| Research Article | ||
Open Vet. J.. 2025; 15(9): 4032-4043 Open Veterinary Journal, (2025), Vol. 15(9): 4032-4043 Research Article Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilumKrasimira Gospodinova1* and Koycho Koev21Department of General and Clinical Pathology, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria 2Department of Veterinary Microbiology, Infectious and Parasitic Diseases, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria *Corresponding Author: Krasimira Gospodinova. Department of General and Clinical Pathology, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria. Email: krasimira.gospodinova [at] trakia-uni.bg Submitted: 20/06/2025 Revised: 12/08/2025 Accepted: 22/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Canine monocytic ehrlichiosis (CME) and canine granulocytic anaplasmosis (CGA), caused by Ehrlichia canis and Anaplasma phagocytophilum, are tick-borne diseases prevalent in dogs. Both trigger systemic inflammation, with C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) as potential severity biomarkers. Aim: This study aimed to compare serum CRP and TNF-α in CME, CGA, and co-infected dogs and assess their relationships with hematological, biochemical, and liver enzyme changes. Methods: In 134 dogs showing clinical signs of CME/CGA, infections were confirmed using SNAP® 4Dx® Plus, indirect immunofluorescence assay, and polymerase chain reaction (PCR). CRP and TNF-α were quantified using canine-specific enzyme-linked immunosorbent assay kits. Results: Of the dogs tested, 112 (83.6%) were seropositive and 77 (68.8%) were PCR-positive (E. canis: 27; A. phagocytophilum: 29; co-infection: 21). The co-infected group had the highest levels of CRP (135.2–154.8 mg/l) and TNF-α (161.5–174.0 pg/ml), significantly exceeding those of CME (104.6–120.9; 148.7–162.2 pg/ml), CGA (88.5–104.4; 140.1–156.5 pg/ml), PCR-negative (33.1–45.8; 12.3–18.9 pg/ml), and control groups (0.9–4.0; 0.2–2.3 pg/ml) with p < 0.001. Thrombocytopenia was common in all infected groups, with the lowest platelet counts in co-infected dogs (median 106.0 × 109/L, p < 0.001). Aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly elevated only in co-infected dogs (ALT: 89.25 U/l; AST: 69.38 U/l; p < 0.001). CRP correlated moderately with ALT/AST; TNF-α showed weaker positive associations. Conclusion: CRP and TNF-α are valuable indicators of systemic inflammation in CME and CGA, with maximal increases and stronger links to liver injury in co-infections, supporting their use in diagnosis and prognosis. Keywords: Ehrlichia canis, Anaplasma phagocytophilum, CRP, TNF-α, Co-infection, Dogs. IntroductionTick-borne diseases, such as Ehrlichia canis-induced canine monocytic ehrlichiosis (CME) and Anaplasma phagocytophilum-induced canine granulocytic anaplasmosis (CGA), are prevalent and pose significant threats to canine health. Both pathogens, members of the Anaplasmataceae family, are transmitted via ticks—Rhipicephalus sanguineus for E. canis and Ixodes species for A. phagocytophilum (Laatamna et al., 2022). Despite substantial advances in understanding their epidemiology and pathogenesis, reliable biomarkers capable of differentiating between mono- and coinfections in naturally infected dogs remain insufficiently defined. Once transmitted, these intracellular bacteria target specific leukocyte populations, eliciting distinct immune and pathological responses (Aziz et al., 2022). In such infections, anemia and thrombocytopenia are largely secondary to the inflammatory response rather than direct cytopathic effects. Ehrlichia canis primarily infects mononuclear cells, leading to systemic inflammation, immune dysregulation, and consequent hematologic abnormalities such as thrombocytopenia and anemia (Ramakant et al., 2020). In contrast, A. phagocytophilum targets neutrophils, impairing their function and promoting inflammation, which clinically manifests as polyarthritis, lethargy, and fever (Khatat et al., 2021). Both infections trigger acute-phase and cytokine-mediated immune responses that, while essential for pathogen control, can exacerbate inflammation and contribute to disease pathogenesis if dysregulated (Cardoso et al., 2020; Ismail, et al. 2022; Nahed et al., 2022). C-reactive protein (CRP), an acute-phase protein synthesized by hepatocytes in response to interleukin-6 (IL-6), is a sensitive biomarker of systemic inflammation and tissue injury (Cray, 2012). Elevated CRP levels are common in bacterial infections, including those caused by tick-borne pathogens, and reflect the inflammatory burden associated with CME (Asawakarn and Taweethavonsawat, 2021; Asawapattanakul et al., 2021). Tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine produced mainly by activated macrophages, monocytes, and dendritic cells, regulates inflammation, apoptosis, and immune cell recruitment via TNFR1 and TNFR2 (Jang et al., 2021). In CME, TNF-α production is stimulated by E. canis, contributing to macrophage and monocyte activation but, when excessive, driving systemic inflammation, tissue damage, and immune dysregulation (Lima et al., 2015). Infection with A. phagocytophilum induces significant upregulation of proinflammatory cytokines, including TNF-α, MIP-2, and IL-6, in murine bone marrow cells (Johns et al., 