| Research Article | ||
Open Vet. J.. 2025; 15(9): 4248-4254
Open Veterinary Journal, (2025), Vol. 15(9): 4248-4254 Research Article Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickensAmir Bashir Kouchey1, Showkat Ahmad Shah1*, Majid Shafi1, Shaheen Farooq2, Shabu Showkat3, Akeel Bashir1, Shayaib Ahmad Kamil1, Masood Saleem Mir1, Mir Nadeem Hassan3, Zahoor Ahmad Wani4 and Mudasir Ali Rather31Division of Veterinary Pathology, FVSc & AH, Shuhama, SKUAST Kashmir, Srinagar, India 2Division of Veterinary Microbiology & Immunology, FVSc & AH, Shuhama, SKUAST Kashmir, Srinagar, India 3Division of Veterinary Public Health, FVSc & AH, Shuhama, SKUAST Kashmir, Srinagar, India 4Division of Veterinary Parasitology, FVSc & AH, Shuhama, SKUAST Kashmir, Srinagar, India *Corresponding Author: Showkat Ahmad Shah. Division of Veterinary Pathology, FVSc & AH, SKUAST-K, Srinagar, India. Email: vetshowkat [at] skuastkashmir.ac.in Submitted: 02/07/2025 Revised: 12/08/2025 Accepted: 18/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: With the significant growth of the global broiler industry, Clostridium perfringens-induced necrotic enteritis (NE) has become an ongoing challenge, causing considerable economic losses, particularly following the ban on antimicrobial growth promoters in animal feeds in many countries. Aim: The present study aimed to study the prevalence and histopathological alterations in NE in broiler chickens in Kashmir, India. Methods: Ninety-five (95) samples (fecal material and intestinal contents) suspected of NE were collected from various outbreaks and processed for identification of C. perfringens type A by polymerase chain reaction (PCR). Results: Out of 95 suspected cases, NE was confirmed in 37 cases, giving an overall occurrence of 1.22%, with the highest prevalence in the 22–28-day age group (n=22; 0.73%), followed by the >29-day age group (n=15; 0.49%). The gross pathological changes were mainly seen in the jejunum and were characterized by friable and thin-walled jejunum with ballooning, small focal blackish spots, and a Turkish towel appearance. Histopathologically, lesions were confined mostly to the jejunum with varying degrees of severity of mucosal necrosis, desquamation of villous epithelial cells into the lumen, coagulative necrosis and villi fusion, mucosal sloughing, thickened muscularis layer, hemorrhages at the tips of villi, Heterophilic infiltration, and severe hemorrhages in the mucosa. Conclusion: NE was confirmed in 37 of 95 suspected broiler chicken cases, with the highest occurrence observed in the 22–28-day age group. The disease predominantly affected the jejunum, exhibiting characteristic gross and histopathological lesions, including mucosal necrosis, villous desquamation, fusion, hemorrhage, and inflammatory cell infiltration. Keywords: Broiler, Clostridium perfringens, Histomorphology, Necrotic enteritis. IntroductionThe poultry sector’s role in addressing malnutrition and poverty is widely acknowledged as a significant contribution to global efforts. Poultry often serves as a farmer’s initial investment in the livestock hierarchy as a means of boosting income and escaping poverty. Additionally, poultry is regarded as the primary source of not only affordable animal protein but also high-quality human food in many regions of the modern world (Haque and Gofur, 2020). Restrictions on the use of sub-therapeutic doses of antimicrobials as AGPs in the broiler industry have resulted in the reemergence of enteric diseases such as necrotic enteritis (NE) (Hughes et al., 2008). NE is a complex, multifactorial condition that can manifest as either an acute clinical disease or a subclinical form (Ficken, 1991). NE is caused by Clostridium perfringens type A and C and primarily affects chickens aged 2 weeks to 6 months (Keyburn et al., 2008). The disease has become more frequent and severe over the years. The significant economic losses associated with NE include reduced production performance, increased mortality rates of up to 1% per day, treatment costs, and carcass condemnation in processing plants due to cholangiohepatitis (Immerseel et al., 2004; Timbermont et al., 2011). Predisposing factors (coccidiosis, wet litter, and immunosuppression) are necessary to produce a conducive environment for the proliferation and colonization of C. perfringens in chickens. These factors act by damaging the intestinal epithelium, increasing mucus secretion, disrupting gut microbiota composition, and altering the host’s immune status (Rodgers et al., 2015; Moore, 2016). The disease was previously managed using antibiotic growth promoters (Lanckriet et al., 2010a) before the ban