| Research Article | ||
Open Vet. J.. 2025; 15(6): 2532-2539 Open Veterinary Journal, (2025), Vol. 15(6): 2532-2539 Research Article Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in ratsNoran Ibrahim Salah1*, Reda M. Abd El-Aziz2, Sameh Elnabtity1 and Wageh Sobhy Darwish31Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Food Hygiene, Safety, and Technology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt * Corresponding Author: Noran Ibrahim Salah. Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: noransalah2162013 [at] gmail.com Submitted: 30/05/2025 Revised: 02/06/2025 Accepted: 07/06/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
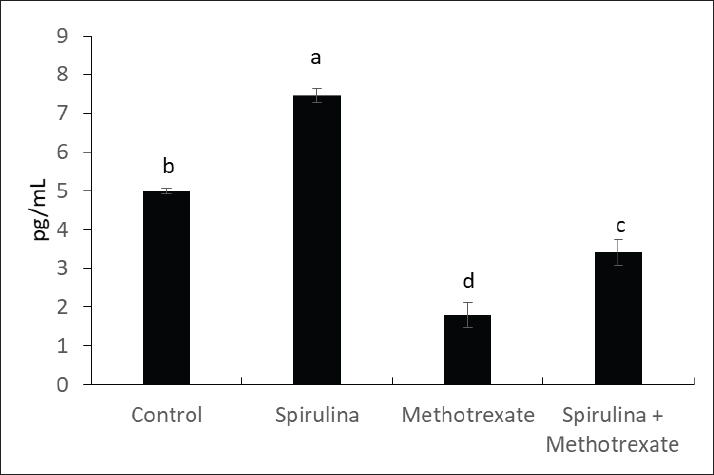
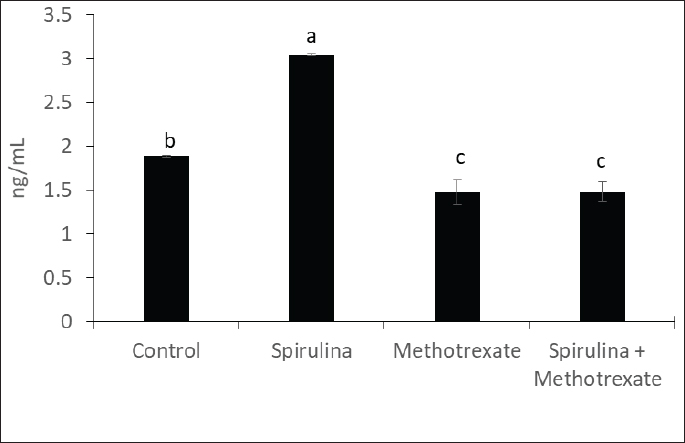
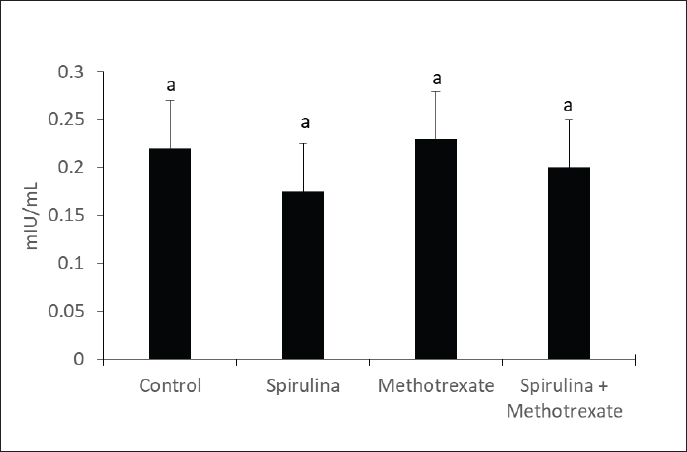
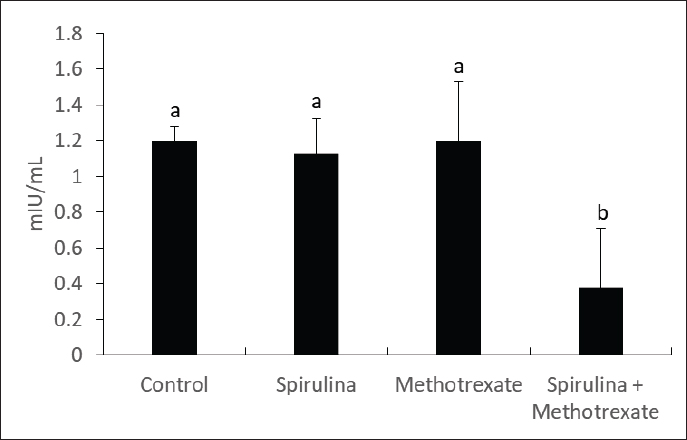
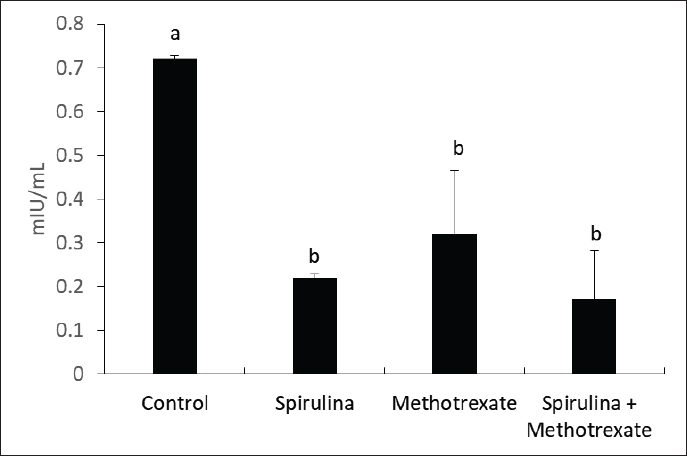
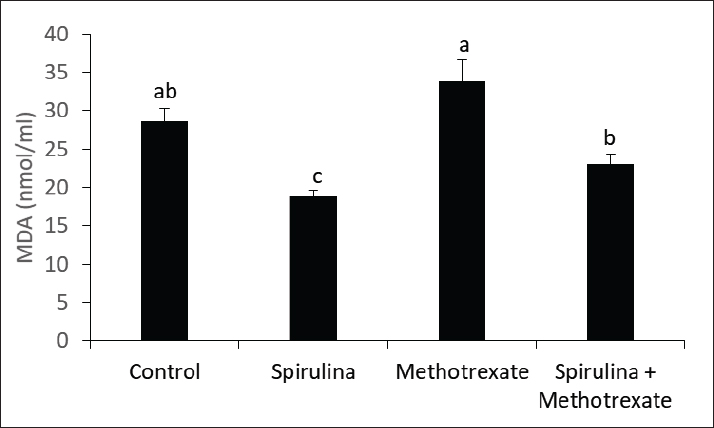
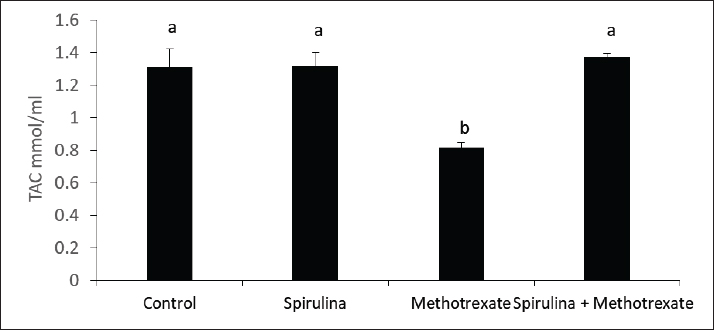
AbstractBackground: Methotrexate is a chemotherapy drug that is used despite having several toxic effects. Spirulina is commonly consumed as a food supplement because of its antioxidant, antiinflammatory, and other beneficial effects. Aim: The present study was conducted to investigate the adverse effects of MTX on male sex hormones and subsequently its effects on oxidant/antioxidant status. The ameliorative effects of spirulina against such MTX-induced adverse effects were also evaluated. Methods: Forty male rats were randomly divided into four groups: control, spirulina, MTX, and MTX + spirulina groups. The levels of testosterone (free and total), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin hormone were evaluated as biochemical markers of MTX-induced infertility. Malondialdehyde (MDA) and total antioxidant capacity (TAC) levels were assayed in liver tissue as oxidative stress markers. Results: The results obtained from the current study showed that Spirulina improved the adverse effects of MTX on testosterone (free and total), LH, FSH, MDA, and TAC. Conclusions: The current study indicated that spirulina can be used to ameliorate MTX-induced adverse effects on male infertility and oxidative stress. Keywords: Methotrexate, Spirulina, Infertility, Oxidative stress. IntroductionThe chemotherapeutic agent methotrexate (4-amino-10-methylfolic acid) (MTX) is commonly prescribed for the treatment of cancer (Chan and Cronstein, 2013). Rheumatological, inflammatory, autoimmune, dermatological, and cancer-related illnesses have long been treated with MTX (Cetinkaya et al., 2006). At doses below 25 mg, the gastrointestinal tract is the primary route of MTX absorption; however, intravenous (IV) administration is advised for doses beyond 25 mg. After IV administration, MTX is excreted from plasma in three distinct phases. Approximately half of the MTX is bound to plasma proteins. Nearly all of the MTX excreted is found in urine (Gilman et al., 2018). After MTX is metabolized in the liver, it is normally removed from the body by the kidneys. The 7-hydroxymethotrexate form of MTX is eliminated from the body within 24 hours of ingestion by renal filtration and tubular reabsorption. One of the main MTX byproducts is 7-hydroxymethotrexate. Bile can excrete a trace amount of MTX (Bedoui et al., 2019). As part of cancer treatment plans, high-dose MTX (HD-MTX; 500 mg) is utilized to reduce the production of DNA and RNA, which in turn