2009). Similarly, increased secretion of TNF-α, IL-1, MIP-2, and IL-6 has been observed in canine models of infection (Stanilov et al., 2024). The dual role of CRP and TNF-α as both mediators of inflammation and biomarkers of immune dysregulation makes them highly relevant for investigating the pathophysiology of CME and CGA (Lima et al., 2015; Asawapattanakul et al., 2021). Their elevated concentrations may assist in distinguishing between mono- and co-infections (Asawakarn and Taweethavonsawat, 2021). However, limited data exist on their concurrent changes in naturally co-infected dogs and their relationship with liver injury indicators such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Cardoso et al., 2020). Moreover, their combined diagnostic and prognostic potential—particularly in co-infections where immune responses may be amplified or dysregulated—remains underexplored (Aziz et al., 2022). This study aimed to evaluate and compare serum levels of CRP and TNF-α in dogs infected with E. canis and A. phagocytophilum. By elucidating their role in the inflammatory and immune responses associated with these infections, this research seeks to provide insights into disease pathogenesis and highlight their potential as biomarkers for improved diagnosis, monitoring, and management of canine tick-borne diseases. Materials and MethodsA total of 134 client-owned dogs were enrolled in this study. All animals were presented to the Small Animal Clinic at the University Hospital of Trakia University in Stara Zagora, Bulgaria, with a documented history of tick infestations. Dogs were eligible for inclusion if they had confirmed infections with E. canis or A. phagocytophilum or if they exhibited clinical signs consistent with CME—such as fever, anemia, and thrombocytopenia—or CGA, characterized by lethargy, polyarthritis, and thrombocytopenia. Each dog underwent complete hematological and biochemical evaluation and was tested for E. canis and A. phagocytophilum by rapid enzyme-linked immunosorbent assay (ELISA; SNAP® 4Dx® Plus, IDEXX Laboratories, Westbrook, ME), indirect immunofluorescence assay (IFA; Mega FLUO® E. canis and Mega FLUO® A. phagocytophilum, Megacor Diagnostik GmbH, Austria), and polymerase chain reaction (PCR) with Anaplasmataceae-specific primers (16S rRNA gene) and species-specific primers for E. canis (16S rRNA gene) and A. phagocytophilum (ankA gene). The dogs ranged in age from 1 to 12 years (mean ± SD: 6.4 ± 3.1 years) and included 72 (53.7%) males and 62 (46.3%) females. Most were mixed-breed dogs (57.5%), followed by German Shepherds (14.9%), Labrador Retrievers (8.2%), and Rottweilers, Beagles, and Setters (19.4%). The majority of respondents originated from rural areas (61.2%), while the remainder lived in urban environments (38.8%). Although cases were recorded year-round, most presentations occurred in spring and summer, coinciding with the region’s peak tick activity. Group distributionDogs that tested positive for antibodies to E. canis and A. phagocytophilum by serology were subsequently examined by uniplex PCR using Anaplasmataceae-specific primers and species-specific primers targeting E. canis and A. phagocytophilum. Based on clinical, hematological, biochemical, serological, and molecular results, the dogs were classified into three groups:
Blood was collected from the external cephalic vein (vena cephalica antebrachii externa) into ethylenediaminetetraacetic acid-coated vacuum tubes for hematological analysis and into plain tubes for serum separation. Hematology was performed using an automated analyzer (MINDRAY BC-5000 Vet; Shenzhen Mindray Animal Medical Technology Co., Ltd.), and serum biochemistry was analyzed using an automated chemistry analyzer (MINDRAY BS-120; Mindray Medical India Pvt. Ltd.). Diagnostic proceduresSerologyRapid diagnostic test (SNAP® 4Dx® Plus)Serum samples were tested for IgG antibodies to A. phagocytophilum, Anaplasma platys, E. canis, and Ehrlichia ewingii and for circulating antigens of Dirofilaria immitis using the SNAP® 4Dx® Plus assay according to the manufacturer’s instructions. Two drops of serum were mixed with horseradish peroxidase–conjugated proteins, and the resulting antigen–antibody complexes were detected using colorimetric analysis. Indirect IFASera were analyzed using Mega FLUO® E. canis and Mega FLUO® A. phagocytophilum kits to detect IgG antibodies specific to E. canis and A. phagocytophilum. Samples were diluted in phosphate-buffered saline (PBS; pH 7.2–7.4) and incubated at 37°C to allow antigen–antibody binding. Nonspecific proteins were removed by washing, and fluorescence was observed under a microscope at 400× magnification. PCRDNA extractionGenomic DNA was extracted from whole blood using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol. DNA was stored at −20°C until further analysis. PCR amplificationGradient PCR (AERIS PCR system, Esco, Singapore) was used to optimize annealing temperatures. Initial screening employed Anaplasmataceae-specific 16S rRNA primers (Parola et al., 2000; Table 1), followed by species-specific primers for a 409 bp E. canis 16S rRNA fragment (Inokuma et al., 2003; Table 1) and a 444 bp A. phagocytophilum ankA fragment (Caturegli et al., 2000; Walls et al., 2000; Table 1). Table 1. PCR primers used in this study.