on antibiotics in poultry farming was imposed by the European Union (Casewell et al., 2003). The pathogenesis of NE is multifactorial and involves both bacterial virulence factors and predisposing conditions that disrupt gut homeostasis. Under normal circumstances, C. perfringens exists in low numbers within the gut, but under favorable conditions—such as dietary changes (high protein or non-digestible carbohydrates), intestinal mucosal damage (e.g., due to coccidiosis), immunosuppression, or poor hygiene—it can rapidly proliferate and produce potent toxins. NetB (NE B-like toxin), a pore-forming toxin that damages intestinal epithelial cells, is a key virulence factor associated with NE, leading to mucosal necrosis, hemorrhage, and impaired nutrient absorption (Keyburn et al., 2008). Other toxins and enzymes, including α-toxin (phospholipase C), also contribute to tissue destruction and disease progression (Cooper and Songer, 2009). Clinically, NE can present in acute or subclinical forms, with the latter often going undetected but resulting in reduced feed efficiency and growth performance. The complex interaction between bacterial toxins, host immune response, and environmental or nutritional stressors makes NE a challenging disease to control, especially in the absence of AGPs. Understanding the pathogenesis is critical for developing effective prevention and control strategies in poultry production systems. In humans, C. perfringens intoxication is the third most common bacterial foodborne illness, with poultry and poultry products being responsible for 30% of these outbreaks. (Grass et al., 2013). The prevalence of NE in broilers varies widely according to management practices, environmental conditions, and diagnostic methods. Subclinical NE is more common and can affect up to 40% or more of broiler flocks globally, often going unnoticed due to the absence of overt symptoms but still impacting performance (Loos Scarth, 2006). Clinical NE, characterized by sudden mortality and severe intestinal lesions, typically has a lower prevalence, with reported incidence rates ranging from 2% to 50% in affected flocks (Paiva and McElroy, 2014). Recently, research into antibiotic alternatives that can enhance the gut health and immune status of poultry has intensified. However, the current alternatives are not as effective as antibiotics in controlling NE. A deeper understanding of the virulence factors of C. perfringens, pathogenesis and pathology of NE, and host responses is essential for developing effective control strategies and a new generation of supplements. Therefore, the present study aimed to investigate the prevalence and histomorphological alterations in NE in broiler chickens in Kashmir, India. Materials and MethodsSamples comprised mortalities from various poultry farms operating in the Srinagar and Ganderbal districts. Out of a total of 100 outbreaks of enteritis that were screened during the study period, fecal material and intestinal contents (n=95) were collected from suspected NE outbreaks in sterile vials and transported to the laboratory on ice for C. perfringens type A identification. DNA extractionThe collected samples were mixed with PBS and subsequently centrifuged at 13,000–16,000 rpm for 2 minutes to pellet the cells. The supernatant was removed, and the obtained pellet was subjected to DNA extraction using a commercially available DNA extraction kit (Promega Wizard Genomic DNA Purification Kit) as per the standard procedure. Polymerase chain reaction (PCR)All PCR assays were performed in a 25 µl reaction volume containing DNA template, GO-green Master MIX (Promega, premixed ready-to-use solution containing bacterially derived Taq DNA polymerase, dNTPs, MgCl2, and reaction buffers), specific primers, and nuclease-free water. Sterile distilled water was used as the negative template control. The lyophilized culture of C. perfringens was supplied by the Division of Veterinary Microbiology and Immunology, FVSc, and AH SKUAST-K was used as the positive control. Detection of C. perfringens type AThe extracted DNA samples were subjected to PCR targeting 16S rRNA. The PCR conditions consisted of initial denaturation at 95oC for 15 minutes, followed by 35 cycles of denaturation at 94oC for 30 seconds, annealing at 55oC for 90 seconds, and extension at 72oC for 90 seconds. Final extension was done at 72oC for 10 minutes (Tonooka et al., 2005). The primers required for the study are listed in Table 1. Electrophoresis and documentation of the gel to analyze the PCR result were performed as per the procedure described by Wani et al. (2018). Table 1. Primer sequence used for the 16S rRNA gene.
