| Research Article | ||
Open Vet. J.. 2025; 15(9): 4265-4275
Open Veterinary Journal, (2025), Vol. 15(9): 4265-4275 Research Article Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix)Iman Ibrahim Al Hacham*, Eman F. Albaghdady, Abdulrazzaq Baqer Kadhim, Hassaneen A. Sharoot and Hazem AlmhannaDepartment of Anatomy and Histology, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Qādisiyyah, Iraq *Corresponding Author: Iman Ibrahim Al Hacham. Department of Anatomy and Histology, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Qādisiyyah, Iraq. Email: iman.alhachem [at] qu.edu.iq Submitted: 05/06/2025 Revised: 01/08/2025 Accepted: 08/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Formalin is a widely used chemical with toxic effects on various organs. Its exposure may harm reproductive organs, especially in quails, which are sensitive models for toxicological research. Aim: This study aimed to investigate the effects of 5% formalin gas exposure on quail testicular health, blood parameters, hormone levels, and tumor protein TP53 (TP53) gene expression. Methods: Twenty male Japanese quails were randomly divided into four groups. The control group received no exposure. The remaining three groups were exposed to 5% formalin gas twice daily for 2 hours over 10, 20, and 30 days. Blood and testicular samples were collected at each time point. Testosterone levels, complete blood count, histopathology, and real-time quantitative polymerase chain reaction (RT-qPCR) for TP53 expression were analyzed. Data were evaluated using one-way analysis of variance (p ≤ 0.05). Results: Exposed quails exhibited anxiety, weight loss, and respiratory distress. Body weight decreased progressively from 200 ± 0.66 g (control) to 130 ± 0.43 g (30-day group). The testicular weight increased from 2.12 ± 0.25 g (control) to 4.45 ± 0.34 g (30-day group), with significant enlargement (p < 0.05). Testosterone dropped from 2.22 ± 0.30 ng/ml (control) to 0.9 ± 0.07 ng/ml (30-day group), showing a time-dependent decline (p < 0.05). wWhite blood cells, red blood cells, and hemoglobin (HGB) levels increased significantly, whereas lymphocytes decreased (p < 0.05). Histology revealed seminiferous tubule damage and necrosis. RT-qPCR revealed upregulated TP53 expression, especially in the 30-day group (p=0.018). Conclusion: Formalin gas exposure harms quail testes by reducing hormones, altering blood counts, damaging tissues, and increasing TP53 gene expression. This suggests the potential testicular toxicity and carcinogenic risk of formalin. Keywords: Formalin, Gene expression TP53, Quail, Testes. IntroductionQuail (Coturnix coturnix) is widely used as a reliable model in toxicological research because of its small size, rapid growth, and high sensitivity to environmental toxins (Barszcz et al., 2024). It is used to conduct many scientific experiments to evaluate the effects of risky diseases, poisons, and drug applications in the poultry industry. Compared with other birds, these birds breed quickly and tolerate challenging conditions (Snow et al., 2014; Egbuniwe et al., 2024; Lansford and Cheng, 2024). Recent studies have expanded our understanding of formaldehyde toxicity in both animals and humans. Formalin exposure has been linked not only to irritation and inflammation but also to deeper tissue damage and cancer risks in various organ systems (Bernardini et al., 2022; La Torre et al., 2023). In mammals, chronic exposure to formaldehyde may contribute to cellular stress and even neurological problems such as dementia (Kou et al., 2022). Additionally, large-scale reviews have highlighted occupational exposure to formaldehyde as a carcinogen affecting several anatomical sites (Xiong et al., 2024). Formalin (CH2O) is a colorless gas that burns at room temperature with high toxicity. Consequently, formalin has a germicidal effect. Its many uses make it in demand by consumers, such as its use in manufacturing paper, fertilizers, and animal tissue fixation for teaching, investigations, and research (Van Agteren et al., 1998). Many studies have confirmed that formalin can be used as a food preservative and included in the ingredients of medicines, antiseptics, and cosmetics (Agarwal et al., 2013). However, exposure to formalin gas and liquid irritates the entire body, especially the eyes and upper and lower respiratory tract, at high or low concentrations and for long or short periods, especially in birds, ducks, and quail (Kuethe, 1988; Hasan et al., 2021; Eladl et al., 2022), leading to suffocation, coughing, and lacrimation. Exposure to formalin may cause testicular damage, decreased sperm count, and altered sperm morphology, which can lead to infertility. Formalin exposure can also cause hormonal imbalances, which can affect the overall function of the male reproductive system (Han et al., 2015). Formalin exposure has direct harmful effects on