Physiologic responses in healthy Labrador Retrievers during field trial training and competition. J. Vet. Intern. Med. 18(2), 147–151. Steiss, J.E. and Wright, J.C. 2008. Respiratory alkalosis and primary hypocapnia in Labrador Retrievers participating in field trials in high–ambient-temperature conditions. Am. J. Vet. Res. 69(10), 1262–1267. Stephen, P. and DiBartola. 2012. 6—Electrolyte and acid-base disorders. In Small animal clinical diagnosis by laboratory methods, 5th ed. Eds., M.D. Willard and H. Tvedten. St. Louis, MO: Elsevier Health Sciences, pp: 112–125. Vorobjeva, N.V., Chelombitko, M.A., Sud’Ina, G.F., Zinovkin, R.A. and Chernyak, B.V. 2023. Role of mitochondria in the regulation of effector functions of granulocytes. Cells 12(18), 2210. Wagner, J.A., Horvath, S.M. and Dahms, T.E. 1977. Cardiovascular, respiratory, and metabolic adjustments to exercise in dogs. J. Appl. Physiol. 42(3), 403–407. Zaid, N.S.N., Muhamad, A.S., Jawis, M.N., Ooi, F.K., Mohamed, M., Mohamud, R., Hamdan, N.F. and Jusoh, N. 2024. The effect of exercise on immune response in population with increased risk factors for cardiovascular disease: a systematic review. MJMS 31(5), 83. | ||
| How to Cite this Article |
| Pubmed Style Simatupang GTTO, Widiyono I, Yanuartono Y, Salsabila RAI. Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Vet. J.. 2025; 15(9): 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 Web Style Simatupang GTTO, Widiyono I, Yanuartono Y, Salsabila RAI. Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. https://www.openveterinaryjournal.com/?mno=264096 [Access: January 11, 2026]. doi:10.5455/OVJ.2025.v15.i9.7 AMA (American Medical Association) Style Simatupang GTTO, Widiyono I, Yanuartono Y, Salsabila RAI. Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Vet. J.. 2025; 15(9): 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 Vancouver/ICMJE Style Simatupang GTTO, Widiyono I, Yanuartono Y, Salsabila RAI. Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Vet. J.. (2025), [cited January 11, 2026]; 15(9): 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 Harvard Style Simatupang, G. T. T. O., Widiyono, . I., Yanuartono, . Y. & Salsabila, . R. A. I. (2025) Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Vet. J., 15 (9), 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 Turabian Style Simatupang, Grace Tabitha Tenggi Olihta, Irkham Widiyono, Yanuartono Yanuartono, and Ridha Avicena Ila Salsabila. 2025. Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Veterinary Journal, 15 (9), 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 Chicago Style Simatupang, Grace Tabitha Tenggi Olihta, Irkham Widiyono, Yanuartono Yanuartono, and Ridha Avicena Ila Salsabila. "Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate." Open Veterinary Journal 15 (2025), 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 MLA (The Modern Language Association) Style Simatupang, Grace Tabitha Tenggi Olihta, Irkham Widiyono, Yanuartono Yanuartono, and Ridha Avicena Ila Salsabila. "Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate." Open Veterinary Journal 15.9 (2025), 4024-4031. Print. doi:10.5455/OVJ.2025.v15.i9.7 APA (American Psychological Association) Style Simatupang, G. T. T. O., Widiyono, . I., Yanuartono, . Y. & Salsabila, . R. A. I. (2025) Effect of training on physiological, hematological, and chemical parameters in Belgian Malinois Dogs in Indonesia’s tropical climate. Open Veterinary Journal, 15 (9), 4024-4031. doi:10.5455/OVJ.2025.v15.i9.7 |