| Review Article | ||
Open Vet. J.. 2025; 15(9): 3912-3930 Open Veterinary Journal, (2025), Vol. 15(9): 3912-3930 Review Article Glanders: Historical military use and potential bioterrorism concernMohammad Sukmanadi1, Aswin Rafif Khairullah2, Bantari Wisynu Kusuma Wardhani3, Imam Mustofa4*, Siti Hamidatul Aliyah5, Ikechukwu Benjamin Moses6, Riza Zainuddin Ahmad2, Andi Thafida Khalisa7, Bima Putra Pratama8, Muhammad Khaliim Jati Kusala2, Dea Anita Ariani Kurniasih9, Adeyinka Oye Akintunde10, Ima Fauziah2, Syahputra Wibowo11, Abdul Hadi Furqoni5, Kartika Afrida Fauzia12, Irma Melati13 and Muhammad ‘Ahdi Kurniawan141Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Center for Biomedical Research, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 7Faculty of Military Pharmacy, Universitas Pertahanan, Bogor, Indonesia 8Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia 9Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Department of Agriculture and Industrial Technology, Babcock University, Ikenne, Nigeria 11Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 12Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 13Research Center for Limnology and Water Resources, National Research and Innovation Agency (BRIN), Bogor, Indonesia 14Medical Biotechnology Research Group, Virtual Research Center for Bioinformatics and Biotechnology, Surabaya, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 15/06/2025 Revised: 05/08/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBurkholderia mallei, the etiological agent of glanders, is a zoonotic bacterium primarily affecting equines and occasionally humans. Although rare today, it remains endemic in certain regions and poses a potential threat to both animal and public health. Historically, B. mallei was among the earliest biological agents used in warfare, and its classification as a Category B bioterrorism agent underscores the need for ongoing vigilance. This review is warranted due to several factors: the limited availability of effective treatments or vaccines, the pathogen’s ability to cause severe systemic infections, its diagnostic challenges, and its re-emerging status in some parts of the world. Moreover, growing concern over its potential misuse in bioterrorism highlights the urgency for a comprehensive understanding of the bacterium. The aim of this review is to provide a consolidated overview of B. mallei and glanders, emphasizing pathogenesis, clinical manifestations in animals and humans, diagnostic methods, differential diagnoses, and current prevention strategies. It also discusses the historical use of B. mallei as a biological weapon and its implications for modern biosecurity. By synthesizing existing knowledge, this review contributes to a more structured understanding of glanders and highlights knowledge gaps that require further research. Raising awareness of this neglected zoonosis is essential to strengthen surveillance, improve diagnostic capabilities, and inform policies aimed at mitigating future outbreaks or misuse. Keywords: B. mallei, Bioterrorist, Glanders, Public health, Zoonosis. IntroductionGlanders is a neglected but highly consequential zoonotic disease caused by Burkholderia mallei, primarily affecting equines such as donkeys, horses, and mules, and occasionally humans (Gaspar et al., 2023; Alikhanov et al., 2024). While the pathogen was first isolated in 1882, and glanders has been eradicated from many industrialized countries, sporadic outbreaks continue to occur in Asia, Africa, and parts of Latin America, underscoring its persistence as a transboundary threat (Kettle and Wernery, 2016; Pal and Paulos Gutama, 2022). This persistence contrasts with the global decline of many other equine diseases, suggesting that glanders is not only an epidemiological relic but also a re-emerging infection that thrives in under-resourced veterinary systems. Unlike strictly historical diseases, its recurrence highlights the need for sustained scientific and policy attention. Beyond its natural epidemiological burden, B. mallei has unique historical and contemporary relevance. It was among the earliest pathogens used as a biological warfare agent during World Wars I and II, and it remains classified as a Tier 1 Select Agent by the Centers for Disease Control and Preventiondue to its aerosol transmissibility, high lethality, and diagnostic challenges (Varga et al., 2012; Cote et al., 2020). Burkholderia mallei remains classified as a Tier 1 Select Agent due to its transmissibility and lethality (CDC, 2000; CDC, 2024). this dual identity—as both a neglected zoonosis and a biothreat agent—distinguishes glanders from many other infectious diseases, placing it at the intersection of veterinary medicine, public health, and international security. The implications extend well beyond equine health, raising concerns about biodefense preparedness, laboratory safety, and global surveillance. Historically, several reviews have summarized the general clinical and epidemiological features of glanders (e.g., Al-Ani and Roberson, 2007; Van Zandt et al., 2013). However, most existing reviews remain descriptive, focusing heavily on history, case reports, and classical pathology, while providing limited synthesis of recent scientific advancements. Over the past decade, important new findings have reshaped our understanding of B. mallei. For example, genomic studies have revealed an unusually high rate of genetic variation in this bacterium, comparable to RNA viruses, which may underlie its capacity to evade host immune defenses and complicate vaccine development (Memisević et al., 2013; Charron et al., 2023). This discovery challenges the long-standing assumption that bacterial pathogens are genetically stable and highlights why glanders research can inform broader questions of microbial evolution. Epidemiologically, glanders has often been considered rare or declining, yet recent serological surveys and outbreak reports from South Asia, the Middle East, and South America reveal patterns of underdiagnosis and underreporting (Singha et al., 2020; Resende et al., 2022). In some cases, countries that declared themselves glanders-free experienced reintroductions, linked to uncontrolled horse movement across borders and insufficient veterinary capacity. This suggests that the official absence of glanders may reflect diagnostic blind spots rather than true eradication, creating a false sense of security in trade and animal health policy. Such analytical insights are largely missing in prior reviews, which tend to present prevalence data without interrogating their reliability or implications. Another critical gap lies in clinical and pathological interpretation. While glanders in animals and humans presents with fever, respiratory distress, nodules, and septicemia, the overlap with other infectious diseases complicates early recognition. Prior reviews list these differential diagnoses, but few provide a comparative analysis that could guide clinicians toward a more accurate distinction in endemic settings. In practice, this diagnostic ambiguity increases both animal and human mortality, especially where laboratory confirmation is limited. Treatment and prevention remain equally challenging. Horses testing positive are typically culled to prevent further spread, and no vaccine is available. For humans, long-term antibiotic therapy has been attempted, often by extrapolating from melioidosis treatment protocols (Wiersinga et al., 2018). Yet systematic evaluation of these regimens is sparse, and recent experimental studies on novel antibiotics and immunological interventions have not been comprehensively reviewed. By integrating these scattered data, this article aims to provide a more evidence-based assessment of therapeutic prospects. The biosecurity dimension further elevates the importance of glanders research. The deliberate weaponization of B. mallei by Germany and Japan during the World Wars, and its continued classification as a potential bioterrorism agent, underscore enduring security concerns (Koenig and Schultz, 2016). However, existing literature often recounts this history without linking it to current biodefense strategies. A more critical discussion is needed, particularly regarding gaps in preparedness, laboratory containment policies, and the integration of glanders surveillance into broader frameworks for high-risk pathogens. Therefore, this review does not merely reiterate the clinical and historical aspects of glanders. Its novelty lies in synthesizing recent molecular insights, critically reassessing epidemiological patterns in the light of underreporting, and analyzing the biosecurity implications in the context of modern biodefense. By highlighting these elements, the review contributes to a more forward-looking understanding of glanders, framing it as both a neglected zoonotic disease and a pathogen of global security significance. In doing so, it underscores the urgent need for interdisciplinary collaboration across veterinary medicine, microbiology, public health, and policy to close the current knowledge and preparedness gaps. EtiologyB. mallei is a facultative intracellular rod-shaped bacterium that is straight or slightly curved, measuring 2–5 µ in length and 0.3–0.8 µ in width (Whitlock et al., 2009). It does not generate spores. These bacteria are required to be aerobes, with the exception of media that include nitrate (Mangalea et al., 2017). Glycerol-containing media are preferred by aerobic-growing bacteria as an enrichment agent. On Glycerol Dextrose Agar, there was a smooth, moist, and thick, cream-colored, unified growth after 24 hours of incubation (Kinoshita et al., 2019). Continued incubation causes the growth to thicken, darken, and harden. Electron microscopy has shown a capsule-like layer. The neutral carbohydrate capsule shields cells from adverse environmental conditions (Llobet et al., 2008). Burkholderia pseudomallei is closely related to this bacterium, but B. mallei is not motile and does not have flagella (Gilad et al., 2007). This organism has a beaded appearance and is challenging to show in tissue slices. The age of the culture and the kind of media influence how they look in cultural media. Older cultures exhibit a great deal of pleomorphism in the organism (Jilani et al., 2023). B. mallei is sensitive to the environment and is killed by the majority of conventional disinfectants, including formalin, chlorine, copper sulfate, potassium permanganate, and phenol, within 24 hours of being exposed to direct sunlight (Shams et al., 2011). The organism can live for up to 4 weeks in clean water, around 6 weeks in contaminated cages, and 3–5 weeks in moist media and decomposing matter (Gaspar et al., 2023). HistoryHippocrates identified the earliest indications of glanders disease when he documented its clinical manifestations between 450 and 425 BC (Júnior et al., 2020). One hundred years later, Aristotle used the broad term for epizootics to describe the sickness and called it “melis.” He also mentioned that humans were unintentional hosts and that horses were the disease’s natural reservoir (Neubauer et al., 2005). Glanders’ relationship with battle was expanded during the fourth century AD, when Apsyrtus worked as a veterinarian in Constantine the Great’s army (Torres, 2025). Furthermore, Vegetius was named by Roman military historians in the fifth century AD, who also referred to the illness as “malleus” Historical laboratory-acquired infections have been documented during World War II (Howe and Miller, 1947; Herr et al., 1985). The French King, Louis XV, commissioned the first veterinary school in Lyons, led by Claude Bourgelat, in 1761 to study Glanders in an attempt to safeguard the French Army’s horses because the region was well-known during the Crusades and other military conflicts (Wilkinson, 1981). Glanders was recognized as an infectious disease in 1876 after Viborg proved its contagiousness in 1797 (Torres, 2025). In 1882, he isolated the bacterium, also known as Burkholderia mallei, from the liver and spleen of afflicted horses in Germany (Lauman and Dennis, 2021). In 1956, Henning named Pfeifferella mallei (Al-Ani and Roberson, 2007). The authors gave it the name Pseudomonas mallei that same year (Wetmore and Gochenour, 1956). Furthermore, Evans named Actinobacillus mallei in 1966 (Evans, 1966). Following that, Merchant & Packer gave it the name Malleomyces mallei (Merchant and Packer, 1967). B. mallei is the common name used today (Yabuvchi et al., 1992). Host rangeSolipeds, or mammals with a single hoof on each foot, are the natural reservoir of B. mallei (Wiersinga et al., 2018). In horses, this condition is typically chronic; in donkeys and mules, it manifests as an acute form that frequently results in death (Van Zandt et al., 2013). Although the majority of other domesticated mammals can contract the disease experimentally, pigs, cattle, and chickens have shown resistance to it (Pal et al., 2022). There have also been reports of the disease developing spontaneously in a number of species, such as cats, dogs, and goats (Khan et al., 2013). Occupational groups at high risk for glanders include laboratory workers, stable keepers, blacksmiths, veterinarians, horse groomers, and horse slaughterers (Kettle and Wernery, 2016). EpidemiologyGlanders is reported to be endemic in horses in the Middle East and in Asian nations like Bangladesh, Iran, and Mongolia (Khaki et al., 2012; Rahman et al., 2018; Erdemsurakh et al., 2020b). According to Office International des Epizooties (OIE) data, Glanders is prevalent in China (Zheng et al., 2019) and Russia (Zakharova et al., 2018), and it has also been documented in Vietnam (Brightman and Locum, 2020), Korea (Lee et al., 2010), and Pakistan (Khan et al., 2012). Rose Bengal Test (RBT) and Complement Fixation Test (CFT) results indicate that the prevalence of the disease among Mongolian horses ranges from 7.7% to 8.3%. They discovered that compared to endemic native Mongolian horses, there was a greater correlation between seropositive horses produced by crossing native Mongolian horses and thoroughbred horses (Erdemsurakh et al., 2020b). Additionally, researchers in Pakistan’s Punjab province used CFT to detect antibodies against B. mallei, followed by western blot analysis (Khan et al., 2012). Table 1 describes the incidence of Glanders by country, endemic status, diagnostic method, and Seroprevalence of glanders in Mongolian horses was reported at 7.7%–8.3% (Erdemsurakh et al., 2020a). Glanders is also endemic in India, and reports of seroprevalence by indirect Enzyme-linked Immunosorbent Assay (ELISA) and CFT of 0.62% and 1.145% have been made in some areas. Scientists have observed that cases have returned to Maharashtra, Haryana, and Punjab after a 10-year period in which these states were free of Glanders. It is believed that glanders may not have been truly eradicated but instead remained undiagnosed and underreported due to low disease awareness, inadequate veterinary services, and uncontrolled movement of horses across state borders (Singha et al., 2020). Furthermore, two strains of B. mallei seemed to have come from the same region by way of the importation of sick animals, as was shown in the Glanders outbreak in Bahrain (Scholz et al., 2014). Table 1. Glanders incidents in several countries that have been reported.