causes cells to die (malignant cells). While the inhibition of DNA and RNA synthesis may not account for the anti-inflammatory effects of low-dose MTX (50 mg) in inflammatory diseases, other potential mechanisms of action include increased adenosine levels leading to decreased neutrophil chemotaxis and the inhibition of polyamine-derived lymphotoxin synthesis (Han et al., 2022). Multiple animal studies have demonstrated the harmful side effects of methotrexate. Aslankoc et al. (2020) reported that it might have negative effects on the reproductive system as well as hepatotoxicity and nephrotoxicity. Yulug et al. (2013) found that male factor infertility was present in 25%–50% of cases. Testicular damage is the most severe possible adverse effect of MTX. Research has connected MTX to an increase in reactive oxygen species (ROS) and oxidative stress. Male fertility is significantly affected by the ROS created by MTX. ROS causes spermatogonia stem cell (SSC) death, severe damage to the seminiferous tubule, endothelial dysfunction, mitochondrial membrane damage, and endothelial dysfunction. Furthermore, ROS damages the mitochondrial membrane, causing apoptosis of the testicular germ cell. After the drug is given, the sperm count and harm to sperm DNA (Pınar et al., 2018). Spirulina, a blue–green algae that can be found in fresh, brackish, or saline water, is one example of an antioxidant substance that has recently been studied for its possible efficacy in mitigating methotrexate’s negative effects (Kumari et al., 2011). The numerous health benefits it provides have propelled it to the ranks of the most consumed foods and dietary supplements on a global scale. Spirulina has a high concentration of amino acids, carotenoids, fatty acids, vitamin E, B-complex vitamins, magnesium, iron, selenium, zinc, copper, manganese, and magnesium (Bath et al., 2014). Spirulina contains 270–535 mcg of folate per 100 grams, according to Kumari et al. (2011). Taking SP supplements may also be helpful for healthy, active people, especially athletes. Kalafati et al. (2010) found that SP could help with oxidative stress management in a few ways. One way is by influencing exercise-induced lipid peroxidation markers such as TBARS, MDA, and PC. Another way is by increasing the activity of redox enzymes such as CAT, GPx, SOD, and TAC. A well-balanced diet that satisfies the caloric, macro-, and micronutrient requirements of high-intensity exercisers is essential for preventing immunological dysfunction and maintaining an optimum redox state (Gleeson, 2016). Early in vitro results demonstrating the high radical scavenging activity of the algae (Bermejo-Bescós et al., 2008) piqued a lot of interest in how the antioxidant effects of supplementing SP may support exercise performance. These findings were most likely related to the inhibition of lipid peroxidation (Bermejo-Bescós et al., 2008) and activation of the NRF2 signaling pathway (Abd El-Baky et al., 2009). Research has shown that infertile men’s semen contains an excess of reactive oxygen species (ROS), which can cause long-term oxidative damage to different types of biological molecules. These molecules include proteins, nucleic acids, and polyunsaturated fatty acids that make up biological membrane lipids. The health and reproductive potential of spermatozoa may be compromised as a result of this. Reactive nitrogen species (RNS) can be