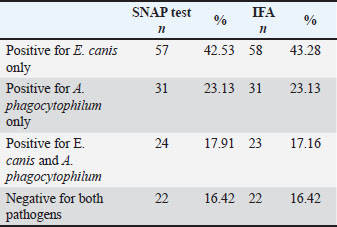
PCR conditionsReactions included positive controls (confirmed DNA from E. canis and A. phagocytophilum) and a negative control (nuclease-free water). Cycling conditions were as follows: 95°C for 5 minute; 35 cycles of 94°C for 30 second, annealing at 55°C–60°C for 30 second (gene-dependent), 72°C for 1 minute; and final extension at 72°C for 10 minute. Gel electrophoresisAmplicons were separated on a 1.5% agarose gel stained with ethidium bromide. A DNA ladder was used to determine the product size. Bands were visualized and documented under UV light. Method validationPCR protocols, including cycling conditions, primer design, and gel electrophoresis, were validated in a previous study (Gospodinova et al., 2024) to ensure reproducibility and accuracy. All dogs enrolled in the study. Biomarker measurementC-reactive proteinPlasma CRP concentrations were measured using a fluorescence immunoassay kit (InSight V-IA Canine CRP Rapid Quantitative Test, Woodley Equipment Company Ltd., UK) according to the manufacturer’s instructions, with analysis performed on the InSight V-IA Veterinary Immunoassay Analyzer. The samples were measured in duplicate, and the mean values were used for statistical analysis. The intra- and inter-assay coefficients of variation were <10% and <15%, respectively. The detection range was 2–250 mg/l, with a reference value for healthy dogs of <10 mg/l. Tumor necrosis factor alphaSerum TNF-α concentrations were determined using a canine-specific sandwich ELISA kit (Canine TNF-α ELISA Kit, FineTest®, China), following the manufacturer’s protocol. Optical density was measured at 450 nm using an ELISA plate reader (LEDETECT 96, Labexim Produkt, Biomed Dr. Weisser GmbH, Austria). All samples were analyzed in duplicate, and the concentrations were calculated from a standard curve. The assay sensitivity was <9.375 pg/ml, with a working range of 15.625–1,000 pg/ml. The intra- and inter-assay coefficients of variation were <8% and <10%, respectively. Statistical analysisData were analyzed using IBM SPSS Statistics version 29.0.2 (IBM Corp., Armonk, NY), GraphPad Prism version 10.0.3 (GraphPad Software, San Diego, CA), and, when appropriate, available online calculators from the StatPages.net website (http://statpages.org/index.html, accessed on June 2, 2024). The Shapiro–Wilk test was used to assess normality. Normally distributed variables are presented as mean ± SE (min–max), and non-normally distributed variables are presented as median (IQR, min–max). Group differences for CRP, TNF-α, hematological, and biochemical parameters were evaluated using one-way analysis of variance with Tukey’s post hoc test, and correlations with liver enzymes were assessed using Pearson’s correlation coefficient. Statistical significance was set at p < 0.05. Ethical approvalThe Local Ethics Committee of the Faculty of Veterinary Medicine, Trakia University, approved the study (FVM-09/protocol, June 9, 2020). Informed consent was obtained from the study population. ResultsSerological findingsOf the 134 blood samples tested with SNAP® 4Dx® Plus (IDEXX Laboratories, Westbrook, ME), 112 (83.6%) were positive for antibodies to Ehrlichia spp. and/or Anaplasma spp., while 22 (16.4%) were negative (Table 2). All samples were negative for Borrelia burgdorferi antibodies and D. immitis antigen, and no dogs were microfilaremic. Table 2. Number and percentage of dogs who were positive in the SNAP and IFA tests according to the evaluated groups.
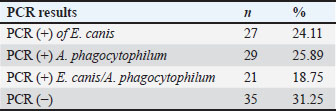
IFA detected IgG antibodies against E. canis (titer ≥1:40) in 58 samples, A. phagocytophilum in 31 samples, and both pathogens in 23 samples. Twenty-two dogs were negative for both the SNAP and IFA tests and served as controls. PCR resultsAll 112 antibody-positive dogs were tested for Anaplasmataceae-specific 16S rRNA sequences by PCR. Seventy-seven (68.8%) were PCR positive and 35 (31.2%) were negative. Species-specific PCR identified E. canis alone in 27 samples, A. phagocytophilum alone in 29, and both pathogens in 21 samples (Table 3). Table 3. Number and percentage of PCR-positive samples according to the evaluation groups.