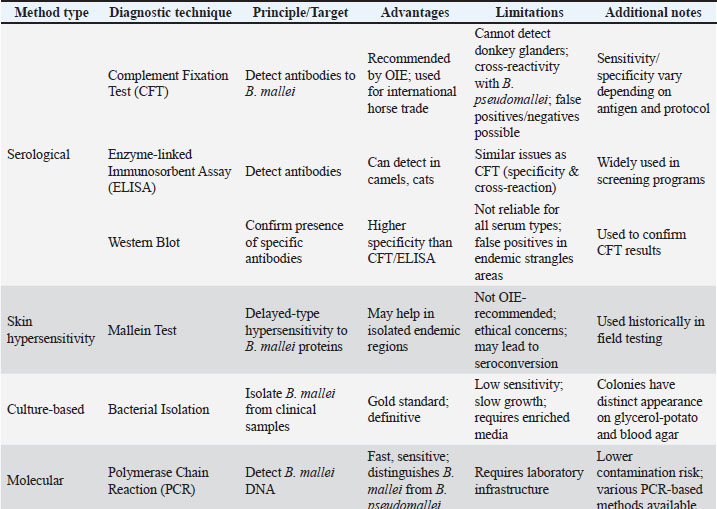
Glanders remains a major issue in Brazil, where many new cases are reported each year, impacting the animal trade and resulting in significant financial losses (Resende et al., 2022). Given the numerous recent outbreaks reported across multiple countries, glanders is regaining attention as a re-emerging infectious disease of concern (Khan et al., 2013). Asia and certain regions of Africa and South America are thought to be endemic for the new disease (Torres, 2025). Horses and camels carrying glanders serve as a reservoir for the return of glanders into nations that were previously designated as glanders-free (Kettle and Wernery, 2016). The Central Veterinary Laboratory (CVRL) in Dubai is the official organization in charge of glanders research, surveillance, and eradication in the Arab world. It is also the official OIE reference laboratory for glanders (Hmood and Al-Amery, 2022). All animal-related procedures were performed using established protocols and in compliance with OIE’s animal welfare guidelines. By the early 20th century, glanders had been exterminated in the majority of developed nations, which was a significant indication of advancements in veterinary medicine, diagnostic capabilities, and disease control measures. Glanders was exterminated in Australia, Japan, North America, and Western Europe (Cárdenas et al., 2019). PathogenesisB. mallei enters the body naturally through the gastrointestinal system, integument, and mucous membranes of the eyes and nose (Syed and Wooten, 2021). Bacteria enter the circulation and internal organs, especially the lungs, by penetrating the mucosa of the colon or oropharynx and traveling via lymphatic vessels to the regional lymph nodes (Whitlock et al., 2009). It then travels via the bloodstream to produce sores on the nose, skin, and nodes. The organism enters the body through the skin and travels into the lymphatic passages, causing lymphangitis (Siggins and Sriskandan, 2021). Terminal signs, including bronchopneumonia and death, are primarily caused by anoxia resulting from progressive respiratory failure Genomic studies highlight the pathogen’s adaptability and virulence (Kyle et al., 2015). Lesions in humans can be found in the skin, skeletal muscle, bones, joints, lymph nodes, spleen, liver, and, less commonly, the brain, meninges, nose, and eyes (Verma et al., 2014). It was once believed that glanders had no effect on human or animal bones, but later research has shown that mules, humans, and experimental hamsters can all develop bone lesions (Fritz et al., 1999). B. mallei’s primary defenses against phagocytosis include intracellular localization and the existence of a capsule and capsular lipopolysaccharide (LPS) (Bzdyl et al., 2022). The two main virulence factors that glanders bacilli produce in vivo during infection are overexpression of type VI secretion protein (T6S) and a functioning type III secretion system (Schell et al., 2007). According to recent in vitro research, B. mallei significantly influences murine macrophages by inducing the expression of inducible nitric oxide synthase (iNOS), which is essential for the elimination of bacteria by activated macrophages (Brett et al., 2008). Once B. mallei has briefly passed through a number of mammalian hosts, including humans, its genome changes at a very high rate (Romero et al., 2006). Since only RNA viruses have the ability to rapidly alter their genomes as a key part of their strategy to elude the host immune response, it is the first and only bacterial pathogen to possess this ability (Memisević et al., 2013). B. mallei may express more virulence genes during in vivo infection as a result of this substantial and quick genomic variation. They also found that B. mallei uses a mutant gene that codes for a penicillin-binding protein (PBP-1c) involved in cell wall production and β-lactam resistance to avoid immunological detection and phagocytic clearance in vivo (Charron et al., 2023). The organism B. mallei is not kept as a clonal population like other organisms, but rather as a population of variant or mutant organisms within the host (Whitlock et al., 2009). The development of vaccines and treatments for glanders disease may be impacted by this genetic instability during transmission. Immune responseAlthough Burkholderia species may utilize different pathogenic mechanisms, invasion and adherence to host epithelial cells are essential stages of infection and appear to play a major role in overall virulence (David et al., 2015). B. mallei depends on the deliberate application of numerous virulence factors and mechanisms to control various host processes and pathways in order to successfully infect host cells recent molecular analyses provide novel insights into virulence pathways (Nikolakakis et al., 2024). Nine B. mallei virulence factors and their interactions with host proteins were recently extensively evaluated using a combination of computational and experimental methods in an effort to clarify the mechanisms behind B. mallei pathogenicity (Memisević et al., 2013). According to topological research of B. mallei–host protein–protein interactions, the parasite targets intracellular multifunctional host proteins, host proteins that engage in mutual contact, and proteins with several interacting partners. The focal adhesion pathway and the ubiquitination degradation system are two host mechanisms that are significantly impacted by these protein-protein interactions (Memišević et al., 2015). These findings are in line with earlier research that documented connections between the TssN protein and the proteins cullin-1a and polyubiquitin-B. This host protein interacts with two elements necessary for Toll-like receptor (TLR) signaling: inhibitor of IκB-α and tumor necrosis factor (TNF) receptor-related factor 6 (Duan et al., 2022). This study sheds some light on B. mallei’s pathophysiology and supports the theory that the pathogen alters the innate immune response by either directly or in concert with other harmful proteins interfering with host ubiquitination. A thorough analysis of murine macrophages infected with a variety of Burkholderia species reveals the production of the cytokines TNFα, interleukin 1β (IL-1β), and the chemokine murine keratinocyte-derived protein, which is the murine counterpart of human IL-8 (Chiang et al., 2015). The fact that B. mallei-infected macrophages release noticeably more IL-6 and IL-10 than B. pseudomallei-infected macrophages suggests that these two pathogens alter host signaling cascades in different ways (Lu et al., 2012). Furthermore, compared to other Burkholderia species, macrophages infected with B. mallei exhibited considerably greater levels of expression of IL-1β, IL-10, TNF receptor superfamily member 1B, and IL-36α mRNA, indicating gene-based variations in the host inflammatory response specific to B. mallei (Chin et al., 2010). Further analysis of the infected macrophages revealed increased phosphorylation of adenosine monophosphate-activated protein kinases, as well as mitogen-activated protein kinases (such as p38, extracellular signal-regulated kinase 1/2, and c-Myc) and regulators of the nuclear factor-kappa B signaling pathway [such as IκB-α inhibitor, glycogen synthase kinase (GSK) 3β, Src, and STAT1] (Aiba et al., 2003). The observed variations in pathogenicity among Burkholderia species are correlated with the degree of modulation of target host proteins or processes. B. mallei is a stronger inducer of interferon-gamma (IFNβ) production and iNOS expression in infected macrophages than B. pseudomallei (Brett et al., 2008). Based on these findings, a representative network of signaling routes and axes was built to explain how signaling cascades are activated in response to Burkholderia spp. infection, in addition to what is currently known about signal transduction. The canonical pathway downstream of TLR4 indicates that the induction of phosphorylated forms of GSK3β, Src, and adenosine monophosphate-activated protein kinase-α1 is crucial in controlling the inflammatory response of Burkholderia spp’s infection (Escobar et al., 2015). LPS is a powerful inducer of the host’s innate immune response and a significant part of the outer membrane of Gram-negative bacteria (Maldonado et al., 2016). Studies of the structure-activity relationship of TLR4 agonists reveal a correlation between the lipid A moiety’s composition and LPS’s biological activity (Matamoros-Recio et al., 2023). The assessment of B. mallei LPS revealed that its biological activity was more influenced by lipid A acylation than by its length (Brett et al., 2007). Therefore, B. mallei LPS, which is comparable to B. pseudomallei LPS and has penta-acylated lipid A with 4-amino-4-deoxyarabinose in nearly half of its molecules, may be linked to the overall differential macrophage activation. It also seems to be a weaker macrophage activator than enterobacterial LPS (Korneev et al., 2015). This is supported by the fact that when macrophages were stimulated with isolated B. mallei LPS as opposed to those treated with E. coli LPS, there was a notable reduction in the mRNA expression or secretion of IL-6, TNFα, and IL-1β (Duan et al., 2023). The production of IFN-dependent genes and mediators (IFNβ and Nitric oxide), as well as cytokines (TNFα, IL-6, IL-10, Granalocyte-macrophage colony stimulating factor, and normal T cells was similarly lower in B. mallei-infected macrophages than in E. coli-infected macrophages (Chen et al., 2023). In order to create a persistent infection, B. mallei needs to get past a number of antibacterial processes and products (such as adenosine monophosphate and reactive oxygen and nitrogen species) that are necessary for innate immunity (Saikh and Mott, 2017). The capacity of B. mallei isolates obtained from mice spleens at Frederick Memorial Hospital (FMH) to multiply and cause cytotoxicity in macrophage tests was diminished 60 days after infection (Bernhards et al., 2017). Because of the loss of O-specific polysaccharide (OPS) during infection, one isolate of B. mallei displayed a change in its LPS phenotype from smooth to rough. It is known that these phenotypic alterations occur when an infection switches from an acute to a chronic or subclinical form, which is less likely to elicit an immunological response from the host. Prior research has indicated that the persistence of B. pseudomallei and B. mallei may be linked to genetic and phenotypic traits (Massey et al., 2014). The genetic basis for the loss of OPS may be revealed by more research, such as sequencing the OPS biosynthetic gene cluster of this FMH B. mallei strain. A key feature of persistent Pseudomonas aeruginosa infection is the alteration or loss of OPS (Bernhards et al., 2017). PathologyThe pathognomonic pathology of Glanders does not provide a conclusive pathological diagnosis. Nodules or ulcers are