synthesized excessively when reactive oxygen species (ROS) are overproduced, leading to oxidative and nitrosative stress. Several human diseases are associated with an excess of reactive oxygen species (ROS) (Amorini et al., 2021). In this work, we aim to investigate how MTX affects male sex hormones and, by extension, how it influences the oxidant/antioxidant balance. In addition, we assessed whether spirulina had any protective effects against these MTX-induced adverse effects. Materials and MethodsAnimalsFrom the Small Animal Breeding Station, Faculty of Veterinary Medicine, Zagazig University, Egypt, 40 adult Wistar rats ranging in weight from 180 to 220 g were obtained. The animals were kept in a room with climate control, light (a 12-hour day-dark cycle), and humidity (50% + 10%). The temperature was maintained between 21°C and 22 °C. Normal laboratory food and water were freely available to the animals. The timing of the testing was from 9:00 AM to 1:00 PM. All procedures followed the ethical standards set forth by Zagazig University in Egypt. ChemicalsAl-Gomhoria Pharmaceutical Company (Cairo, Egypt) supplied the methotrexate (Mylan) injection vials and spirulina algae. Chemicals and reagents were purchased from M/s Merck India Ltd., Bangalore, India, and were of analytical grade for this work. Experimental designAfter a 2-week period of adaptation to the laboratory setting, 40 adult male rats were randomly divided into four equal groups as outlined below: Group I (control group) received 0.5 ml of normal saline daily for 60 days. Group II, the Spirulina-treated group (SP), was administered S. platensis (300 mg/kg) dissolved in normal saline once daily for 2 weeks. Group III, the MTX-treated group, received a single intramuscular dose of methotrexate (25 mg) once a week. Group IV, the SP+MTX group, was given S. platensis before and concurrently with Methotrexate at the aforementioned doses and duration. The doses used for the experimental groups were according to Arisha (2017). At the end of the experiment, blood samples were collected from each group without the use of anticoagulants, allowing the blood to clot for 30 minutes at 25 °C. The samples were then centrifuged at 3,000 rpm for 15 minutes to separate the serum, which was used to measure levels of testosterone (both free and total), LH, FSH, and prolactin hormones. Subsequently, the animals were euthanized via neck dislocation, and the liver was extracted for assessing TAC and MDA levels. Measurement of serum hormonesTotal testosterone level was detected through Enzyme Linked Immunosorbent Assay (ELISA) as described by Joshi et al. (1979). Free testosterone level was determined by ELISA as described by McCann and Kirkish (1985). LH determination through ELISA as described by Pierce and Parson (1981). FSH determination through ELISA as described by Pierce and Parson (1981). determination of Prolactin hormone (Serafín et al., 2014). MDA and TAC examinationHepatic malondialdehyde (MDA) content was measured in order to evaluate lipid peroxidation (Uchiyama and Mihara, 1978). Tissue levels of total antioxidant capacity (TAC) were determined (Aebi, 1984). Statistical analysisIn order to conduct the statistical analysis, SPSS (SPSS Inc., Chicago, IL, USA, version 11.5) was used. To compare the parameters between the groups for normally distributed data, one-way analysis of variance (ANOVA) was used. Multiple