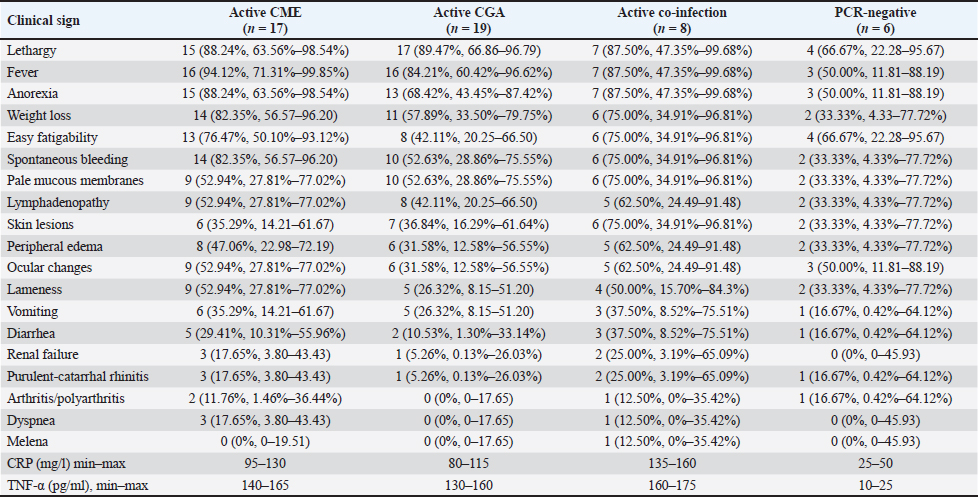
Clinical signsThe distribution of clinical signs among PCR-positive and PCR-negative dogs, along with CRP and TNF-α concentration ranges, is summarized in Table 4. The most frequent signs in the CME group included fever (94.1%), lethargy (88.2%), anorexia (88.2%), spontaneous bleeding (82.4%), and weight loss (82.4%). Similar trends were observed in the CGA group, where lethargy (89.5%), fever (84.2%), anorexia (68.4%), and spontaneous bleeding (52.6%) predominated. Dogs with co-infections exhibited a broader spectrum of abnormalities, including lethargy (87.5%), fever (87.5%), spontaneous bleeding (75.0%), skin lesions (75.0%), and weight loss (75.0%), often accompanied by ocular changes (62.5%). Less frequent but clinically relevant signs included arthritis/polyarthritis (up to 12.5%), renal insufficiency (up to 25.0%), and melena (12.5%). PCR-negative dogs had fewer and milder signs, most often lethargy (66.7%) and easy fatigability (66.7%), with occasional fever, ocular changes, or gastrointestinal symptoms. Plasma CRP and TNF-α levels tended to be higher in dogs with more severe or multiple clinical manifestations, particularly in the co-infected group. Table 4. Clinical signs, CRP and TNF-α levels in dogs naturally infected with E. canis, A. phagocytophilum, or both pathogens, and PCR-negative dogs.
CRP and TNF-α levelsShapiro–Wilk testing confirmed that CRP and TNF-α concentrations followed a normal distribution (p > 0.05). CRP levels were significantly different among the groups (ANOVA, F(4, 129)=187.77, p < 0.0001). The co-infected group had the highest CRP concentrations, ranging from 135.2 to 154.8 mg/l, exceeding those observed in dogs with CME (104.6–120.9 mg/l, p < 0.001), CGA (88.5–104.4 mg/l, p < 0.001), PCR-negative dogs (33.1–45.8 mg/l, p < 0.001), and clinically healthy controls (0.9–4.0 mg/l, p < 0.001). CRP values in CME and CGA groups were also significantly higher than those in PCR-negative and control dogs (p < 0.001) (Fig. 1).
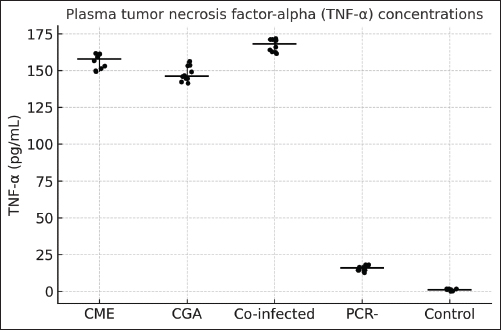
Fig. 1. Plasma CRP concentrations in dogs with active CME, CGA, co-infection, PCR-negative dogs (post-infection or seropositive animals), and healthy controls. Horizontal bars represent the median values, and whiskers indicate the minimum–maximum range. TNF-α concentrations also differed significantly among groups (ANOVA, F(4, 129)=493.96, p < 0.0001). Co-infected dogs had TNF-α levels ranging from 161.5 to 174.0 pg/ml, which were higher than those in CGA (140.1–156.5 pg/ml, p < 0.01), CME (148.7–162.2 pg/ml, p < 0.05), PCR-negative (12.3–18.9 pg/ml, p < 0.001), and control dogs (0.2–2.3 pg/ml, p < 0.001). PCR-negative dogs also had significantly higher TNF-α than controls (p < 0.01) (Fig. 2).
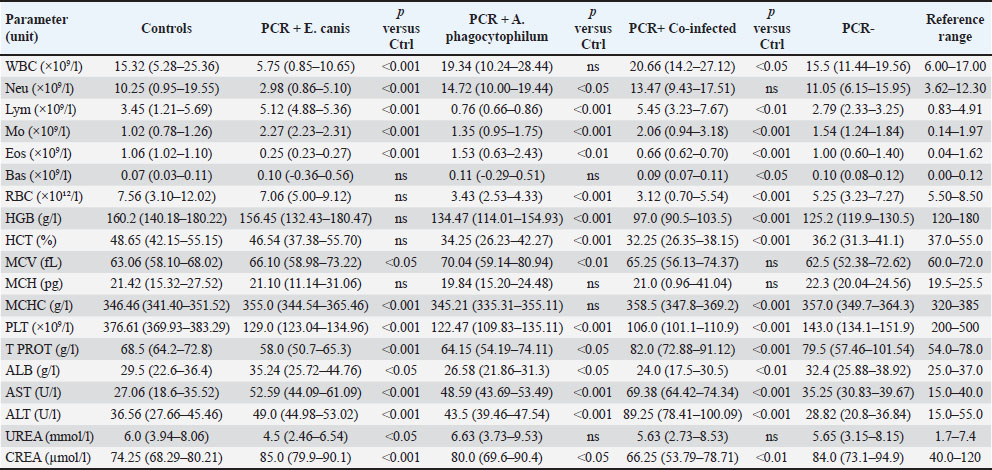
Fig. 2. Plasma TNF-α concentrations in dogs with CME, CGA, co-infection, PCR-negative, and healthy control groups Horizontal bars represent the median values, and whiskers indicate the minimum–maximum range. Hematological and biochemical parametersThrombocytopenia, leukopenia, lymphopenia, and neutropenia were common in E. canis-positive dogs (Table 5). Elevated AST level was the most frequent biochemical abnormality. Table 5. Hematological and biochemical parameters (median, min–max) with p-values is controls.