typically present in a variety of tissues, including the liver, spleen, pleura, lungs, and upper respiratory tract (Mota et al., 2010). Fibrosis, congestion, or nodules may expand the lymph nodes. Infected stallions have also been reported to develop orchitis due to systemic spread of the infection (Spickler, 2018). Histopathology demonstrating hemorrhage and vasculitis together with skin glanders (Carlson, 2010). Also common are lymph node foci of pyogranulomatous inflammation (Mota et al., 2010). Nasal glanders are linked to damage to the nasal epithelium and septum, as well as significant widespread purulent inflammation (López and Martinson, 2017). Similar to the skin glands, the nasal glands can also experience hemorrhage, vasculitis, and abscesses that progress into granulomatous lesions (Zachary, 2017). The lungs of pulmonary glanders have granulomatous or pyogranulomatous lesions (Fritz et al., 1999). Inflammatory cells, such as macrophages, giant cells, lymphocytes, and plasma cells, encircle the lesion, which has a central region of caseous necrosis (Kumar et al., 2013). The lung tissue of horses with pulmonary glanders has also been seen to exhibit intra-alveolar fibrin deposits, localized bleeding, and areas of edema (López and Martinson, 2017). Clinical symptomsIn animalsHorse glanders can manifest as nasal, pulmonary, or cutaneous, contingent on the location of the initial lesion. The illness could be chronic or acute. Acutely affected horses pass away in a matter of days to weeks (Raj et al., 2024). Critically ill animals frequently experience respiratory symptoms, septicemia, a high temperature, weight loss, and thick mucopurulent nasal discharge after an incubation period of 3–2 weeks (Pal et al., 2016). The nasal form is characterized by a high temperature and lack of appetite, coughing and shortness of breath, eye discharge, sticky yellowish green nasal discharge, nasal nodules and ulcers, and star-shaped crusted ulcers (Pal and Paulos Gutama, 2022). The most prevalent kind, the pulmonary form, develops more slowly than the nasal form but is nonetheless intense. The most prevalent clinical signs of the pulmonary form are as follows: upper respiratory tract infections, pneumonia, pulmonary nodules or abscesses, dry cough, and shortness of breath (Siddique et al., 2023). In contrast to the other two skin types, this one is a chronic infection that begins with minor to undetectable signs before becoming a crippling illness (Torres, 2025). The most typical symptoms include exacerbation episodes, cough, fever, ulcerating and rupturing skin nodules, fluid draining from nodules, sluggish nodule healing, swollen lymph nodes, and joint swelling (Khan et al., 2013). The clinical manifestations of spontaneous infection in dromedary camels are similar to those observed in horses (Khalafalla, 2016). Cats that have consumed contaminated meat may develop nodules and ulcers in their nasal passages, on the conjunctiva, and deeper in their respiratory tract (Rahman et al., 2020). Infected cats also have a purulent yellowish nasal discharge, which can occasionally be bloody. Dyspnea and enlarged lymph nodes are further symptoms, and infected cats typically pass away in 1–2 weeks (Morrow and Ruggiero, 2025). Glanders were discovered on five dead lions at the Gulhane Zoo in Istanbul, Turkey. Clinical indicators include nasal mucosal lesions, anorexia, lethargy, ocular discharge, epistaxis, sinusitis, and facial and head edema (Alibasoglu et al., 1986). In humansHuman glanders may present as bacteremia, acute lung infections, persistent cutaneous infections, or localized skin lesions (Júnior et al., 2020). Common symptoms include headache, muscle stiffness, chest pain, fever, and muscle aches. Other symptoms have been recorded, including diarrhea, light sensitivity, and excessive eye tearing (Van Zandt et al., 2013). Localized infections are typically limited to a particular region and are distinguished by suppuration foci. An abscess can drain liquids and ulcerate for a lengthy time (Kianfara et al., 2018). However, localized infections have the potential to spread and result in multitissue infections, septicemia, or pulmonary infections (Virk et al., 2023). The following clinical characteristics were present in eight Fort Detrick laboratory-acquired infections: In order of most frequent occurrence, low-grade fever in the afternoon to evening, malaise, exhaustion, headache, myalgia, including back pain, lymphadenopathy, and chest discomfort (Van Zandt et al., 2013). About 50% of patients have a temporary improvement in their clinical condition following the initial wave of illness symptoms. After this time, which can range from a few days to 2 months, patients exhibit clinical signs of infection (Nasiri et al., 2023). DiagnosisAnimal glanders can be identified by immunological response (serological testing and mallein test) and antigen detection [bacterial culture/culture and molecular detection/polymerase chain reaction (PCR)] (Abreu et al., 2020). The selection of glanders diagnostic techniques is carried out depending on the intent and purpose, whether for surveillance, disease confirmation, or an eradication program. Table 2 explains the comparison of glanders diagnostic methods summarized from your description, including the type of method, working principle, advantages, and limitations. The gold standard method for diagnosing the genus Burkholderia is culture-based bacterial isolation; however, even with newly obtained, sterile samples, isolating these bacteria is extremely challenging (Li et al., 2022). B. mallei isolates can be obtained from blood samples, skin lesions, and exudates from the nasal and upper respiratory tracts (Rocha et al., 2023). B. mallei bacteria require specialized media with the addition of enrichment media, like glycerol, because they grow very slowly and can be readily contaminated by other bacteria (Kinoshita et al., 2019). The B. mallei colony on blood agar or Loeffler serum agar is thick, white, semi-translucent, and about 1 mm in diameter. It will turn yellow on the colony. After 3 days, the bacterial growth on glycerin-potato media will resemble a honey coating before turning brown or reddish brown (Abnaroodheleh et al., 2023). The World Organization for Animal Health does not advise the Mallein test, a hypersensitivity skin test based on water-soluble proteins isolated from microbes, because of concerns about animal welfare (Karimi and Mosavari, 2019). Furthermore, cultural isolation is not a preferred method for glanders diagnosis due to the test’s low sensitivity and time-consuming nature, and it is not approved for use in commercial testing (Elschner et al., 2021). Additionally, as supplementary diagnostic techniques like the CFT, malleinized animals may undergo seroconversion and thereafter exhibit positive results (Abreu et al., 2020). However, in isolated endemic locations, the mallein test might be helpful in eliminating glanders The CFT is recommended by the OIE for glanders surveillance in horses (Elschner et al., 2011). The PCR test is one molecular detection method for glanders that can lower the risk of environmental and human bacterial contamination (Tikmehdash et al., 2024). The species B. mallei and B. pseudomallei can be differentiated by PCR techniques (restriction fragment length polymorphism, pulse-field gel electrophoresis, and 16S rRNA sequencing) (Janesomboon et al., 2021). Other commonly used glander detection techniques include immunofluorescence and latex agglutination (Frolov et al., 2020). The CFT and the ELISA are serological tests that can be used to identify glanders antibodies in horses. Other animals, such as cats and camels, can have glanders detected using the CFT and ELISA tests; however, donkey glanders cannot be detected with CFT (Dehghan Rahimabadi et al., 2023). The OIE recommends the CFT test as a means of checking for glanders in horses that are traded internationally (Elschner et al., 2021). The CFT test’s specificity and sensitivity vary based on the antigen and technique (Elschner et al., 2021). False positives and false negatives are issues with CFT and ELISA serological testing for glander diagnosis. They also cannot distinguish between antibodies from B. mallei and B. pseudomallei (Elschner et al., 2019). Compared to CFT, the Westernblot test is more effective and specific for glander detection using serological means (Elschner et al., 2019). This test can be used in conjunction with other tests and shows great promise in identifying glanders. The western blot test is used to confirm CFT because it is unreliable for testing animal serum with anti-complementary activity or reacting alone with normal antigens; it cannot detect antibodies after 40 days of the disease onset or produce false negatives; and it can cross-react with serum infected with strangles in endemic areas, producing false positives (Elschner et al., 2021). Differential diagnosisClinically, glanders can be mistaken for strangles, sporotrichosis, ulcerative lymphangitis, and epizootic lymphangitis. These diseases are as follows, and due to the stringent control measures mandated by legislation, differential diagnosis must be performed. Table 2. Diagnostic approaches for glanders.
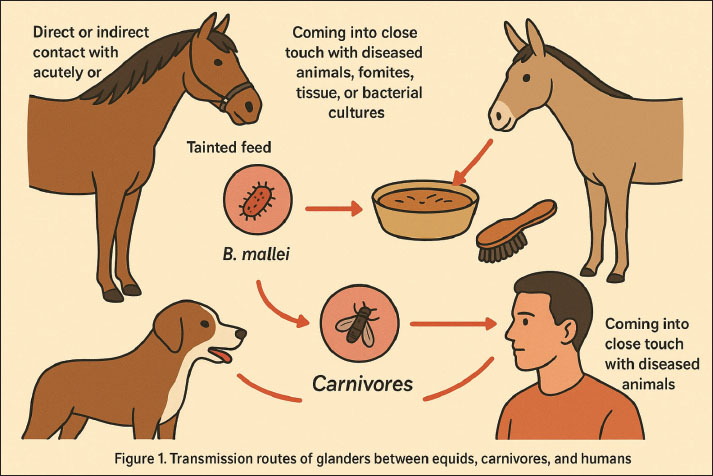