comparisons were conducted using Tukey’s post hoc test. The data are presented as the average plus or minus the standard deviation (SD). For statistical purposes, a p-value less than 0.05 was deemed significant. Ethical approvalExperiments were performed according to the ethical guidelines of Zagazig University, Egypt. ResultsThe effect of methotrexate administration in male rats resulted in a significant decrease in both testosterone (free) and testosterone (total) levels compared with the control group, whereas methotrexate administration with spirulina improved the testosterone level (Figs. 1, 2). However, methotrexate treatment did not alter the levels of either LH (Fig. 3) or prolactin (Fig. 4). Using methotrexate on the rats produced a significant decrease in FSH compared with the control group, and using spirulina with methotrexate did not improve such case (Fig. 5). The effects of MTX on the oxidant and antioxidant status were further investigated. The obtained results IN Fig. 6 revealed that MTX caused significant MDA production. Such MDA levels were significantly reduced when rats received both MTX and spirulina. In the same way, the TAC was significantly reduced in rats receiving MTX, while in those rats that received both MTX and spirulina, showed significant improvement in TAC (Fig. 7). DiscussionThis research investigated the impact of MTX on sex hormones and antioxidant status in male rats. The ameliorative effects of spirulina on the MTX-induced alterations in the levels of sex hormones and TAC were further investigated. The obtained findings revealed that in the MTX group, both free and total testosterone levels, as well as follicle-stimulating hormone (FSH), were reduced, while both luteinizing hormone (LH) and prolactin levels remained unchanged when compared to the control group. Additionally, MTX treatment resulted in a significant increase in MDA levels relative to the control group, with a significant reduction in TAC. Interestingly, co-exposure of experimental rats to both MTX and spirulina showed significant improvement in the production of either free or total testosterone and reduction of the MDA production, and enhancement of the TAC. In agreement with the obtained results, the research conducted by Cao et al. (2014) indicated a decline in both enzymatic and non-enzymatic antioxidant levels within interstitial cells, which subsequently leads to reduced testosterone synthesis and secretion. This reduction adversely affects spermatogenesis and results in a decreased number of sperm in the epididymis. Methotrexate, a chemotherapy agent known for its toxic effects, can directly and indirectly harm the seminiferous epithelium and interstitial cells (Howell et al., 1999). When interstitial cells undergo atrophy, the secretion of the steroid hormone, testosterone, diminishes (Hosseini et al., 2011). Oxidative damage resulting from MTX administration impairs cellular functions and interstitial cell steroidogenesis (Luo et al., 2006). This goes in line with Reichman and Green (1994) noted that MTX induced biochemical alterations and caused the destruction of testicular tissue, ultimately leading to a decrease in testosterone levels.
Fig. 1. The effect of methotrexate and spirulina administration or their combination on free testosterone level.
Fig. 2. The effect of methotrexate and spirulina administration or their combination on total testosterone level.