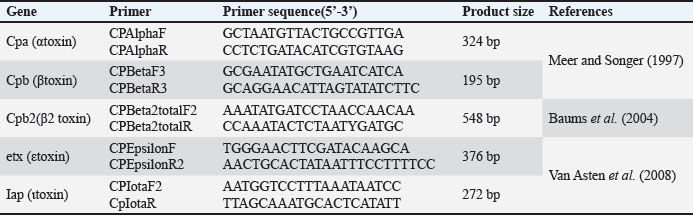
Toxinotyping of C. perfringens type ASamples positive for C. perfringens were subjected to toxinotyping for the presence of various toxigenic genes such as cpa (α-toxin), cpb (β-toxin), cpb2 (β2-toxin), etx (ε-toxin), and iap (ι-toxin), by employing a multiplex PCR assay as per standard procedures (Meer and Songer, 1997; Baums et al., 2005; Van Asten et al., 2008) with some modifications. Screening of toxin genes was used for C. perfringens typing. The primers used in the study are shown in Table 2. Table 2. Primers used for toxinotyping of C. perfringens.
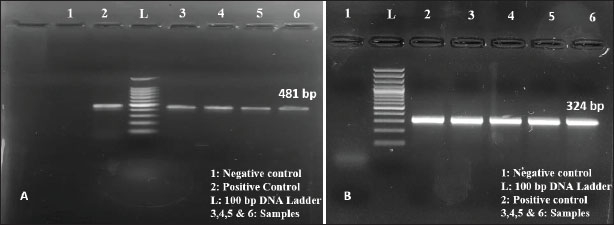
Pathological studiesThe carcasses were subjected to systematic necropsy examination. Different parts of the intestine were examined grossly for external appearance, mucosal changes, intestinal contents, and lesion distribution. Based on the gross pathology, NE was suspected in the samples and, therefore, subjected to further evaluation. For histopathological examination, different parts of the intestines were collected in 10% formalin and processed using the paraffin embedding technique. Sections were stained with Harris’ hematoxylin and eosin technique for routine examination (Luna, 1968). Ethical statementEthical clearance was not required as the study involved cases of natural infection and excluded any animal experimental procedures. ResultsOccurrenceIn the present study, 3024 broiler chickens were necropsied and examined for the presence and nature of intestinal lesions. The overall mortality rate during the study period was 17.58% (3024/105202). NE was suspected in 95 cases based on gross lesions in the GI tract and was confirmed in 37 cases by polymerase chain reaction, giving an overall occurrence of 1.22%. The disease was found mostly in the 22–28-day age group (n=22; 0.73%), followed by the >29-day age group (n=15; 0.49%). Detection and confirmation of C. perfringens type A in broiler chicken by polymerase chain reactionOf the 1,382 samples, 95 samples suspected of NE were screened for the 16S rRNA gene fragment of C. perfringens type A. Primers targeting C. perfringens 16S rRNA amplified an expected size of 481 bp amplicon (Fig. 1A) in PCR products that confirmed the identity of C. perfringens. The DNA extracted from the samples was subjected to PCR with the primers for the detection of the alpha toxin gene (cpa) of C. perfringens type A. An expected size of 324 bp was obtained in PCR products in all samples, which confirmed the alpha toxin gene of C. perfringens type A (Fig. 1B).