Epizootic lymphangitisHorses are the main victims of epizootic lymphangitis, a persistent granulomatous and suppurative fungal illness (Rebuma et al., 2024). The fungus Histoplasma farciminosum is the cause (Wernery et al., 2025). This condition affects the lymphatic system of the skin, can be minor or asymptomatic, and can appear to resolve yet still be a hidden infection (Seid et al., 2019). Identifying H. farciminosum by direct smear and/or culture allows for a quick diagnosis (Abdela et al., 2021). SporotrichosisOne type of chronic subcutaneous lymphatic mycosis is sporotrichosis (Mahajan, 2014). This disease differs from glanders in that it occurs sporadically and does not follow the consistent epidemiological trends commonly seen in glanders. The gram-positive fungus Sporotrichum schenki, which produces single-walled spores, can be used to positively identify sporotrichosis (Barros et al., 2011). Ulcerative lymphangitisThe bacterial condition known as ulcerative lymphangitis affects horses and cattle and is brought on by Corynebacterium pseudotuberculosis (El-Karim et al., 2024). The development of nodules in the subcutaneous tissue, particularly in the vicinity of the fetlock joint, is a hallmark of this illness (Abebaw, 2024). The causal organism is isolated in order to confirm the diagnosis. StranglesStreptococcus equi is the causative agent of strangles, an acute bacterial disease in horses (Paillot et al., 2010). Abscesses in the surrounding lymph nodes and inflammation of the upper respiratory tract are the hallmarks of this illness (Boyle et al., 2018). Penicillin medication works well for this illness (Pringle et al., 2020). MelioidosisBurkholderia pseudomallei is the cause of melioidosis, often known as Whitmore’s sickness (Wiersinga et al., 2018). Melioidosis and glanders are related conditions. Their epidemiology differs, but their pathophysiological effects are comparable. The disease is indigenous to Southeast Asia, the Philippines, Indonesia, and other tropical regions, where B. pseudomallei flourishes (San Martin et al., 2018; Anggraini et al., 2024). The condition is particularly common in Thailand, where it was the cause of 40% of community-acquired septicemia deaths and 19% of community-acquired sepsis deaths in one hospital (Jatapai et al., 2018). This disease can infect humans as well as other vulnerable animals (Torres, 2023). Tropical regions have a large distribution of this organism in both soil and water (Swe et al., 2021). Humans can contract the disease via inhaling dust or aerosols or by coming into close contact with infected sources (Hsueh et al., 2018). TransmissionAnimals contract glanders primarily through direct or indirect contact with acutely or chronically infected horses, donkeys, or mules (Khan et al., 2013). The most frequent way that the germs are exposed is by tainted feed or water that contains respiratory secretions (Torres, 2025). The animals most commonly seen to contract B. mallei infections from eating meat tainted with the bacteria are carnivores (Libera et al., 2022). B. mallei is transmitted by contact with contaminated fomites (e.g., horse bridles or grooming tools), inhalation of aerosols, damaged skin, or mucous membranes (Peacock et al., 2008). Animals with the infection will excrete microorganisms in their saliva, urine, tears, and feces (Gaspar et al., 2023). Bacterial shedding can happen intermittently or persistently in horses. The number of animals in the cage, their closeness to diseased animals, and high levels of stress brought on by the surroundings are all risk factors for the spread of glanders in animals (Cárdenas et al., 2019). B. mallei can be transferred from sick to healthy animals by the biological vector fly (Musca domestica) (Lopez et al., 2003). Humans can get glanders infections by coming into close touch with diseased animals, fomites, tissue, or bacterial cultures (Van Zandt et al., 2013). Bacteria can enter the body through the mouth, nose, or skin abrasions or sores (Whitlock et al., 2007). The majority of human glander infections occur in lab settings when handling and processing animal materials or B. mallei cultures (Virk et al., 2023). These primary transmission routes emphasize the interconnected risk between equids, carnivores, and humans, as visualized in Figure 1. Military relevanceWhile the historical weaponization of B. mallei is well documented, the contemporary bioterrorism landscape requires a more nuanced analysis. Unlike in the early 20th century, modern bioterrorism threats are shaped by advances in synthetic biology, genetic engineering, and global laboratory accessibility. The possibility of manipulating B. mallei strains to enhance antibiotic resistance or alter virulence factors cannot be overlooked (Christopher et al., 1997; Cote et al., 2020). Such modifications could render existing treatment protocols ineffective, complicate diagnosis, and increase the pathogen’s persistence in both human and animal hosts. This underscores the necessity of including B. mallei in present-day biodefense surveillance frameworks, even though it has not yet the historical weaponization of B. mallei has been well documented (Wheelis, 1998; Regis, 1999; Riedel, 2004). Preparedness challenges are amplified by the pathogen’s clinical ambiguity. As emphasized by Van Zandt et al. (2013), glanders presents with non-specific symptoms that mimic pneumonia or tuberculosis, making delayed diagnosis a likely outcome in non-endemic regions. This diagnostic delay is not merely a clinical problem but also a national security vulnerability, since early detection is crucial for containment and effective response. Modern biodefense strategies, therefore, need to invest in point-of-care diagnostic platforms capable of differentiating glanders from other respiratory infections under field conditions. Another underexplored issue is the gap between international treaties and practical enforcement. The Biological Weapons Convention of 1972 (Smart, 1997) prohibits the development and stockpiling of pathogens such as B. mallei, yet history demonstrates that state-level violations occurred decades after ratification (Alibek and Handelman, 1999). This raises questions about verification mechanisms, transparency in research involving select agents, and the dual-use dilemma of scientific advances. For example, work conducted to understand virulence mechanisms or develop medical countermeasures can unintentionally provide knowledge that could be repurposed for hostile use. The role of veterinary surveillance and animal movement control also deserves critical attention in biodefense planning. As Noor and Ariyanti (2019) noted, equids remain susceptible reservoirs, and clandestine use of infected animals could prolong pathogen circulation after an intentional release. Unlike anthrax, which produces spores with environmental persistence, B. mallei relies on living hosts for transmission, making surveillance of equine populations an integral yet often neglected component of biodefense. Thus, international cooperation in animal health monitoring is as important as investment in human clinical preparedness. Finally, preparedness gaps remain in post-exposure management and public communication. Although glanders is theoretically treatable if identified early (Torres, 2025), the high mortality rate—even under therapy (Barnes et al., 2022)—highlights the need for stockpiling effective antibiotics and developing candidate vaccines. In parallel, public health messaging frameworks must be prepared for scenarios where glanders is deliberately released, as misinformation and panic could exacerbate the impact. Integrating glanders into broader biodefense exercises, alongside pathogens such as anthrax and smallpox, would help policymakers stress-test their response capacity and identify weak points in current systems.
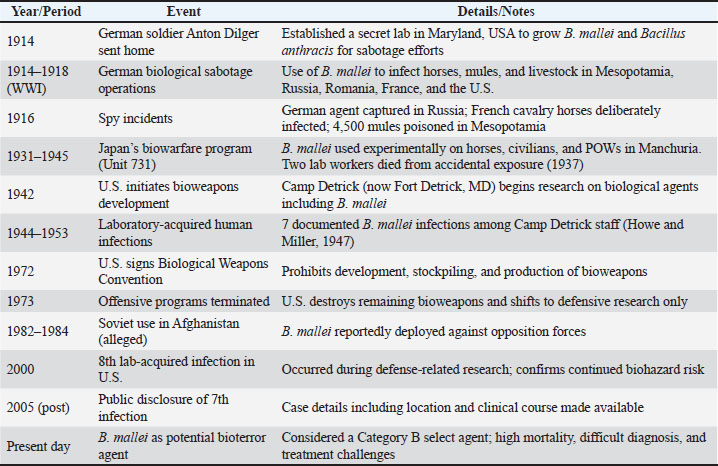
Fig. 1. Transmission routes of glanders between equids, carnivores, and human. Public health importanceBuilding on the epidemiological and pathogenic characteristics described earlier, glanders represents a significant public health concern due to its high zoonotic potential and severe clinical outcomes (Noor and Ariyanti, 2019). Several transboundary zoonotic diseases continue to pose significant global threats (Clemmons et al., 2021). environments where humans and animals interact closely—such as farms, military operations, or research laboratories—are at elevated risk of transmission (Van Zandt et al., 2013). The major challenge lies in the difficulty of early diagnosis, since the symptoms can mimic other diseases like pneumonia or tuberculosis, often leading to delayed treatment and higher mortality (Kettle and Wernery, 2016). This diagnostic challenge underscores the need for enhanced clinical awareness and laboratory capacity. As there is still no vaccine available, public health management relies solely on rapid case detection, targeted antibiotic therapy, and strict isolation protocols (Torres, 2025). Beyond the clinical sphere, glanders also poses broader biosecurity challenges, as B. mallei is recognized as a potential bioterrorism agent (Pal and Paulos Gutama, 2022). Consequently, public health preparedness must go beyond medical treatment, incorporating emergency planning, risk communication, and surveillance strategies to prevent potential outbreaks or deliberate misuse. To strengthen resilience, robust monitoring systems, continuous training for healthcare and veterinary personnel, and community education are essential. Such integrated measures are critical not only for controlling natural outbreaks but also for mitigating risks associated with intentional release or laboratory exposure (Van Zandt et al., 2013). TreatmentInfected mules, donkeys, and horses should not be treated with antibiotics. B. mallei is resistant to many antibiotics and requires long-term combination antibiotic therapy (Lim et al., 2022). Chronic or latent infections may also arise as a result of ineffective antibiotic treatment. A localized outbreak of glanders affecting 23 horses in Lahore, Pakistan, prompted the implementation of a 12-week treatment program that included oral doxycycline, parenteral enrofloxacin, and trimethoprim/sulfadiazine (Saqib et al., 2012). The fact that there was no relapse during the 360-day follow-up period after antibiotic treatment suggests that long-term antibiotics might be beneficial. This treatment plan is costly, though, and more research with bigger sample numbers and longer-term monitoring is required to ascertain the efficacy of this antibiotic regimen. It is generally not advised to treat diseased horses outside of endemic areas because of the zoonotic risk (Khan et al., 2012). Table 3. Historical timeline of B. mallei in military and biowarfare use.
Antibiotics are the main treatment used for glanders in humans. Since human cases of this bacteria are uncommon, there aren’t many precise, research-based treatments available. Nonetheless, a similar therapeutic strategy is frequently employed, as for melioidosis, another bacterially induced infectious condition (Wiersinga et al., 2018). Common medicines that must be used for a sufficient amount of time to guarantee that the B. mallei bacteria are totally removed from the body include ciprofloxacin, gentamicin, streptomycin, and doxycycline (Kenny et al., 1999). A combination of antibiotics and hospitalization may be required in cases of severe or persistent infection. Delaying treatment might raise the risk of major complications, such as organ failure and death, because glanders’ symptoms frequently mimic those of other illnesses, like pneumonia or tuberculosis. For this reason, early diagnosis is crucial (Torres, 2025). Medical professionals who treat sick patients must wear personal protective equipment (PPE) to reduce the risk of infection, and infected patients must be isolated to stop the disease from spreading (Rahman et al., 2020). VaccinationThe development of a vaccine against B. mallei, the causative agent of glanders, remains one of the most pressing challenges in biodefense research. Despite extensive investigations, no licensed vaccine is currently available (Torres, 2025). This reflects both the biological complexity of the pathogen and the limited translational success of candidate vaccines. A critical evaluation of past and recent strategies provides valuable insights into the barriers that continue to comprehensive overviews of glanders in biodefense