Fig. 3. Effect of methotrexate and spirulina administration or their combination on LH level.
Fig. 4. Effect of methotrexate and spirulina administration or their combination on prolactin level.
Fig. 5. Effect of methotrexate and spirulina administration or their combination on FSH level.
Fig. 6. Effect of methotrexate and spirulina administration or their combination on MDA level.
Fig. 7. Effect of methotrexate and spirulina administration or their combination on TAC level. MDA is a key chain reaction byproduct that facilitates the oxidation of polyunsaturated fatty acids, making it a dependable indicator of lipid peroxidation associated with oxidative stress (Ozguner et al., 2005). Research by Jahovic et al. (2003) showed that MTX administration led to elevated MDA levels in the blood, liver, and kidneys, whereas the antioxidant melatonin was able to counteract these effects. In the current study, MTX was found to increase MDA levels in the cerebellum. Uzar et al. (2006) reported that Wistar albino rats administered a single dose of MTX at 20 mg/kg experienced peroxidative stress. This stress may arise from MTX inducing lipid peroxidation or increasing reactive oxygen species (ROS), which could elevate MDA levels. Alternatively, MTX might inhibit specific antioxidant enzymes, resulting in reduced activity of these protective enzymes and consequently increasing lipid peroxidation. Sabik and Abd El-Rahman, (2009) indicated that MTX administration resulted in a reduction of catalase and superoxide dismutase activity, accompanied by an increase in malondialdehyde levels. This suggests the presence of free radicals, oxidative stress, and lipid peroxidation. Additionally, Oktar et al. (2010) demonstrated that following MTX treatment, there was a significant reduction of the TAC and total oxidative stress in the liver was significantly reduced compared with the control group. ConclusionThis study demonstrated that MTX had clear adverse effects on testosterone production, indicating an impairment in male fertility, possibly via oxidative damage induction. Interestingly, coadministration of spirulina during treatment with MTX could enhance TAC and improve male fertility. FundingNot available. Authors’ contributionsAll authors contributed equally. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll related data are included in the manuscript. ReferencesAbd El-Baky, H.H., El Baz, F.K. and El-Baroty, G.S. 2009. Enhancement of antioxidant production in Spirulina platensis under oxidative stress. Acta Physiol. Plant. 31, 623–631. Aebi, H. 1984. Catalase in vitro. Methods Enzymol. 105, 121–126. Academic press. Amorini, A.M., Listorti, I., Bilotta, G., Pallisco, R., Saab, M.W., Mangione, R., Manca, B., Lazzarino, G., Tavazzi, B., Lazzarino, G. and Bilotta, P. 2021. Antioxidant-based therapies in male infertility: Do we have sufficient evidence supporting their effectiveness? Antioxidants 10(2), 220. Arisha, S.M. 2017. Effect of grape seed extract, Gervital, against methotrexate induced histological and ultrastructural alterations in TESTES of albino rats. World J. Pharm. Res. 6(7), 98–126. Aslankoc, R., Ozmen, O. and Ellidag, H.Y. 2020. Ameliorating effects of agomelatine on testicular and epididymal damage induced by methotrexate in rats. J. Biochem. Mol. Toxicol. 