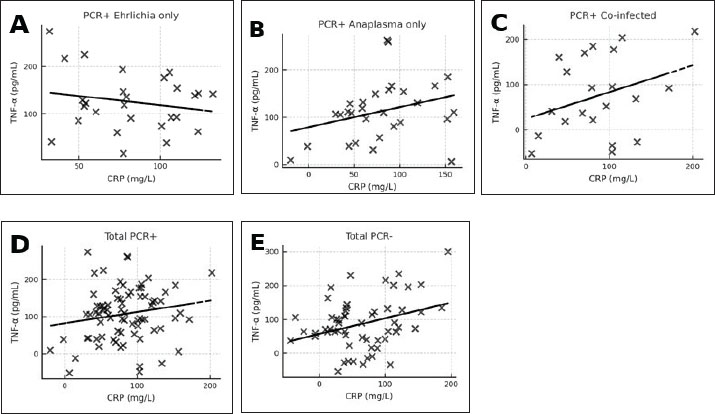
A. phagocytophilum-positive dogs most often showed erythropenia, low hematocrit, thrombocytopenia, leukopenia, lymphopenia, and neutropenia, with occasional AST elevation. Co-infected dogs exhibited marked thrombocytopenia, leukocytosis, granulocytosis, low hematocrit, erythropenia, hemoglobinemia, and lymphocytosis. Platelets (in hematology/clinical context) (PLT) was significantly reduced in all infected groups compared with controls (p < 0.001), with the lowest values observed in co-infected dogs (median 106.0 × 109/;, range 101.1–110.9). Biochemical changes were more frequent in the co-infected group, with increased creatinine, urea, ALT, and AST levels and occasional hyperproteinemia and hyperglobulinemia with hypoalbuminemia. Increases in ALT and AST activities were predominantly observed in co-infected dogs, with median values exceeding the upper reference limits [ALT: 89.25 (78.41–100.09) U/l, reference range: 15.0–55.0 U/l; AST: 69.38 (64.42–74.34) U/l, reference range: 15.0–40.0 U/l; both p < 0.001 vs. controls]. No significant elevations were detected in the CME, CGA, or PCR-negative groups. PCR-negative dogs had mild thrombocytopenia, anemia, and slight hyperglobulinemia (Table 5). Correlation analysesCorrelation between CRP and TNF-α levels in the different infection groupsPearson’s correlation analysis was conducted to evaluate the relationship between plasma CRP and TNF-α concentrations within each infection category (Fig. 3A–E; Table 6).
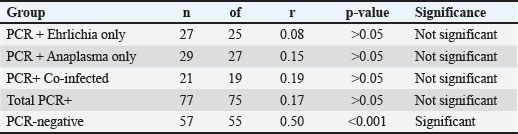
Fig. 3. (A–E) Correlation between CRP and TNF-α concentrations in different infection groups: (7A) PCR-positive Ehrlichia only (r=0.08, p > 0.05, ns), (7B) PCR-positive Anaplasma only (r=0.15, p > 0.05, ns), (7C) PCR-positive co-infection with E. canis and A. phagocytophilum (r=0.19, p > 0.05, ns), (7D) combined PCR-positive group (r=0.17, p > 0.05, ns); and (7E) PCR-negative dogs (seropositive, post-infection animals) (r=0.50, p < 0.001, *Statistical significance: is=not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001. Table 6. Pearson’s correlation coefficients (r), p-values, and degrees of freedom (df) for the relationship between CRP and TNF-α concentrations in different infection groups.
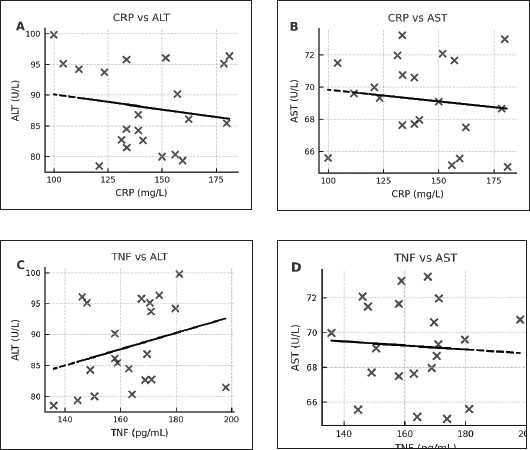
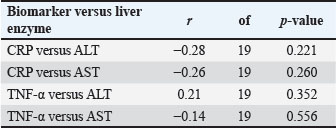
In the E. canis-positive group (PCR+ Ehrlichia only; n=27), CRP and TNF-α values showed a weak, non-significant positive correlation (r=0.08, df=25, p > 0.05). Similarly, in the A. phagocytophilum-positive group (PCR+ Anaplasma only; n=29), the correlation remained low and non-significant (r=0.15, df=27, p > 0.05). The co-infected group (PCR+ for both pathogens; n=21) exhibited a slightly stronger correlation (r=0.19, df=19, p > 0.05), which also did not reach statistical significance. When all PCR-positive dogs were analyzed together (Total PCR+; n=77), the correlation remained weak and non-significant (r=0.17, df=75, p > 0.05). In contrast, the PCR-negative group (n=57), consisting of seropositive dogs without active infection, demonstrated a moderate and statistically significant positive correlation between CRP and TNF-α (r=0.50, df=55, p < 0.001). These results indicate that while CRP and TNF-α levels show limited correlation during active E. canis or A. phagocytophilum infection, their association is more pronounced in PCR-negative but seropositive dogs, possibly reflecting post-infectious immune modulation. Correlation between inflammatory markers and liver enzyme activity in co-infected dogsWithin the co-infected group, Pearson’s correlation analysis revealed moderate negative associations between CRP and both ALT and AST activities, whereas TNF-α levels demonstrated a weak positive correlation with ALT and a weak negative correlation with AST (Table 7, Fig. 4A–D). These findings indicate that cytokine-mediated inflammation may contribute to liver enzyme alterations in co-infected dogs, although the association strength was generally low.