contexts are available (Welkos et al., 2018). Killed and inactivated vaccinesHistorically, killed or chemically inactivated B. mallei preparations were among the first candidates evaluated. While these formulations were relatively simple to produce, they consistently failed to induce long-lasting or protective immunity in animal models (Avril et al., 2024). Their immunogenicity was limited to humoral responses, with insufficient activation of cell-mediated immunity, which is essential to combat an intracellular pathogen such as B. mallei (Saikh and Mott, 2017). Thus, despite their early promise in experimental settings, inactivated vaccines have been deemed inadequate for practical use. Live-attenuated vaccinesLive-attenuated vaccines have attracted considerable interest due to their ability to mimic natural infection and stimulate both humoral and cellular immunity. Studies using attenuated B. pseudomallei (a closely related organism) demonstrated partial protection in murine models (Khakhum et al., 2019). However, the risk of reversion to virulence and potential safety concerns in immunocompromised hosts remain significant barriers (Ghattas et al., 2021). For a pathogen considered a high-priority bioterrorism agent, the safety profile of live-attenuated strains represents an unacceptable risk, limiting their potential for deployment (Johnson and Ainslie, 2017). Subunit and recombinant protein vaccinesA more targeted approach has involved subunit vaccines based on outer membrane proteins, type VI secretion system components, and LPSs (Swietnicki, 2021). These antigens have shown the capacity to generate measurable humoral immune responses, sometimes combined with partial survival benefit in experimental challenges (Waag et al., 2012). Nevertheless, the protective efficacy remains inconsistent, and immunity tends to wane without booster immunization (Johnson and Ainslie, 2017). Furthermore, reliance on humoral responses alone has been insufficient, as protective immunity against B. mallei likely requires a strong T-cell-mediated component (Wang et al., 2020). DNA and vector-based vaccinesMore recent research (2020–2025) has explored DNA vaccines and viral-vector platforms encoding immunodominant B. mallei antigens (Wang et al., 2020; Chapartegui-González et al., 2021; Badten and Torres, 2024; Sengyee et al., 2025). These strategies aim to provide durable cellular responses and overcome some limitations of protein-based vaccines. Preclinical data indicate induction of antigen-specific T-cell activity, yet survival outcomes following challenge have varied across different models. Moreover, challenges related to stability, delivery systems, and scale-up for biodefense applications persist (Lafontaine et al., 2018). Comparative evaluationWhen compared systematically, it becomes clear that each vaccine strategy offers distinct strengths and weaknesses: inactivated vaccines provide safety but poor efficacy; live-attenuated strains offer stronger immunity but unacceptable risks; subunit and recombinant approaches are safer but lack durable protection; while DNA/vector-based methods are innovative yet remain in early developmental stages. Importantly, the failures and partial successes across platforms highlight that B. mallei’s intracellular lifestyle, immune evasion strategies, and ability to establish chronic infection present formidable barriers to vaccine design. Critical outlookFrom a biodefense and bioterrorism preparedness perspective, the lack of a licensed vaccine against glanders represents a significant vulnerability. The consensus emerging from recent research suggests that no single antigen or approach will be sufficient. Instead, a multiantigen, multiplatform strategy, potentially combining subunit vaccines with nucleic acid or vector-based systems, may offer the best chance of achieving protective and durable immunity. Furthermore, a stronger emphasis on comparative studies across standardized animal models is urgently needed to identify correlates of protection. Without such critical advances, vaccine development against B. mallei will continue to lag behind other priority pathogens, leaving populations at risk in both natural and intentional exposure scenarios (Bondi and Goldberg, 2008). ControlSince there is currently no vaccine to prevent glanders in humans or animals, all positive instances of the bacteria must be swiftly discovered, killed, and disposed of appropriately to guarantee efficient prevention and control (Torres, 2025). Extreme caution, including the use of the proper PPE, should be used when handling sick animals and contaminated fomites (Pal and Paulos Gutama, 2022). If glanders is suspected to be the cause of death, the animal carcass should be buried or incinerated without post-mortem examination to avoid the risk of environmental contamination and disease transmission (Verma et al., 2014). Strict disinfection procedures must be followed for the grounds, feed and water troughs, and other locations. B. mallei should be disinfected because it is extremely sensitive to common disinfectants such benzalkonium chloride, iodine, potassium permanganate, 1% sodium hypochlorite, 70% ethanol, mercuric chloride in alcohol, and 2% glutaraldehyde (EFSA Panel on Animal Health and Welfare (AHAW) et al., 2022). It is less susceptible to phenolic disinfectants. Additionally, these bacteria can be eliminated by subjecting them to UV light or heating them to 55°C for ten minutes (Rito et al., 2024). Isolation, hygiene, and sanitation protocols should be followed. Contaminated materials should be cleaned using a solution of one part household bleach (0.5% sodium hypochlorite solution) to nine parts water (Mohapatra and Mishra, 2022). Targeted awareness campaigns on glanders should be prioritized in endemic regions, especially among equine veterinarians, animal handlers, and regulatory authorities. These efforts are particularly important during trade seasons or outbreaks, and should focus on early detection, biosecurity measures, and mandatory reporting procedures. ConclusionIn conclusion, glanders disease is still a major zoonotic illness that has a major impact on public safety and horse health, especially in regions where it is still endemic. B. mallei’s use as a biological weapon emphasizes the necessity of ongoing monitoring, investigation, and biosecurity protocols to stop its spread and safeguard populations of humans and animals. AcknowledgmentsThe authors would like to thank Universitas Airlangga and the National Research and Innovation Agency (BRIN) for providing institutional and technical support throughout the preparation of this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Author’s contributionsMS and ARK served as the primary authors of this review, drafting the major sections including epidemiology, pathogenesis, and clinical aspects. BWKW prepared treatment and control strategies, while IM contributed to the introduction and military relevance and helped coordinate the draft. BPP also worked on revising, and editing the draft. SHA, IBM, and RZA revised the pathology, public health, and vaccination sections, respectively. ATK revised the biosecurity and biodefense section. MKJK, DAAK, AOA, IF, and SW critically reviewed key sections, including epidemiology, zoonoses, pathogenesis, and biosecurity. AHF, KAF, IMe, and M’AK managed and edited references. All authors reviewed and approved the final manuscript. Data availabilityNo data were generated in this study. All cited information is available in the original open access publications referenced. ReferencesAbdela, M.G., Teshale, S., Gobena, M.M., Zewde, A., Jaleta, H., Gumi, B. and Ameni, G. 2021. Epidemiology of epizootic lymphangitis among carthorses in Ethiopia. Front. Vet. Sci. 8(1), 762937. Abebaw, B. 2024. Ulcerative and spreading nodular lesion of epizootic lymphangitis in adult black horse in Gondar, Ethiopia: case report and wound treatment. Case Rep. Vet. Med. 2024(1), 2478774. Abnaroodheleh, F., Mosavari, N., Pourbakhsh, S.A., Tadayon, K. and Jamshidian, M. 2023. Identification of Burkholderia mallei isolates with polymerase chain reaction-restriction fragment length polymorphism. Arch. Razi. Inst. 78(4), 1305–1312. Abreu, D.C., Gomes, A.S., Tessler, D.K., Chiebao, D.P., Fava, C.D., Romaldini, A.H.C.N., Araujo, M.C., Pompei, J., Marques, G.F., Harakava, R., Pituco, E.M. and Nassar, A.F.C. 2020. Systematic monitoring of glanders-infected horses by Complement Fixation Test, bacterial isolation, and PCR. Vet. Anim. Sci. 10(1), 100147. Aiba, S., Manome, H., Nakagawa, S., Mollah, Z.U.A., Mizuashi, M., Ohtani, T., Yoshino, Y. and Tagami, H. 2003. p38 mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J. Invest. Dermatol. 120(3), 390–399. Al-Ani, F.K. and Roberson, J. 2007. Glanders in horses: a review of the literature. Vet. Arhiv. 77(3), 203–218. Alibasoglu, M., Yesildere, T., Calislar, T., Inal, T. and Calsikan, U. 1986. Malleus-ausbruch bei löwen im zoologischen garten istanbul [Glanders outbreak in lions in the Istanbul zoological garden]. Berl. Munch. Tierarztl. Wochenschr. 99(2), 57–63. Alibek, K. and Handelman, S. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world. New York, NY: Random House. Alikhanov, K., Abultdinova, A., Sansyzbay, A., Nussupova, S., Yespembetov, B. and Syrym, N. 2024. Epizootological monitoring of glanders in the republic of Kazakhstan. Int. J. Vet. Sci. 13(4), 514–520. Anggraini, D., Siregar, F.M., Rosdiana, D., Kemal, R.A., Yovi, I., Triani, Z.D., Jasmin, N., Dwijelita, N., Webb, J.R., Mayo, M., Kaestli, M. and Currie, B.J. 2024. Epidemiology and genetic diversity of Burkholderia pseudomallei from Riau Province, Indonesia. PLoS Negl. Trop. Dis. 18(5), e0012195. Avril, A., Guillier, S. and Rasetti-Escargueil, C. 2024. Development of effective medical countermeasures against the main biowarfare agents: the importance of antibodies. Microorganisms 12(12), 2622. Badten, A.J. and Torres, A.G. 2024. Burkholderia pseudomallei complex subunit and glycoconjugate vaccines and their potential to elicit cross-protection to Burkholderia cepacia complex. Vaccines 12(3), 313. Barnes, K.B., Bayliss, M., Davies, C., Richards, M.I., Laws, T.R., Vente, A. and Harding, S.V. 2022. Efficacy of finafloxacin in a murine model of inhalational glanders. Front. Microbiol. 13(1), 1057202. Barros, M.B., De Almeida Paes, R. and Schubach, A.O. 2011. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 24(4), 633–654. Bernhards, R.C., Cote, C.K., Amemiya, K., Waag, D.M., Klimko, C.P., Worsham, P.L. and Welkos, S.L. 2017. Characterization of in vitro phenotypes of Burkholderia pseudomallei and Burkholderia mallei strains potentially associated with persistent infection in mice. Arch. Microbiol. 199(2), 277–301. Bondi, S.K. and Goldberg, J.B. 2008. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev. Vaccines 7(9), 1357–1365. Boyle, A.G., Timoney, J.F., Newton, J.R., Hines, M.T., Waller, A.S. and Buchanan, B.R. 2018. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles-revised consensus statement. J. Vet. Intern. Med. 32(2), 633–647. Brett, P.J., Burtnick, M.N., Snyder, D.S., Shannon, J.G., Azadi, P. and Gherardini, F.C. 2007. Burkholderia mallei expresses a unique lipopolysaccharide mixture that is a potent activator of human Toll-like receptor 4 complexes. Mol. Microbiol. 63(2), 379–390. Brett, P.J., Burtnick, M.N., Su, H., Nair, V. and Gherardini, F.C. 2008. iNOS activity is critical for the clearance of Burkholderia mallei from infected RAW 264.7 murine macrophages. Cell Microbiol. 10(2), 487–498. Brightman, C. and Locum. 2020. Melioidosis: the Vietnamese time bomb. Trends Urol. Men’s Health 11(3), 30–32. Busl, K.M. and Bleck, T.P. 2012. Treatment of neuroterrorism. Neurotherapeutics 9(1), 139–157. Bzdyl, N.M., Moran, C.L., Bendo, J. and Sarkar-Tyson, M. 2022. Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 13(1), 1945–1965. Cárdenas, N.C., Galvis, J.O.A., Farinati, A.A., Grisi-Filho, J.H.H., Diehl, G.N. and Machado, G. 2019. Burkholderia mallei: the dynamics of networks and disease transmission. Transbound. Emerg. Dis. 66(2), 715–728. Carlson, J.A. 2010. The histological assessment of cutaneous vasculitis. Histopathology 56(1), 3–23. Centers for Disease Control and Prevention (CDC). 2024. Glanders (Burkholderia mallei). In U.S. Department of Health & Human Services. Available via https://www.cdc.gov/glanders/ Centers for Disease Control and Prevention (CDC). 2000. Laboratory-acquired human glanders--Maryland, May 2000. MMWR. Morb. Mortal. Wkly. Rep. 49(24), 532–535. Chapartegui-González, I., Bowser, S., Torres, A.G. and Khakhum, N. 2021. Recent progress in Shigella and Burkholderia pseudomallei vaccines. Pathogens 10(11), 1353. Charron, P., Gao, R., Chmara, J., Hoover, E., Nadin-Davis, S., Chauvin, D., Hazelwood, J., Makondo, K., Duceppe, M.O. and Kang, M. 2023. Influence of genomic variations on glanders serodiagnostic antigens using integrative genomic and transcriptomic approaches. Front. Vet. Sci. 10(1), 1217135. Chen, S., Saeed, A.F.U.H., Liu, Q., Jiang, Q., Xu, H., Xiao, G.G., Rao, L. and Duo, Y. 2023. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 8(1), 207. Chiang, C.Y., Ulrich, R.L., Ulrich, M.P., Eaton, B., Ojeda, J.F., Lane, D.J., Kota, K.P., Kenny, T.A., Ladner, J.T., Dickson, S.P., Kuehl, K., Raychaudhuri, R., Sun, M., Bavari, S., Wolcott, M.J., Covell, D. and Panchal, R.G. 2015. Characterization of the murine macrophage response to infection with virulent and avirulent Burkholderia species. BMC Microbiol. 15(1), 259. Chin, C.Y., Monack, D.M. and Nathan, S. 2010. Genome wide transcriptome profiling of a murine acute melioidosis model reveals new insights into how Burkholderia pseudomallei overcomes host innate immunity. BMC. Genomics 11(1), 672. Christopher, G.W., Cieslak, T.J., Pavlin, J.A. and Eitzen, E.M. Jr. 1997. Biological warfare. A historical perspective. JAMA 278(5), 412–417. Clemmons, E.A., Alfson, K.J. and Dutton, J.W. 3rd. 2021. Transboundary animal diseases, an overview of 17 diseases with potential for global spread and serious consequences. Animals (Basel). 11(7), 2039. Cote, C.K., Blanco, I.I., Hunter, M., Shoe, J.L., Klimko, C.P., Panchal, R.G. and Welkos, S.L. 2020. Combinations of early generation antibiotics and antimicrobial peptides are effective against a broad spectrum of bacterial biothreat agents. Microb. Pathog. 142(1), 104050. David, J., Bell, R.E. and Clark, G.C. 2015. Mechanisms of disease: host-pathogen interactions between Burkholderia species and lung epithelial cells. Front. Cell. Infect. Microbiol. 5(1), 80. Rahimabadi, P., Nazari, A., Kamyabi, M. and Mosavari, N. Serological and bacteriological surveillance of glanders among horses in Central Region of Iran. SSRN. Electron. J. 127(1), 104535. Duan, H., Wang, L., Huangfu, M. and Li, H. 2023. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: mechanisms and therapeutic potentials. Biomed. Pharmacother. 165(1), 115276. Duan, T., Du, Y., Xing, C., Wang, H.Y. and Wang, R.F. 2022. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 13(1), 812774. EFSA Panel on Animal Health and Welfare (AHAW), Nielsen, S.S., Alvarez, J., Bicout, D.J., Calistri, P., Canali, E., Drewe, J.A., Garin-Bastuji, B., Rojas, J.L.G., Schmidt, C.G., Herskin, M., Michel, V., Chueca, M.A.M., Padalino, B., Pasquali, P., Spoolder, H., Ståhl, K., Velarde, A., Viltrop, A., Winckler, C., Gubbins, S., Laroucau, K., Antoniou, S.E., Aznar, I., Broglia, A., Lima, E., Van der Stede, Y., Zancanaro, G. and Roberts, H.C. 2022. Assessment of the control measures of the category a diseases of animal health law: Burkholderia mallei (Glanders). EFSA. J. 20(1), e07069. El-Karim, D.R.S.G., El-Amrawi, G. and Salama, A.R. 2024. Clinicopathological studies on ulcerative lymphangitis in cattle: alterations in serum inflammatory cytokines, anti-microbial, organs functions, and oxidative stress-related biomarkers. Open Vet. J. 14(1), 25–31. Elschner, M.C., Laroucau, K., Singha, H., Tripathi, B.N., Saqib, M., Gardner, I., Saini, S., Kumar, S., El-Adawy, H., Melzer, F., Khan, I., Malik, P., Sauter-Louis, C. and Neubauer, H. 2019. Evaluation of the comparative accuracy of the Complement Fixation Test, Western blot and five enzyme-linked immunosorbent assays for serodiagnosis of glanders. PLoS One 14(4), e0214963. Elschner, M.C., Melzer, F., Singha, H., Muhammad, S., Gardner, I. and Neubauer, H. 2021. Validation of a commercial glanders ELISA as an alternative to the CFT in international trade of equidae. Front. Vet. Sci. 8(1), 628389. Elschner, M.C., Scholz, H.C., Melzer, F., Saqib, M., Marten, P., Rassbach, A., Dietzsch, M., Schmoock, G., De Assis Santana, V.L., de Souza, M.M., Wernery, R., Wernery, U. and Neubauer, H. 2011. Use of a Western blot technique for the serodiagnosis of glanders. BMC. Vet. Res. 7(1), 4. Erdemsurakh, O., Ochirbat, K., Gombosuren, U., Tserendorj, B., Purevdorj, B., Vanaabaatar, B., Aoshima, K., Kobayashi, A. and Kimura, T. 2020b. Seroprevalence of equine glanders in horses in the central and eastern parts of Mongolia. J. Vet. Med. Sci. 82(9), 1247–1252. Erdemsurakh, O., Purevdorj, B., Ochirbat, K., Adilbish, A., Vanaabaatar, B., Aoshima, K., Kobayashi, A. and Kimura, T. 2020a. Pathological and immunohistochemical analyses of naturally occurring equine glanders using an anti-BpaB antibody. Vet. Pathol. 57(6), 807–811. Escobar, D.A., Botero-Quintero, A.M., Kautza, B.C., Luciano, J., Loughran, P., Darwiche, S., Rosengart, M.R., Zuckerbraun, B.S. and Gomez, H. 2015. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J. Surg. Res. 194(1), 262–272. Evans, D.H. 1966. Utilization of carbohydrates by Actinobacillus mallei. Can. J. Microbiol. 12(4), 625–639. Fritz, D.L., Vogel, P., Brown, D.R. and Waag, D.M. 1999. The Hamster model of Intraperitoneal Burkholderia mallei (Glanders). Vet. Pathol. 36(1), 276–291. Frolov, D.M., Teteryatnikova, N.N., Bui, T.L.A., Zakharova, I.B. and Khrapova, N.P. 2020. Development of a latex agglutination test for detecting pathogenic Burkholderia and its approbation in the endemic regions of Vietnam. Probl. Partic. Danger. Infect. 1(4), 133–138. Gaspar, E.B., Santos, L.R.D., Egito, A.A.D., Santos, M.G.D., Mantovani, C., Rieger, J.D.S.G., Abrantes, G.A.D.S., Suniga, P.A.P., Favacho, J.D.M., Pinto, I.B., Nassar, A.F.D.C., Santos, F.L.D. and Araújo, F.R.D. 2023. Assessment of the virulence of the Burkholderia mallei strain BAC 86/19 in BALB/c mice. Microorganisms 11(10), 2597. Ghattas, M., Dwivedi, G., Lavertu, M. and Alameh, M.G. 2021. Vaccine technologies and platforms for infectious diseases: current progress, challenges, and opportunities. Vaccines (Basel) 9(12), 1490. Gilad, J., Schwartz, D. and Amsalem, Y. 2007. Clinical features and laboratory diagnosis of infection with the potential bioterrorism agents Burkholderia mallei and Burkholderia pseudomallei. Int. J. Biomed. Sci. 3(3), 144–152. Herr, S., Huchzermeyer, H.F., Te Brugge, L.A., Williamson, C.C., Roos, J.A. and Schiele, G.J. 1985. The use of a single Complement Fixation Test technique in bovine brucellosis, Johne’s disease, dourine, equine piroplasmosis and Q fever serology. Onderstepoort J. Vet. Res. 52(4), 279–282. Hmood, A. and Al-Amery, M. 2022. A review of glanders regarded Iraq and surrounding countries. Basrah J. Vet. Res. 21(S1), 263–275. Howe, C. and Miller, W.R. 1947. Human glanders; report of six cases. Ann. Intern. Med. 26(1), 93–115. Hsueh, P.T., Huang, W.T., Hsueh, H.K., Chen, Y.L. and Chen, Y.S. 2018. Transmission modes of melioidosis in Taiwan. Trop. Med. Infect. Dis. 3(1), 26. Janesomboon, S., Muangsombut, V., Srinon, V., Meethai, C., Tharinjaroen, C.S., Amornchai, P., Withatanung, P., Chantratita, N., Mayo, M., Wuthiekanun, V., Currie, B.J., Stevens, J.M. and Korbsrisate, S. 2021. Detection and differentiation of Burkholderia species with pathogenic potential in environmental soil samples. PLoS One 16(1), e0245175. Jatapai, A., Gregory, C.J., Thamthitiwat, S., Tanwisaid, K., Bhengsri, S., Baggett, H.C., Sangwichian, O., Jorakate, P. and MacArthur, J.R. 2018. Hospitalized bacteremic melioidosis in rural Thailand: 2009-2013. Am. J. Trop. Med. Hyg. 98(6), 1585–1591. Jilani, M.S.A., Farook, S., Bhattacharjee, A., Barai, L., Ahsan, C.R., Haq, J.A. and Tuanyok, A. 2023. Phylogeographic characterization of Burkholderia pseudomallei isolated from Bangladesh. PLoS Negl. Trop. Dis. 17(12), e0011823. Johnson, M.M. and Ainslie, K.M. 2017. Vaccines for the prevention of melioidosis and glanders. Curr. Trop. Med. Rep. 4(3), 136–145. Júnior, E.L.d.S., Moura, J.d.C.R., Protásio, B.K.P.F., Parente, V.A.S. and Veiga, M.H.N.D. 2020. Clinical repercussions of Glanders (Burkholderia mallei infection) in a Brazilian child: a case report. J. Braz. Soc. Trop. Med. 53(1), e20200054. Karim, D. and Amrawi, G. Clinicopathological studies on ulcerative lymphangitis in cattle: alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Vet. J. 14, 25–31. Karimi, A. and Mosavari, N. 2019. Development of Rose Bengal Test against mallein test for rapid diagnosis of equine glanders. Trop. Anim. Health Prod. 51(7), 1969–1974. Kenny, D.J., Russell, P., Rogers, D., Eley, S.M. and Titball, R.W. 1999. In vitro susceptibilities of Burkholderia mallei in comparison to those of other pathogenic Burkholderia spp. Antimicrob. Agents Chemother. 43(11), 2773–2775. Kettle, A.N. and Wernery, U. 2016. Glanders and the risk for its introduction through the international movement of horses. Equine Vet. J. 48(5), 654–658. Khakhum, N., Bharaj, P., Myers, J.N., Tapia, D., Kilgore, P.B., Ross, B.N., Walker, D.H., Endsley, J.J. and Torres, A.G. 2019. Burkholderia pseudomallei ΔtonB Δhcp1 live attenuated vaccine strain elicits full protective immunity against aerosolized melioidosis infection. mSphere 4(1), 570. Khaki, P., Mosavari, N., Khajeh, N.S., Emam, M., Ahouran, M., Hashemi, S., Taheri, M.M., Jahanpeyma, D. and Nikkhah, S. 2012. Glanders outbreak at Tehran Zoo, Iran. Iran. J. Microbiol. 