34(3), e22445. Bath, R.K., Brar, N.K., Forouhar, F.A. and Wu, G.Y. 2014. A review of methotrexate-associated hepatotoxicity. J. Digest. Dis. 15(10), 517–524. Bedoui, Y., Guillot, X., Sélambarom, J., Guiraud, P., Giry, C., Jaffar-Bandjee, M.C., Ralandison, S. and Gasque, P. 2019. Methotrexate an old drug with new tricks. Inter. J. Mol. Sci. 20(20), 5023. Bermejo-Bescós, P., Piñero-Estrada, E. and del Fresno, á.M.V. 2008. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro, 22(6), 1496–1502. Cao, L., Leers-Sucheta, S. and Azhar, S. 2004. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol. 88, 61–67. Cetinkaya, A., Bulbuloglu, E., Kurutas, E.B. and Kantarceken, B. 2006. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med. Sci. Mon. 12(8), BR274-BR278. Chan, E.S. and Cronstein, B.N. 2013. Mechanisms of action of methotrexate. Bull. NYU Hosp. Joint Dis. 71(suppl 1), S5. Gilman, A.L., Leung, W., Cowan, M.J., Cannon, M., Epstein, S., Barnhart, C., Shah, K., Hyland, M., Fukes, T. and Ivanova, A. 2018. Donor lymphocyte infusion and methotrexate for immune recovery after T-cell depleted haploidentical transplantation. Amer. J. Hematol. 93(2), 169–178. Gleeson, M. 2016. Immunological aspects of sport nutrition. Immunol. Cell Boil. 94(2), 117–123. Han, J.M., Choi, K.H., Lee, H.H. and Gwak, H.S. 2022. Association between SLCO1B1 polymorphism and methotrexate-induced hepatotoxicity: a systematic review and meta-analysis. Anti-Cancer Drugs, 33(1), 75–79. Hosseini, A., Ahmadi, A., Ghaderi Pakdel, F. and Zare, S. 2011. Effects of long-term use of low dose cyclophosphamide on testicular tissue in rats. Physiol. Pharmacol. 15, 351–360. Howell, S.J., Radford, J.A., Ryder, W.D. and Shalet, S.M. 1999. Testicular function after cytotoxic chemotherapy: evidence of Leydig cell insufficiency. J. Clin. Oncol. 17, 1493–1498. Jahovic, N., Çevik, H., şehirli, A.ö., Yeğen, B.Ç. and şener, G. 2003. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J. Pin. Res. 34(4), 282–287. Joshi, U.M., Shah, H.P. and Sudhama, S.P. 1979. A sensitive and specific enzymeimmunoassay for serum testosterone. Steroids, 34(1), 35–46. Kalafati, M., Jamurtas, A.Z., Nikolaidis, M.G., Paschalis, V., Theodorou, A.A., Sakellariou, G.K., Koutedakis, Y. and Kouretas, D. 2010. Ergogenic and antioxidant effects of spirulina supplementation in humans. Med. Sci. Sports Exerc. 42(1), 142–151. Kumari, D.J., Babitha, B., Jaffar, S., Prasad, M.G., Ibrahim, M.D. and Khan, M.S. 2011. Potential health benefits of Spirulina platensis. Int. J. Adv. Pharm. Sci. 2, 417–422. Luo, L., Chen, H., Trush, M.A., Show, M.D., Anway, M.D. and Zirkin, B.R. 2006. Aging and the brown Norway rat leydig cell antioxidant defense system. J. Androl. 27, 240–247. McCann, D. and Kirkish, L. 1985. Immunoassay. race. the cardia male hormone study. Cancer J. Clin. 8, 234–236. Oktar, S., GökÇe, A., Aydin, M., Davarci, M., Meydan, S., Oztürk, O.H. and KoÇ, A. 2010. Beneficial effect of erdosteine on methotrexate-induced testicular toxicity in mice. Toxicol. Ind. Health 26(7), 433–438. Ozguner, F., Oktem, F., Armagan, A., Yilmaz, R., Koyu, A., Demirel, R., Vural, H. and Uz, E. 2005. Comparative analysis of the protective effects of melatonin and caffeic acid phenethyl ester (CAPE) on mobile phone-induced renal impairment in rat. Mol. Cell. Biochem. 276, 31–37. Pierce, J.G. and Parsons, T.F. 1981. Glycoprotein hormones: structure and function. Ann. Rev. Biochem. 50(1), 465–495. Pınar, N., Çakırca, G., özgür, T. and Kaplan, M. 2018. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed. Pharm. 97, 1486–1492. Reichman, B.S. and Green, K.B. 1994. Breast cancer in young women: effect of chemotherapy on ovarian function, fertility, and birth defects. J. Natl. Cancer Inst. Monogr. 1994, 125–129. Sabik, L.M. and Abd El-Rahman, S.S. 2009. Alpha-tocopherol and ginger are protective on Cyclophosphamide-induced gonadal toxicity in adult male albino rats. Basic Appl. Pathol. 2, 21–29. Serafín, V., Agüí, L., Yáñez-Sedeño, P.I. and Pingarrón, J.M. 2014. Determination of prolactin hormone in serum and urine using an electrochemical immunosensor based on poly (pyrrolepropionic acid)/carbon nanotubes hybrid modified electrodes. Sens. Actu. B: Chem. 195, 494–499. Uchiyama, M. and Mihara, M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86(1), 271–278. Uzar, E., Koyuncuoglu, H.R., Uz, E., Yilmaz, H.R., Kutluhan, S., Kilbas, S. and Gultekin, F. 2006. The activities of antioxidant enzymes and the level of malondialdehyde in cerebellum of rats subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol. Cell. Biochem. 291, 63–68. Yuluğ, E., Türedi, S., Alver, A., Türedi, S. and Kahraman, C. 2013. Effects of resveratrol on methotrexate-induced testicular damage in rats. Sci. World J. 2013(1), 489659. | ||
| How to Cite this Article |
| Pubmed Style Salah NI, El-aziz RMA, Elnabtity S, Darwish WS. Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Vet. J.. 2025; 15(6): 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 Web Style Salah NI, El-aziz RMA, Elnabtity S, Darwish WS. Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. https://www.openveterinaryjournal.com/?mno=267987 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i6.25 AMA (American Medical Association) Style Salah NI, El-aziz RMA, Elnabtity S, Darwish WS. Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Vet. J.. 2025; 15(6): 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 Vancouver/ICMJE Style Salah NI, El-aziz RMA, Elnabtity S, Darwish WS. Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Vet. J.. (2025), [cited January 24, 2026]; 15(6): 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 Harvard Style Salah, N. I., El-aziz, . R. M. A., Elnabtity, . S. & Darwish, . W. S. (2025) Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Vet. J., 15 (6), 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 Turabian Style Salah, Noran Ibrahim, Reda M. Abd El-aziz, Sameh Elnabtity, and Wageh Sobhy Darwish. 2025. Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Veterinary Journal, 15 (6), 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 Chicago Style Salah, Noran Ibrahim, Reda M. Abd El-aziz, Sameh Elnabtity, and Wageh Sobhy Darwish. "Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats." Open Veterinary Journal 15 (2025), 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 MLA (The Modern Language Association) Style Salah, Noran Ibrahim, Reda M. Abd El-aziz, Sameh Elnabtity, and Wageh Sobhy Darwish. "Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats." Open Veterinary Journal 15.6 (2025), 2532-2539. Print. doi:10.5455/OVJ.2025.v15.i6.25 APA (American Psychological Association) Style Salah, N. I., El-aziz, . R. M. A., Elnabtity, . S. & Darwish, . W. S. (2025) Ameliorative effect of spirulina on methotrexate-induced male fertility and oxidative stress in rats. Open Veterinary Journal, 15 (6), 2532-2539. doi:10.5455/OVJ.2025.v15.i6.25 |