Fig. 4. (A–D) Scatter plots illustrating the relationships between CRP and ALT (A), CRP and AST (B), TNF-α and ALT (C), and TNF-α and AST (D) in dogs co-infected with Ehrlichia canis and A. phagocytophilum. Linear regression lines illustrate the observed overall trends between the variables. Table 7. Correlation between inflammatory biomarkers (CRP, TNF-α) and liver enzymes (ALT and AST) in PCR-positive dogs.
DiscussionCRP and acute phase proteinsCRP remains the principal acute-phase protein in dogs and a sensitive, nonspecific biomarker of systemic inflammation (Malin and Witkowska-Piłaszewicz, 2022). CRP concentrations varied significantly across infection categories (ANOVA, p < 0.0001), with co-infected dogs showing the highest values (135.2–154.8 mg/l), significantly exceeding those in CME (104.6–120.9 mg/l, p < 0.001) and CGA (88.5–104.4 mg/l, p < 0.001). These levels were also significantly higher than those in PCR-negative (33.1–45.8 mg/l, p < 0.001) and control dogs (0.9–4.0 mg/l, p < 0.001). This gradient mirrors disease severity and aligns with previous reports that dual infections exacerbate systemic inflammatory responses (Sainz et al., 2015). Our findings reinforce the clinical utility of CRP in identifying dogs with active and particularly severe vector-borne disease, supporting its routine inclusion in diagnostic panels in endemic regions. TNF-α and cytokine dynamicsTNF-α is a pleiotropic cytokine with a dual role: it activates antimicrobial immune mechanisms and contributes to tissue injury when excessively produced. We observed significantly elevated TNF-α in all infected groups (ANOVA, p < 0.0001), with the highest values observed in co-infected dogs (161.5–174.0 pg/ml), surpassing CGA (140.1–156.5 pg/ml, p < 0.01), CME (148.7–162.2 pg/ml, p < 0.05), PCR-negative (12.3–18.9 pg/ml, p < 0.001), and controls (0.2–2.3 pg/ml, p < 0.001). Higher TNF-α levels in co-infected animals were correlated with more severe clinical signs, including bleeding tendencies, skin lesions, and ocular changes, suggesting that TNF-α elevation is clinically relevant rather than a subclinical finding. The source of this TNF-α likely includes macrophages and monocytes, but also CD8+ cytotoxic T lymphocytes, which are strongly activated during E. canis and A. phagocytophilum infections (Scorpio et al., 2006; Castro et al., 2022). Upon antigen recognition, CD8+ T cells secrete TNF-α alongside perforin and granzyme, promoting pathogen clearance but also contributing to hepatocyte apoptosis and endothelial damage. This mechanism may explain the elevated levels of hepatic enzymes observed in co-infected dogs. Hepatic involvement and inflammatory markersALT and AST activities were significantly increased only in co-infected dogs (ALT: median 89.25 U/l, AST: median 69.38 U/l; both p < 0.001 vs. controls). Moderate positive correlations between CRP and both ALT (r ≈ 0.45) and AST (r ≈ 0.42), and weaker positive associations for TNF-α (ALT: r ≈ 0.30, AST: r ≈ 0.28), suggest that systemic inflammation exacerbates hepatocellular stress. TNFα itself can directly impair hepatocyte function through apoptosis and proinflammatory cascade induction, potentially creating a feedback loop linking immune activation to hepatic pathology in severe coinfections (Tiegs and Horst, 2022). Since CRP synthesis is largely IL-6–driven in hepatocytes, liver injury may amplify acute-phase protein production. IL-6 is recognized as the primary inducer of hepatic CRP gene expression (Sproston and Ashworth, 2018). TNF-α itself can directly impair hepatocyte function through apoptosis and proinflammatory cascade induction, potentially creating a feedback loop linking immune activation to hepatic pathology in severe coinfections. Specifically, TNF-α sensitizes hepatocytes to FasL-induced apoptosis via c-Jun N-terminal kinase activation and Nuclear Factor kappa-light-chain-enhancer of activated B cells mediated Fas upregulation (Faletti et al., 2018). Clinical implications of co-infectionsIn this study, co-infected dogs exhibited the most severe hematological and biochemical changes, including marked thrombocytopenia (median 106.0 × 109/l), leukocytosis, granulocytosis, anemia, and hypoalbuminemia. These findings indicate that E. canis and A. phagocytophilum infection synergistically amplifies inflammatory, immune-mediated, and metabolic disturbances. From a clinical perspective, high CRP and TNF-α values—especially when combined with elevated liver enzymes—should prompt consideration of co-infection, guide more intensive monitoring, and potentially influence therapeutic decisions, including early anti-inflammatory or hepatoprotective interventions. While earlier work (Rikihisa, 2000; Mylonakis et al., 2004) documented cytokine and acute-phase responses in experimental ehrlichiosis and anaplasmosis, our results from naturally infected dogs confirm that co-infection amplifies these responses in a real-world