4(1), 3–7. Khalafalla, A.I. 2016. Emerging infectious diseases in camelids. Emerg. Re-Emerg. Infect. Dis. Livest. 1(1), 425–441. Khan, I., Wieler, L.H., Butt, M.A., Elschner, M.C., Cheema, A.H., Sprague, L.D. and Neubauer, H. 2012. On the current situation of glanders in various districts of the Pakistani Punjab. J. Equine Vet. Sci. 32(12), 783–787. Khan, I., Wieler, L.H., Melzer, F., Elschner, M.C., Muhammad, G., Ali, S., Sprague, L.D., Neubauer, H. and Saqib, M. 2013. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound. Emerg. Dis. 60(3), 204–221. Kianfara, N., Ghasemianb, A., Al-Marzoqic, A.H., Eslamid, M., Vardanjanie, H.R., Mirforughif, S.A. and Vardanjani, H.R. 2018. The reemergence of glanders as a zoonotic and occupational infection in Iran and neighboring countries. Rev. Med. Microbiol. 29(1), 1–6. Kinoshita, Y., Cloutier, A.K., Rozak, D.A., Khan, M.S.R., Niwa, H., Uchida-Fujii, E., Katayama, Y. and Tuanyok, A. 2019. A novel selective medium for the isolation of Burkholderia mallei from equine specimens. BMC. Vet. Res. 15(1), 133. Koenig, K.L. and Schultz, C.H. 2016. Clinical management. In Koenig and Schultz’s disaster medicine: comprehensive principles and practices. Cambridge, UK:Cambridge University Press, pp: 463–736. Korneev, K.V., Arbatsky, N.P., Molinaro, A., Palmigiano, A., Shaikhutdinova, R.Z., Shneider, M.M., Pier, G.B., Kondakova, A.N., Sviriaeva, E.N., Sturiale, L., Garozzo, D., Kruglov, A.A., Nedospasov, S.A., Drutskaya, M.S., Knirel, Y.A. and Kuprash, D.V. 2015. Structural relationship of the lipid A acyl groups to activation of murine Toll-like receptor 4 by lipopolysaccharides from pathogenic strains of Burkholderia mallei, Acinetobacter baumannii, and Pseudomonas aeruginosa. Front. Immunol. 6(1), 595. Kumar, S., Prasad, T., Narayan, P. and Muruganandhan, J. 2013. Granuloma with langhans giant cells: an overview. J. Oral Maxillofac. Pathol. 17(3), 420–423. Kyle, R.A., Steensma, D.P. and Shampo, M.A. 2015. Friedrich August Johannes Löffler (Loeffler), German Bacteriologist. Mayo Clin. Proc. 90(12), 135. Lafontaine, E.R., Chen, Z., Huertas-Diaz, M.C., Dyke, J.S., Jelesijevic, T.P., Michel, F., Hogan, R.J. and He, B. 2018. The autotransporter protein BatA is a protective antigen against lethal aerosol infection with Burkholderia mallei and Burkholderia pseudomallei. Vaccine X. 1(1), 100002. Lauman, P. and Dennis, J.J. 2021. Advances in phage therapy: targeting the Burkholderia cepacia complex. Viruses 13(7), 1331. Lee, S.K., Han, J.I., Yun, S.J. and Kang, H.G. 2010. A survey of epidemic diseases in horses imported into South Korea between 2003 and 2008. J. Vet. Clin. 27(3), 268–272. Li, J., Zhong, Q., Shang, M.Y., Li, M., Jiang, Y.S., Zou, J.J., Ma, S.S., Huang, Q. and Lu, W.P. 2022. Preliminary Evaluation of rapid visual identification of Burkholderia pseudomallei using a newly developed lateral flow strip-based recombinase polymerase amplification (LF-RPA) system. Front. Cell. Infect. Microbiol. 11(1), 804737. Libera, K., Konieczny, K., Grabska, J., Szopka, W., Augustyniak, A. and Pomorska-Mól, M. 2022. Selected livestock-associated zoonoses as a growing challenge for public health. Infect. Dis. Rep. 14(1), 63–81. Lim, Y.M., Vadivelu, J., Mariappan, V., Venkatraman, G. and Vellasamy, K.M. 2022. Effective therapeutic options for melioidosis: antibiotics versus phage therapy. Pathogens 12(1), 11. Llobet, E., Tomás, J.M. and Bengoechea, J.A. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology (Reading) 154(Pt 12), 3877–3886. López, A. and Martinson, S.A. 2017. Respiratory system, mediastinum, and pleurae. Pathol. Basis Vet. Dis. 2017(1), 471–560.e1. Lopez, J., Copps, J., Wilhelmsen, C., Moore, R., Kubay, J., St-Jacques, M., Halayko, S., Kranendonk, C., Toback, S., DeShazer, D., Fritz, D.L., Tom, M. and Woods, D.E. 2003. Characterization of experimental equine glanders. Microbes Infect. 5(12), 1125–1131. Lu, R., Popov, V., Patel, J. and Eaves-Pyles, T. 2012. Burkholderia mallei and Burkholderia pseudomallei stimulate differential inflammatory responses from human alveolar type II cells (ATII) and macrophages. Front. Cell. Infect. Microbiol. 2(1), 165. Mahajan, V.K. 2014. Sporotrichosis: an overview and therapeutic options. Dermatol. Res. Pract. 2014(1), 272376. Maldonado, R.F., Sá-Correia, I. and Valvano, M.A. 2016. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 40(4), 480–493. Mangalea, M.R., Plumley, B.A. and Borlee, B.R. 2017. Nitrate sensing and metabolism inhibit biofilm formation in the opportunistic pathogen Burkholderia pseudomallei by reducing the intracellular concentration of c-di-GMP. Front. Microbiol. 8(1), 1353. Massey, S., Yeager, L.A., Blumentritt, C.A., Vijayakumar, S., Sbrana, E., Peterson, J.W., Brasel, T., Leduc, J.W., Endsley, J.J. and Torres, A.G. 2014. Comparative Burkholderia pseudomallei natural history virulence studies using an aerosol murine model of infection. Sci. Rep. 4(1), 4305. Matamoros-Recio, A., Merino, J., Gallego-Jiménez, A., Conde-Alvarez, R., Fresno, M. and Martín-Santamaría, S. 2023. Immune evasion through Toll-like receptor 4: the role of the core oligosaccharides from α2-proteobacteria atypical lipopolysaccharides. Carbohydr. Polym. 318(1), 121094. Memišević, V., Zavaljevski, N., Pieper, R., Rajagopala, S.V., Kwon, K., Townsend, K., Yu, C., Yu, X., Deshazer, D., Reifman, J. and Wallqvist, A. 2013. Novel Burkholderia mallei virulence factors linked to specific host-pathogen protein interactions. Mol. Cell Proteomics 12(11), 3036–3051. Memišević, V., Zavaljevski, N., Rajagopala, S.V., Kwon, K., Pieper, R., DeShazer, D., Reifman, J. and Wallqvist, A. 2015. Mining host-pathogen protein interactions to characterize Burkholderia mallei infectivity mechanisms. PLoS Comput. Biol. 11(3), e1004088. Merchant, I.A. and Packer, R.A. 1967. Veterinary bacteriology and virology, 7th ed. Ames, Iowa: Iowa State University Press. Mohapatra, P.R. and Mishra, B. 2022. Prevention of melioidosis. J. Fam. Med. Prim. Care 11(9), 4981–4986. Morrow, B.L. and Ruggiero, D. 2025. Burkholderia-associated ocular disease in cats. J. Shelter Med. Commun. Anim. Health 4(1), 117. Mota, R.A., Oliveira, A.A.D.F., Pinheiro Junior, J.W., Silva, L.B.G.D., Brito, M.D.F. and Rabelo, S.S.A. 2010. Glanders in donkeys (Equus asinus) in the state of Pernambuco, Brazil: a case report. Braz. J. Microbiol. 41(1), 146–149. Nasiri, M., Zarrin, A., Roshankarrudsari, S. and Khodadadi, J. 2023. Glanders (Burkholderia mallei infection) in an Iranian man: a case report. IDCases 32(1), e01779. Neubauer, H., Sprague, L.D., Zacharia, R., Tomaso, H., Al Dahouk, S., Wernery, R., Wernery, U. and Scholz, H.C. 2005. Serodiagnosis of Burkholderia mallei infections in horses: state-of-the-art and perspectives. J. Vet. Med. B Infect. Dis. Vet. Public Health 52(5), 201–205. Nikolakakis, I., Michaleas, S.N., Panayiotakopoulos, G., Papaioannou, T.G. and Karamanou, M. 2024. Instances of biowarfare in World War I (1914–1918). Cureus 16(4), 59329. Noor, S.M. and Ariyanti, T. 2019. Awareness of Emerging Glanders in Horses in Indonesia. Indonesian Bull. Anim. Vet. Sci. 29(3), 109–118. Paillot, R., Robinson, C., Steward, K., Wright, N., Jourdan, T., Butcher, N., Heather, Z. and Waller, A.S. 2010. Contribution of each of four Superantigens to Streptococcus equi-induced mitogenicity, gamma interferon synthesis, and immunity. Infect. Immun. 78(4), 1728–1739. Pal, M. and Paulos Gutama, K. 2022. Glanders: a potential bioterrorism weapon disease. Am. J. Infect. Dis. Microbiol. 10(3), 98–101. Pal, M., Gutama, K.P., Gerbaba, N.D., Shuramo, M.Y. and Shifera, F. 2022. Glanders: a highly infectious re-emerging serious zoonotic bacterial disease. J. Adv. Microbiol. Res. 3(2), 25–28. Pal, M., Shimelis, S., Parmar, B.C., Nayak, J.B. and Dasgupta, R. 2016. Glanders : a highly infectious re-emerging fatal bacterial zoonosis. J. Nat. Hist. 12(1), 13–19. Peacock, S.J., Schweizer, H.P., Dance, D.A.B., Smith, T.L., Gee, J.E., Wuthiekanun, V., DeShazer, D., Steinmetz, I., Tan, P. and Currie, B.J. 2008. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg. Infect. Dis. 14(7), e2. Pringle, J., Storm, E., Waller, A. and Riihimäki, M. 2020. Influence of penicillin treatment of horses with strangles on seropositivity to Streptococcus equi ssp. equi-specific antibodies. J. Vet. Intern. Med. 34(1), 294–299. Rahman, M.S., Bhattacharjee, P.K., Sarker, R.R., Parvin, M.S., Tasnin, S., Sarker, M.A.S., Neubauer, H., Khatun, F., Wares, M.A., Nishidate, I. and Elschner, M.C. 2018. Glanders in horses in some selected areas of Bangladesh and comparison between CFT and immunoblot used for the screening of glanders. Indian J. Anim. Res. 54(5), 631–634. Rahman, M.T., Sobur, M.A., Islam, M.S., Ievy, S., Hossain, M.J., El Zowalaty, M.E., Rahman, A.T. and Ashour, H.M. 2020. Zoonotic diseases: etiology, impact, and control. Microorganisms 8(9), 1405. Raj, A., Pathak, A., Karuppusamy, S., Tripathi, B.N., Tripathi, H. and Singha, H. 2024. Knowledge, awareness and perception about equine glanders among veterinarians and medical professionals in India. Front. Vet. Sci. 11(1), 1334485. Rebuma, T., Tariku, F., Regassa, M., Bulo, F. and Teshoma, M. 2024. Epizootic lymphangitis - a major fungal disease of equines. Int. J. Livest. Res. 14(4), 1–6. Regis, E. 1999. The biology of doom. New York, NY: Henry Holt. Resende, C.F., Santos, A.M.D., Filho, P.M.S., De Souza, P.G., Issa, M.D.A., Filho, M.B.D.C., Victor, R.M., Câmara, R.J.F., Gonçalves, G.P., Lima, J.G., Maciel E Silva, A.G., Leite, R.C. and Reis, J.K.P.D. 2022. Glanders and brucellosis in equids from the Amazon region, Brazil. Acta Tropica 231(1), 106429. Riedel, S. 2004. Biological warfare and bioterrorism: a historical review. Proc. (Bayl. Univ. Med. Cent). 17(4), 400–406. Rito, B., Matos, L., Proença, D.N. and Morais, P.V. 2024. Kinetics of inactivation of bacteria responsible for infections in hospitals using UV-LED. Heliyon 10(10), e30738. Rocha, L.S., Oliveira, A.L.F., Arruda, F.P., Pitchenin, L.C., Dutra, V., Nakazato, L., Furlan, F.H. and Colodel, E.M. 2023. Pathology, microbiology, and molecular evaluation of tissues from equids serologically positive for Burkholderia mallei in Midwestern Brazil. Pesq. Vet. Bras. 43(1), e07172. Romero, C.M., DeShazer, D., Feldblyum, T., Ravel, J., Woods, D., Kim, H.S., Yu, Y., Ronning, C.M. and Nierman, W.C. 2006. Genome sequence alterations detected upon passage of Burkholderia mallei ATCC 23344 in culture and in mammalian hosts. BMC Genomics 7(1), 228. Rosebury, T. and Kabat, E.A. 1947. Bacterial warfare, a critical analysis of the available agents, their possible military applications, and the means for protection against them. J. Immunol. 