Fig. 1. Amplification of the 16S rRNA gene. B. Amplification of the cpa gene. Histopathological studiesGross changes in NE consisted of friable and thin-walled jejunum with ballooning (Fig. 2A). Some cases revealed the presence of small focal blackish spots, whereas in severe cases, the entire jejunum was blackish (Fig. 2B). On incision, the intestinal lumen contained a brownish, viscous, foul-smelling fluid. The mucosa was covered by either a brownish or a grayish-white diphtheritic membrane adherent to the mucosa, giving it a “Turkish towel” appearance. (Fig 2C). The duodenum and ileum appeared mostly normal except for slight congestion (Fig. 2D). Histopathologically, lesions were confined mostly to the jejunum with varying degrees of mucosal necrosis severity. In less severe cases, villous epithelial cell desquamation into the jejunal lumen was evident (Fig. 3A). The tips of the villi revealed coagulative necrosis, and villi fusion was also noticed as a consistent feature (Fig. 3B). Sloughing of the mucosa, thickened muscularis layer, and hemorrhages at the tips of the villi were also observed. (Fig. 3C, D). Heterophilic infiltration and severe mucosal hemorrhages were consistently observed in severe cases (Fig. 3E, F, and G). No severe histopathological lesions were noted in the duodenum and ileum, except for mucosal congestion (Fig. 3H, 3I).
Fig. 2. Gross pathological changes in NE: A. Jejunum of a bird showing ballooning. B. Jejunum showing small blackish necrotic spots. C. Jejunum showing Turkish towel appearance. D. Duodenum showing congestion.
Fig. 3. Histopathological changes in intestines in NE. A. Severe necrosis of mucosa. B. Fusion of villi and hemorrhage in the lamina propria (arrow). C. Thickened muscularis (star) with Heterophilic infiltration in the mucosa. D and E. Severe hemorrhage and Heterophilic infiltration in the mucosa. F. Disruption of villi. G. Severe necrosis and infiltration of mucosa at higher magnification. H and I. Vascular congestion in the duodenum and ileum. H&E scale bar length 100 µm. DiscussionNE is among the most prevalent and financially devastating diseases in poultry, affecting approximately 40% of commercial broiler flocks and resulting in mortality rates ranging from 2% to 50% (Loos Scarth, 2006). NE can substantially impact performance by impairing nutrient absorption, growth rate, feed conversion, and overall animal welfare (Paiva and McElroy, 2014). In the present study, C. perfringens was detected in 37 out of 3024 birds examined for enteritis from 95 birds that were suspected of NE, giving an overall occurrence of 1.22% (37/3024). Helal et al. (2019) also reported that not all intestinal lesions observed in field conditions were caused by C. perfringens infection. However, other reports have shown a higher prevalence of NE in broiler chickens (Miah et al., 2011). This variation in the occurrence of disease may be due to variations in the number and nature of samples, predisposing factors, and range of antibiotic administration. Effective management practices, including stringent biosecurity measures, optimized nutrition, and proper litter management, may have played a significant role in minimizing disease prevalence. Additionally, the use of feed additives, such as probiotics, prebiotics, organic acids, or essential oils, may have contributed to the enhancement of gut health and resilience against C. perfringens infection. Vaccination programs, if implemented, could also have helped reduce susceptibility to NE. Furthermore, timely identification and treatment of early enteric disturbances may have prevented progression to clinical NE. Lastly, seasonal or regional variations in pathogen pressure, as well as differences in farm management or genetic resistance among bird strains, may also account for the relatively low incidence reported in this study (Williams, 2005; Huyghebaert et al., 2011; Hofacre et al., 2018). In this study, NE was more prevalent in age groups 22–28 days and >29 days. This is in agreement with previous studies (Malmarugan et al., 2012; Dar et al., 2017). The low occurrence of NE in this study can also be attributed to the extensive use of AGPs and coccidiostats in the feeds. Coccidial infection is a well-established predisposing factor for NE. Coccidial infection causes mucosal damage that creates an environment conducive to the proliferation of C. perfringens (Dierick et al., 2021). Although not directly assessed in this study, the potential off-label use of AGPs, despite regulatory bans, could also contribute to disease suppression and may partially explain the absence of clinical NE in some suspected cases (Casewell et al., 2003). However, without residue testing, this remains speculative. The lack of lesion scoring and histopathological grading limits the ability to quantify disease severity and fully correlate gross pathology with bacterial presence. Future investigations should integrate these scoring systems and examine predisposing factors such as diet composition, co-infection with coccidiosis, and farm-level antimicrobial use to establish causal links between observed pathology and C. perfringens infection. NE primarily involved the jejunum in affected broiler chickens, which appeared friable, thin-walled, and ballooned, with some showing focal blackish spots or entirely blackish segments in severe cases. The intestinal lumen contained foul-smelling, brownish fluid, and the mucosa was often covered with a diphtheritic membrane, giving a characteristic “Turkish towel” appearance. Histologically, the lesions ranged from mild villous epithelial desquamation to severe mucosal necrosis, villous fusion, and sloughing. Additional findings included thickened muscularis, hemorrhages at villous tips, and marked Heterophilic infiltration in the mucosa. The gross and microscopic changes observed in this study are largely in agreement with earlier reports (Olkowski et al., 2006; Olkowski et al., 2008; Miah et al., 2011; Abid et al., 2016). The initial pathological changes in NE are mainly due to collagenolytic activity (Timbermont et al., 2011). In conclusion, the present study confirmed NE in 37 out of 95 suspected cases among 3024 necropsied broiler chickens, with the highest prevalence in the 22–28-day age group. Clostridium perfringens type A was identified and confirmed through PCR by amplification of 16S rRNA and alpha toxin (cpa) genes. Gross and histopathological findings revealed that the jejunum was predominantly affected, showing characteristic lesions such as mucosal necrosis, villous desquamation and fusion, hemorrhages, and diphtheritic membranes giving a “Turkish towel” appearance. AcknowledgmentThe authors are thankful to the Sheri-Kashmir University of Agricultural Sciences and Technology, Shalimar, for providing the necessary facilities to conduct the research. FundingThe authors declare that no funds, grants, or other support were received for this study and during the preparation of this manuscript. Authors contributionsA B Kouchey conducted the research. S A Shah contributed to the conceptualization, methodology, supervision, and writing of the original manuscript. M. Shafi, S. A. Kamil, M. S. Mir, and A. Bashir contributed to the histopathological evaluation. S Farooq, S Showkat, M, N, Hassan, M A Rather contributed to the isolation and identification of bacteria. Z A Wani contributed to data curation and editing. All authors have read and approved the final version of the manuscript. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper. Data availabilityAll data are provided in the manuscript. ReferencesAbid, S.A., Azeem, T., Chaudhary, Z.I., Rehman, Z.U. and Umar, S. 2016. Emerging threat of necrotic enteritis in poultry and its control without use of antibiotics: a review. J. Anim. Plant Sci. 26(6), 1556–1667. Baums, C.G., Schotte, U., Amtsberg, G. and Goethe, R. 2004. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol. 100, 11–16. Casewell, M., Friis, C., Marco, E., McMullin, P. and Phillips, I. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52(2), 159–161. Cooper, K.K. and Songer, J.G. 2009. Virulence of Clostridium perfringens in the pathogenesis of necrotic enteritis in poultry. Anaerobe 15(1–2), 144–148. Dar, P.S., Wani, S.A., Wani, A.H., Hussain, I., Maqbool, R., Ganaie, M.Y. and Qureshi, S. 2017. Isolation, identification and molecular characterization of Clostridium perfringens from poultry in Kashmir valley. J. Entomol. Zool. Stud. 5(5), 409–414. Dierick, E., Ducatelle, R., Van Immerseel, F. and Goossens, E. 2021. Research Note: the administration schedule of coccidia is a major determinant in broiler necrotic enteritis models. Poultry Sci. 100(3), 100806. Ficken, M.D. 1991. Necrotic enteritis. In Diseases of Poultry; Iowa State University Press: Uppsala, IA, USA, 1; pp. 264–267. Grass, J.E., Gould, L.H. and Mahon, B.E. 2013. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog. Dis. 10, 131–136. Haque, M.N. and Gofur, M.R. 2020. Epidemiologic surveillance of Newcastle disease in Sonali chicken in Naogaon district, Bangladesh. Bangladesh J. Agric. Life Sci. 1(1), 41–46. Helal, S., Khalaf, N., El Menisy, A. and Lebdah, M. 2019. Clostridium perfringens type A Causing Necrotic Enteritis Outbreaks among Chickens in Egypt. Zagazig Vet. J. 47(4), 398–407. Hofacre, C.L., Smith, J.A. and Mathis, G.F. 2018. An optimist’s view on necrotic enteritis vaccines. Avian Pathol. 47(1), 1–3. Hughes, L., Hermans, P. and Morgan, K. 2008. Risk factors for the use of prescription antibiotics on UK broiler farms. J. Antimicrob. Chemotherapy. 61(4), 947–952. Huyghebaert, G., Ducatelle, R. and Van Immerseel, F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187(2), 182–188. Immerseel, F.V., Buck, J.D., Pasmans, F., Huyghebaert, G., Haesebrouck, F. and Ducatelle, R. 2004. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 33, 537–549. Keyburn, A.L., Boyce, J.D., Vaz, P., Bannam, T.L., Ford, M.E., Parker, D., Di Rubbo, A., Rood, J.I. and Moore, R.J. 2008. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4(2), e26. Lanckriet, A., Timbermont, L., De Gussem, M., Marien, M., Vancraeynest, D., Haesebrouck, F. and Van Immerseel, F. 2010. The effect of commonly used anticoccidial and antibiotics in a subclinical necrotic enteritis model. Avian Pathol. 39(1), 63–68. Loos Scarth, L. 2006. The Merck Veterinary Manual Online, 8th edition, Emerald Group Publishing Limited, England. Luna, L.G. 1968. Manual of histologic staining method of armed forces Institute of Pathology. 3rd ed., New York: McGraw Hill Book Company. Malmarugan, S., Boobalan, A. and Dorairajan, N. 2012. Necrotic Enteritis in broiler and layer farms in Tamil Nadu, India. Int. J. Agro. Vet. Med. Sci. 6(4), 241–249. Meer, R.R. and Songer, J.G. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58, 702–705. Miah, M.S., Asaduzzaman, M., Sufian, M.A. and Hossain, M.M. 2011. Isolation of Clostridium perfringens, Causal agents of necrotic enteritis in chickens. J. Bangladesh Agric. Univ. 9(1), 97–102. Moore, R.J. 2016. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 45, 275–281. Olkowski, A.A., Wojnarowicz, C., Chirino-Trejo, M. and Drew, M.D. 2006. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 81(1), 99–108. Olkowski, A.A., Wojnarowicz, C., Chirino-Trejo, M., Laarveld, B. and Sawicki, G. 2008. Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res. Vet. Sci. 85(3), 543–553. Paiva, D. and McElroy, A. 2014. Necrotic enteritis: applications for the poultry industry. J. Appl. Poultry Res. 23(3), 557–566. Rodgers, N.J., Swick, R.A., Geier, M.S., Moore, R.J., Choct, M. and Wu, S.A. 2015. Multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 59, 38–45. Timbermont, L., Haesebrouck, F., Ducatelle, R. and Van Immerseel, F. 2011. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 40, 341–347. Tonooka, T., Sakata, S., Kitahara, M., Hanai, M., Ishizeki, S. and Takada, M. 2005. Detection and quantification of four species of the genus Clostridium in infant feces. Microbiol. Immunol. 49, 987–992. Van Asten, A.J., Allaart, J.G., Meeles, A.D., Gloudemans, P.W., Houwers, D.J. and Gröne, A. 2008. A new PCR followed by MboI digestion for the detection of all variants of the Clostridium perfringens cpb2 gene. Vet. Microbiol. 127, 412–416. Wani, N., Wani, S.A., Munshi, Z.H., Shah, S.A., Rather, M.A., Hussain, A., Kashoo, Z. and Khan, N.N. 2018. Isolation and virulence gene profiling of Clostridium perfringens from freshwater fish. J. Entomol. Zool. 6(3), 176–181. Williams, R.B. 2005. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 34(3), 159–180. | ||
| How to Cite this Article |
| Pubmed Style Kouchey AB, Shah SA, Shafi M, Farooq S, Showkat S, Bashir A, Kamil SA, Mir MS, Hassan MN, Wani ZA, Rather MA. Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Vet. J.. 2025; 15(9): 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 Web Style Kouchey AB, Shah SA, Shafi M, Farooq S, Showkat S, Bashir A, Kamil SA, Mir MS, Hassan MN, Wani ZA, Rather MA. Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. https://www.openveterinaryjournal.com/?mno=268266 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.31 AMA (American Medical Association) Style Kouchey AB, Shah SA, Shafi M, Farooq S, Showkat S, Bashir A, Kamil SA, Mir MS, Hassan MN, Wani ZA, Rather MA. Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Vet. J.. 2025; 15(9): 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 Vancouver/ICMJE Style Kouchey AB, Shah SA, Shafi M, Farooq S, Showkat S, Bashir A, Kamil SA, Mir MS, Hassan MN, Wani ZA, Rather MA. Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 Harvard Style Kouchey, A. B., Shah, . S. A., Shafi, . M., Farooq, . S., Showkat, . S., Bashir, . A., Kamil, . S. A., Mir, . M. S., Hassan, . M. N., Wani, . Z. A. & Rather, . M. A. (2025) Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Vet. J., 15 (9), 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 Turabian Style Kouchey, Amir Bashir, Showkat Ahmad Shah, Majid Shafi, Shaheen Farooq, Shabu Showkat, Akeel Bashir, Shayaib Ahmad Kamil, Masood Saleem Mir, Mir Nadeem Hassan, Zahoor Ahmad Wani, and Mudasir Ali Rather. 2025. Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Veterinary Journal, 15 (9), 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 Chicago Style Kouchey, Amir Bashir, Showkat Ahmad Shah, Majid Shafi, Shaheen Farooq, Shabu Showkat, Akeel Bashir, Shayaib Ahmad Kamil, Masood Saleem Mir, Mir Nadeem Hassan, Zahoor Ahmad Wani, and Mudasir Ali Rather. "Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens." Open Veterinary Journal 15 (2025), 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 MLA (The Modern Language Association) Style Kouchey, Amir Bashir, Showkat Ahmad Shah, Majid Shafi, Shaheen Farooq, Shabu Showkat, Akeel Bashir, Shayaib Ahmad Kamil, Masood Saleem Mir, Mir Nadeem Hassan, Zahoor Ahmad Wani, and Mudasir Ali Rather. "Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens." Open Veterinary Journal 15.9 (2025), 4248-4254. Print. doi:10.5455/OVJ.2025.v15.i9.31 APA (American Psychological Association) Style Kouchey, A. B., Shah, . S. A., Shafi, . M., Farooq, . S., Showkat, . S., Bashir, . A., Kamil, . S. A., Mir, . M. S., Hassan, . M. N., Wani, . Z. A. & Rather, . M. A. (2025) Prevalence, molecular detection, and histopathological analysis of necrotic enteritis in chickens. Open Veterinary Journal, 15 (9), 4248-4254. doi:10.5455/OVJ.2025.v15.i9.31 |