setting. More recent studies (Saito and Walker, 2016; Castro et al., 2022) have similarly highlighted the role of Th1-skewed immunity and CD8+ T-cell activation in driving both pathogen control and immunopathology, particularly in the liver. Our data extend this understanding by providing evidence of direct statistical associations between inflammatory mediators and biochemical markers of hepatic injury. ConclusionOur results show that co-infection with A. phagocytophilum and E. canis in naturally infected dogs produces the highest CRP and TNF-α concentrations, the most pronounced hematological alterations, and significant liver enzyme elevations, reflecting severe systemic and hepatic involvement. The combined assessment of CRP, TNF-α, and liver function tests can help identify high-risk patients and may guide prognosis and treatment intensity. Future research should validate these biomarkers in larger populations, assess additional cytokines, such as procalcitonin and interleukins, and explore immunomodulatory strategies targeting CD8+ T-cell–driven TNF-α production to mitigate hepatic injury. LimitationsAlthough this study provides valuable insights into the inflammatory response to E. canis and A. phagocytophilum, the following limitations must be considered: The sample size was adequate; it could be expanded to improve the generalizability of the results. A broader range of inflammatory markers and cytokines could be examined to deepen our understanding of the immune response. This study did not assess the effect of treatment on CRP and TNF-α levels, which could provide insights into the therapeutic efficacy of the treatment. AcknowledgmentsThis research was supported by the Bulgarian Ministry of Education and Science under the National Program “Young Scientists and Posdoctoral Students – 2”. Conflict of interestThe authors declare that they have no conflict of interests. FundingThis work was supported by the Bulgarian Ministry of Education and Science (MES) in the National Program “Young Scientists and Posdoctoral Students – 2”. Authors’ contributionsConceptualization, K.G. and K.K.; methodology, software, validation, formal analysis investigation, K.G.; resources and data curation, K.K. and K.G.; writing—original draft preparation and writing—review and editing, K.K.; supervision, K.K.; program administration, K.G.; funding acquisition, K.G. and K.K. All authors have read and approved the published version of the manuscript. Data availabilityThe data presented in this study are available upon request from the corresponding author due to privacy concerns. ReferencesAsawapattanakul, T., Pintapagung, T., Piratae, S., Juntautsa, S. and Chancharoen, P. 2021. Erythrocyte sedimentation rate, C-reactive protein, and interleukin-6 levels as inflammatory biomarkers in dogs naturally infected with Ehrlichia canis: a case report. Vet. World 14(9), 2325. Asawakarn S, Taweethavonsawat P. 2021. Serum protein electrophoresis patterns and C-reactive protein in canine tick-borne diseases. Vet. World 14(8), 2150. Aziz, M.U., Hussain, S., Song, B., Ghauri, H.N., Zeb, J. and Sparagano, O.A. 2022. Ehrlichiosis in dogs: a comprehensive review of the pathogen and its vectors with emphasis on South and East Asian countries. Vet. Sci. 10(1). Cardoso, S.P., Paludo, G.R., da Silva, J.N., Honório-França, A. and França. E.L. 2020. Hemorheological evaluation and cytokine production in dogs naturally infected with Anaplasmataceae In: Parasitology and microbiology research. London, UK: IntechOpen, pp: 1–25. Castro, M.B.D., Szabó, M.P.J., Aquino, L.P.C.T.D., Dagnoni, A.S., Alessi, A.C., Costa, M.T., Nakaghi, A.C., Santi, M., Calchi, A.C., André, M.R. and Machado, R.Z. 2022. Alterações imunofenotípicas e patológicas em cães experimentalmente infectados por Ehrlichia canis. Revista Brasileira de Parasitol. Vet. 31, e021621. Caturegli, P., Asanovich, K.M., Walls, J.J., Bakken, J.S., Madigan, J.E., Popov, V.L. and Dumler, J.S. 2000. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. J. Biol. Biotechnol. 3, 69. Cray, C. 2012. Acute protein phase in animals. Prog. Mol. Biol. Transl. Sci. 105, 113–150; doi: 10.1080/23144599.2023.2247250 El Hamiani Khatat, S., Daminet, S., Duchateau, L., Elhachimi, L., Kachani, M. and Sahibi, H. 2021. Epidemiological and clinicopathological features of Anaplasma phagocytophilum infection in dogs: a systematic review. Front. Vet. Sci. 8, 686644. Faletti, L., Peintner, L., Neumann, S., Sandler, S., Grabinger, T., Mac Nelly, S. and Borner, C. 2018. TNFα sensitizes hepatocytes to FasL-induced apoptosis by NFκB-mediated Fas upregulation. Cell Death and Dis. 9(9), 909; doi: 10.1016/j.cdx.2018.09.019 Gospodinova K, Stanilov I, Miteva L, Tsachev, I. and Petrov, V. 2024. Molecular detection of Ehrlichia canis and Anaplasma phagocytophilum in blood samples from dogs in Bulgaria. Bulgarian J. Vet. Med. 27. Inokuma, H., Beppu, T., Okuda, M., Shimada, Y. and Sakata, Y. 2003. Epidemiological survey of A. platys and E. canis using ticks collected from dogs in Japan. Vet. Parasitol. 