56(1), 7–96. Saikh, K.U. and Mott, T.M. 2017. Innate immune response to Burkholderia mallei. Curr. Opin. Infect. Dis. 30(3), 297–302. San Martin, P.F.M., Chua, J.C., Bautista, R.L.P., Nailes, J.M., Panaligan, M.M. and Dance, D.A.B. 2018. Melioidosis in the Philippines. Trop. Med. Infect. Dis. 3(3), 99. Saqib, M., Muhammad, G., Naureen, A., Hussain, M.H., Asi, M.N., Mansoor, M.K., Toufeer, M., Khan, I., Neubauer, H. and Sprague, L.D. 2012. Effectiveness of an antimicrobial treatment scheme in a confined glanders outbreak. BMC Vet. Res. 8(1), 214. Schell, M.A., Ulrich, R.L., Ribot, W.J., Brueggemann, E.E., Hines, H.B., Chen, D., Lipscomb, L., Kim, H.S., Mrázek, J., Nierman, W.C. and Deshazer, D. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64(6), 1466–1485. Scholz, H.C., Pearson, T., Hornstra, H., Projahn, M., Terzioglu, R., Wernery, R., Georgi, E., Riehm, J.M., Wagner, D.M., Keim, P.S., Joseph, M., Johnson, B., Kinne, J., Jose, S., Hepp, C.M., Witte, A. and Wernery, U. 2014. Genotyping of Burkholderia mallei from an outbreak of glanders in Bahrain suggests multiple introduction events. PLoS Negl. Trop. Dis. 8(9), e3195. Seid, A., Fedlu, M. and Mama, A. 2019. Review on epizootic lymphangitis: epidemiology and its diagnosis. J. Dairy Vet. Sci. 12(1), JDVS.MS.ID.555830. Sengyee, S., Weiby, S.B., Rok, I.T., Burtnick, M.N. and Brett, P.J. 2025. Melioidosis vaccines: recent advances and future directions. Front. Immunol. 16(1), 1582113. Shams, A.M., O’Connell, H., Arduino, M.J. and Rose, L.J. 2011. Chlorine dioxide inactivation of bacterial threat agents. Lett. Appl. Microbiol. 53(2), 225–230. Siddique, M.H., Samad, M.A., Memoon, A., Naqvi, S.Z.H., Rehman, F.U., Kalim, F., Ali, A., Qureshi, M.A. and Khawar, W. 2023. Burkholderia (mallei and pseudomallei) related zoonosis drastic zoonotic and biological warfare potential. Zoonosis 4(1), 82–99. Siggins, M.K. and Sriskandan, S. 2021. Bacterial lymphatic metastasis in infection and immunity. Cells 11(1), 33. Singha, H., Shanmugasundaram, K., Tripathi, B.N., Saini, S., Khurana, S.K., Kanani, A., Shah, N., Mital, A., Kanwar, P., Bhatt, L., Limaye, V., Khasa, V., Arora, R., Gupta, S., Sangha, S., Sharma, H., Agarwal, S.K., Tapase, J., Parnam, S., Dubey, P., Baalasundaram, S.K., Mandal, B.N., Virmani, N., Gulati, B.R. and Malik, P. 2020. Serological surveillance and clinical investigation of glanders among indigenous equines in India from 2015 to 2018. Transbound. Emerg. Dis. 67(3), 1336–1348. Smart, J. and K. 1997. History of chemical and biological warfare: an American perspective.In Medical aspects of biological and chemical warfare. Eds Zajtchuk, R. and Bellamy, R.F. Washington, DC: Borden Institute, 16, p: 64. Spickler, A. 2018. Glanders. Iowa, USA: Centre for Food Security and Public Health. Swe, M.M.M., Win, M.M., Cohen, J., Phyo, A.P., Lin, H.N., Soe, K., Amorncha, P., Wah, T.T., Win, K.K.N., Ling, C., Parker, D.M., Dance, D.A.B., Ashley, E.A. and Smithuis, F. 2021. Geographical distribution of Burkholderia pseudomallei in soil in Myanmar. PLoS Negl. Trop. Dis. 15(5), e0009372. Swietnicki, W. 2021. Secretory system components as potential prophylactic targets for bacterial pathogens. Biomolecules 11(6), 892. Syed, I. and Wooten, R.M. 2021. Interactions between pathogenic Burkholderia and the complement system: a review of potential immune evasion mechanisms. Front. Cell. Infect. Microbiol. 11(1), 701362. Tikmehdash, H.T., Dehnad, A., Mosavari, N., Hokmabadi, B.N. and Mahmazi, S. 2024. Isolation, serological and molecular methods in screening of Burkholderia mallei in East Azerbaijan province, Iran. Vet. Res. Forum 15(5), 231–236. Torres, A.G. 2023. The public health significance of finding autochthonous melioidosis cases in the continental United States. PLoS Negl. Trop. Dis. 17(8), e0011550. Torres, A.G. 2025. Glanders: an ancient and emergent disease with no vaccine or treatment on site. PLoS Negl. Trop. Dis. 19(6), e0013160. Van Zandt, K.E., Greer, M.T. and Gelhaus, H.C. 2013. Glanders: an overview of infection in humans. Orphanet J. Rare Dis. 8(1), 131. Varga, J.J., Vigil, A., DeShazer, D., Waag, D.M., Felgner, P. and Goldberg, J.B. 2012. Distinct human antibody response to the biological warfare agent Burkholderia mallei. Virulence 3(6), 510–514. Verma, A.K., Saminathan, M., Tiwari, R., Dhama, K. and Singh, S.V. 2014. Glanders-a re-emerging zoonotic disease: a review. J. Biol. Sci. 14(1), 38–51. Virk, H.S., Fhogartaigh, C.N. and Dance, D.A.B. 2023. Glanders and melioidosis: a zoonosis and a sapronosis. In Zoonoses: infections affecting humans and animals. Sing, A. (Ed.). Cham, Switzerland: Springer, pp: 1151–1173. Wiersinga, W.J., Virk, H.S., Torres, A.G., Currie, B.J., Peacock, S.J., Dance, D.A. and Limmathurotsakul, D. 2018. Melioidosis. Nat. Rev. Dis. Primers 4(1), 17107. Waag, D.M., England, M.J. and Deshazer, D. 2012. Humoral immune responses in a human case of glanders. Clin. Vaccine Immunol. 19(5), 814–816. Wang, G., Zarodkiewicz, P. and Valvano, M.A. 2020. Current advances in Burkholderia vaccines development. Cells 9(12), 2671. Welkos, S.L., Gregory, B.C., Waag, D.M. and Burtnick, M.N. 2018. Chapter 8: Glanders. In Medical Aspects of Biological Warfare. Dembek, Z.F. (Ed.). Fort Detrick, MD: Office of The Surgeon General, Borden Institute, pp: 178–179. Wernery, U., Joseph, S., Cavalleri, J.M. and Al Mheiri, F.G. 2025. Equine epizootic lymphangitis: a synopsis and current development. Ger. J. Vet. Res. 5(1), 30–39. Wetmore, P.W. and Gochenour Jr, W.S. 1956. Comparative studies on the genus Malleomyces and selected Pseudomonas species. J. Bacteriol. 72(1), 79–89. Wheelis, M. 1998. First shots fired in biological warfare. Nature 395(6699), 213. Whitlock, G.C., Mark Estes, D. and Torres, A.G. 2007. Glanders: off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 277(2), 115–122. Whitlock, G.C., Valbuena, G.A., Popov, V.L., Judy, B.M., Estes, D.M. and Torres, A.G. 2009. Burkholderia mallei cellular interactions in a respiratory cell model. J. Med. Microbiol. 58(Pt 5), 554–562. Wilkinson, L. 1981. Glanders: medicine and veterinary medicine in common pursuit of a contagious disease. Med. Hist. 25(4), 363–384. Yabuvchi, E., Kosako, Y., Oyaizu, H., Yano, I., Hotta, H., Hashimoto, Y., Ezaki, T. and Arakawa, M. 1992. Proposal of Burkholderia genus and transfer of seven species of the genus Pseudomonas homology group II to the new genus. J. Microbiol. Immunol. 36(12), 1251–1275. Zachary, J.F. 2017. Mechanisms of microbial infections. Pathologic. Basis. Vet. Dis. 1(1), 132–241.e1. Zakharova, I.B., Toporkov, A.V. and Viktorov, D.V. 2018. Melioidosis and glanders: current state and actual issues of epidemiological surveillance. J. Microbiol. Epidemiol. Immunobiol. 95(6), 103–110. Zheng, X., Xia, Q., Xia, L. and Li, W. 2019. Endemic melioidosis in Southern China: past and present. Trop. Med. Infect. Dis. 4(1), 39. | ||
| How to Cite this Article |
| Pubmed Style Sukmanadi M, Khairullah AR, Wardhani BWK, Mustofa I, Aliyah SH, Moses IB, Ahmad RZ, Khalisa AT, Kusala MKJ, Kurniasih DAA, Akintunde AO, Fauziah I, Wibowo S, Furqoni AH, Fauzia KA, Melati I, Pratama BP, Kurniawan M�. Glanders: Historical military use and potential bioterrorism concern. Open Vet. J.. 2025; 15(9): 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 Web Style Sukmanadi M, Khairullah AR, Wardhani BWK, Mustofa I, Aliyah SH, Moses IB, Ahmad RZ, Khalisa AT, Kusala MKJ, Kurniasih DAA, Akintunde AO, Fauziah I, Wibowo S, Furqoni AH, Fauzia KA, Melati I, Pratama BP, Kurniawan M�. Glanders: Historical military use and potential bioterrorism concern. https://www.openveterinaryjournal.com/?mno=264563 [Access: November 22, 2025]. doi:10.5455/OVJ.2025.v15.i9.1 AMA (American Medical Association) Style Sukmanadi M, Khairullah AR, Wardhani BWK, Mustofa I, Aliyah SH, Moses IB, Ahmad RZ, Khalisa AT, Kusala MKJ, Kurniasih DAA, Akintunde AO, Fauziah I, Wibowo S, Furqoni AH, Fauzia KA, Melati I, Pratama BP, Kurniawan M�. Glanders: Historical military use and potential bioterrorism concern. Open Vet. J.. 2025; 15(9): 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 Vancouver/ICMJE Style Sukmanadi M, Khairullah AR, Wardhani BWK, Mustofa I, Aliyah SH, Moses IB, Ahmad RZ, Khalisa AT, Kusala MKJ, Kurniasih DAA, Akintunde AO, Fauziah I, Wibowo S, Furqoni AH, Fauzia KA, Melati I, Pratama BP, Kurniawan M�. Glanders: Historical military use and potential bioterrorism concern. Open Vet. J.. (2025), [cited November 22, 2025]; 15(9): 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 Harvard Style Sukmanadi, M., Khairullah, . A. R., Wardhani, . B. W. K., Mustofa, . I., Aliyah, . S. H., Moses, . I. B., Ahmad, . R. Z., Khalisa, . A. T., Kusala, . M. K. J., Kurniasih, . D. A. A., Akintunde, . A. O., Fauziah, . I., Wibowo, . S., Furqoni, . A. H., Fauzia, . K. A., Melati, . I., Pratama, . B. P. & Kurniawan, . M. �. (2025) Glanders: Historical military use and potential bioterrorism concern. Open Vet. J., 15 (9), 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 Turabian Style Sukmanadi, Mohammad, Aswin Rafif Khairullah, Bantari Wisynu Kusuma Wardhani, Imam Mustofa, Siti Hamidatul Aliyah, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Adeyinka Oye Akintunde, Ima Fauziah, Syahputra Wibowo, Abdul Hadi Furqoni, Kartika Afrida Fauzia, Irma Melati, Bima Putra Pratama, and Muhammad ‘ahdi Kurniawan. 2025. Glanders: Historical military use and potential bioterrorism concern. Open Veterinary Journal, 15 (9), 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 Chicago Style Sukmanadi, Mohammad, Aswin Rafif Khairullah, Bantari Wisynu Kusuma Wardhani, Imam Mustofa, Siti Hamidatul Aliyah, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Adeyinka Oye Akintunde, Ima Fauziah, Syahputra Wibowo, Abdul Hadi Furqoni, Kartika Afrida Fauzia, Irma Melati, Bima Putra Pratama, and Muhammad ‘ahdi Kurniawan. "Glanders: Historical military use and potential bioterrorism concern." Open Veterinary Journal 15 (2025), 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 MLA (The Modern Language Association) Style Sukmanadi, Mohammad, Aswin Rafif Khairullah, Bantari Wisynu Kusuma Wardhani, Imam Mustofa, Siti Hamidatul Aliyah, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Dea Anita Ariani Kurniasih, Adeyinka Oye Akintunde, Ima Fauziah, Syahputra Wibowo, Abdul Hadi Furqoni, Kartika Afrida Fauzia, Irma Melati, Bima Putra Pratama, and Muhammad ‘ahdi Kurniawan. "Glanders: Historical military use and potential bioterrorism concern." Open Veterinary Journal 15.9 (2025), 3912-3930. Print. doi:10.5455/OVJ.2025.v15.i9.1 APA (American Psychological Association) Style Sukmanadi, M., Khairullah, . A. R., Wardhani, . B. W. K., Mustofa, . I., Aliyah, . S. H., Moses, . I. B., Ahmad, . R. Z., Khalisa, . A. T., Kusala, . M. K. J., Kurniasih, . D. A. A., Akintunde, . A. O., Fauziah, . I., Wibowo, . S., Furqoni, . A. H., Fauzia, . K. A., Melati, . I., Pratama, . B. P. & Kurniawan, . M. �. (2025) Glanders: Historical military use and potential bioterrorism concern. Open Veterinary Journal, 15 (9), 3912-3930. doi:10.5455/OVJ.2025.v15.i9.1 |