115(4), 343–348. Ismail, N., Sharma, A., Soong, L. and Walker, D.H. 2022. Protective immunity and immunopathology of ehrlichiosis. Zoonoses (Burlington, Mass) 2(1), 10–15212. Jang, D.I., Lee, A.H., Shin, H.Y., Song, H.R., Park, J.H., Kang, T.B., Lee, S.R. and Yang, S.H. 2021. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 22(5), 2719; doi: 10.3390/ijms22052719 Johns, J.L., Macnamara, K.C., Walker, N.J., Winslow, G.M. and Borjesson, D.L. 2009. Infection with A. phagocytophilum induces the production of KC, MIP-2, JE, TNF-α, and IL-6 in murine bone marrow cells. Infect Immun. 77(11), 4439–4448; doi:10.1128/IAI.00570-09 Laatamna, A., Strube, C., Bakkes, D.K., Schaper, S., Aziza, F.Z., Ben Chelef, H., Amrane, N.E.H., Bedraoui, R., Dobler, G. and Chitimia-Dobler, L. 2022. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus sensu stricto collected from dogs in the steppe and high plateau regions of Algeria. Acta Trop. 234, 106582; doi: 10.1016/j.actatropica.2022.106582 Lima, A.L., Santos, G.J.L., Roatt, B.M., Reis, A.B., Freitas, J.C.C. and Nunes-Pinheiro, D.C.S. 2015 Serum TNF-α and IL-10 in Ehrlichia spp. naturally infected dogs. Act. Sci. Vet. 43, 1322. Malin, K. and Witkowska-Piłaszewicz, O. 2022. C-reactive protein as a diagnostic marker in dogs: a review. Animals. 12, 2888. Mylonakis, M.E., Koutinas, A.F., Breitschwerdt, E.B., Hegarty, B.C., Billinis, C. and Leontides, L.S. 2004. Hematological and acute phase protein changes in dogs experimentally infected with A. platys Vet. Clin. Pathol. 33(4), 238–243; doi:10.1111/j.1939-16 Ramakant, R.K., Verma, H.C. and Diwakar, R.P. 2020. Canine ehrlichiosis: a review. J. Entomol. Zool. Stud. 8(2), 1849–1852. Rikihisa, Y. 2000. Diagnosis of emerging ehrlichial diseases in dogs, horses, and humans. J. Vet. Intern. Med. 14, 250–251. Sainz, Á., Roura, X., Miró, G., Estrada-Peña, A., Kohn, B., Harrus, S. and Solano-Gallego, L. 2015. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 8, 75. Saito, T.B. and Walker, D.H. 2016. Ehrlichioses: an important one health opportunity. Vet. Sci. 3, 20. Scorpio, D.G., von Loewenich, F.D., Göbel, H., Bogdan, C. and Dumler, J.S. 2006. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin. Vaccine Immunol. 13(7), 806–809; doi: 10.1128/CVI.00092-06 Sproston, N.R. and Ashworth, J.J. 2018. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754–756. Stanilov, I., Gospodinova, K., Petrov, V., Miteva, L., Tsachev, I. and Stanilova, S. 2024. Enhanced production of IL-10 in PCR-positive dogs infected with E. canis and A. phagocytophilum facilitate specific immune responses. Microorganisms 12(12), 2516; doi: 10.3390/microorganisms12122516 Tiegs, G. and Horst, A.K. 2022. TNF in the liver: targeting a central player in inflammation. Semin Immunopathol. 44(4), 445–459; doi: 10.1007/s00281-022-00910-2 Walls, J.J., Caturegli, P., Bakken, J.S., Asanovich, K.M. and Dumler, J.S. 2000. I proved the sensitivity of PCR for the diagnosis of human granulocytic ehrlichiosis using the epank1 gene of Ehrlichia phagocytophila-group ehrlichia. J. Clin. Microbiol. 38, 354–356. | ||
| How to Cite this Article |
| Pubmed Style Gospodinova K, Koev K. Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Vet. J.. 2025; 15(9): 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 Web Style Gospodinova K, Koev K. Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. https://www.openveterinaryjournal.com/?mno=265605 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.8 AMA (American Medical Association) Style Gospodinova K, Koev K. Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Vet. J.. 2025; 15(9): 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 Vancouver/ICMJE Style Gospodinova K, Koev K. Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 Harvard Style Gospodinova, K. & Koev, . K. (2025) Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Vet. J., 15 (9), 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 Turabian Style Gospodinova, Krasimira, and Koycho Koev. 2025. Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Veterinary Journal, 15 (9), 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 Chicago Style Gospodinova, Krasimira, and Koycho Koev. "Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum." Open Veterinary Journal 15 (2025), 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 MLA (The Modern Language Association) Style Gospodinova, Krasimira, and Koycho Koev. "Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum." Open Veterinary Journal 15.9 (2025), 4032-4043. Print. doi:10.5455/OVJ.2025.v15.i9.8 APA (American Psychological Association) Style Gospodinova, K. & Koev, . K. (2025) Evaluation of serum levels of CRP and TNF-α in dogs infected with Ehrlichia canis and Anaplasma phagocytophilum. Open Veterinary Journal, 15 (9), 4032-4043. doi:10.5455/OVJ.2025.v15.i9.8 |