the male reproductive system. It can disrupt hormone production, damage testicular tissue, and reduce fertility. Formalin interferes with testosterone synthesis and alters the structure of seminiferous tubules in birds and mammals. These changes lead to reduced sperm count and abnormal sperm shapes. The activation of the TP53 gene, which responds to cellular damage by halting cell growth or triggering cell death, is one key mechanism. This response helps prevent the proliferation of damaged cells and reflects high levels of tissue stress. These reactions occur even with low or short-term exposure. If exposure continues for a long time, more serious problems can occur. Studies have shown that formalin may increase the risk of certain cancers. These include leukemia and cancer of the nose and throat area, such as nasopharyngeal cancer (Duong et al., 2021). Formalin can damage the testes in male animals. This damage can lower sperm numbers and change their shape. These changes reduce the chances of fertility (Grillo et al., 2015). Wearing protective gear is very important when handling formalin. To avoid health risks, good ventilation and safety rules must always be followed (Kim et al., 2011). The TP53 gene plays a key role in regulating cell growth and division inside the body. The TP53 protein produced by this gene stops cells from becoming cancerous (Kumar et al., 2011). When cells are stressed or damaged, TP53 levels go up (Söderberg-Nauclér, 2006). This protein activates other genes that repair DNA or cause damaged cells to die (Gupta et al., 2019). DNA damage caused by chemicals such as formalin can activate this TP53 response (Kadhim et al., 2023). This helps prevent the spread of damaged cells. It stops the cell cycle, repairs DNA, or triggers apoptosis and cell aging (Zhan, 2005; Mallette and Ferbeyre, 2007). This action protects tissues and prevents the development of cancer (Kastan and Bartek, 2004). However, if the TP53 gene is mutated, this protection is lost. Cancer cells can grow more easily. Many human cancers have TP53 mutations (Hanel and Moll, 2012; Leroy et al., 2014). Owing to its importance, many scientists now study TP53 in cancer research. They hope to use this knowledge to create better treatments (Baugh et al., 2018). In this study, we aimed to determine whether formalin gas affects TP53 expression in quail testes. We exposed quails to 5% formalin gas for different periods and measured TP53 gene expression in their testicular tissues. Materials and MethodsExperimental designExperiments were performed using 20 healthy adult Japanese Quail (Coturnix japonica) maintained in a controlled environment with optimal feeding, lighting, and ventilation. Experimental and treatment groupsThis study employed four groups of quails (5 animals each). The control group experienced standard rearing conditions without exposure to formalin gas. The three experimental groups (Groups 1–3) underwent daily exposure to 5% formalin gas for 2 hours, both in the morning and in the evening, for 30 days. Groups 1–3 were euthanized at 10, 20, and 30 days, respectively, for subsequent analysis. Absolute formalin was diluted with distilled water to achieve a 5% formalin gas concentration. Euthanasia and sample collectionQuails were humanely euthanized via intravenous administration of 80 mg/kg sodium pentobarbital. This dose is consistent with the AVMA guidelines, which recommend 60–100 mg/kg sodium pentobarbital for euthanasia in birds (American Veterinary Medical Association, 2020). After euthanasia, tissue and blood samples were collected from each experimental group. Testes were weighed and immersed in 10% neutral buffered formalin for subsequent histological examination. Blood samples were processed to obtain serum. Testicular tissue designated for RNA analysis was immediately stored in TRIzol reagent (SRCr Green-Zol reagent, Qadisiyah Province, Iraq) at −80°C for 30 days prior to RNA extraction and subsequent real-time quantitative polymerase chain reaction (RT-qPCR) analysis. Serum samples were subjected to analysis to determine the concentration of testosterone. The testes were weighed individually using a digital precision balance with an accuracy of ±0.01 g immediately after dissection. Hematological studyAvian blood samples were obtained from the cardiac region of subjects within a control group and three exposure groups. Centrifugation at 3,000 revolutions per minute for 20 minutes facilitated serum isolation. Subsequently, the resulting supernatant was collected and preserved at −20°C. Subsequent analyses encompassed a range of biochemical parameters, including circulating hormone concentrations and hematological profiles, specifically white blood cell (WBC) counts, red blood cell (RBC) counts, and HGB levels, all of which were determined within the isolated serum. Total testosterone levels were measured using a fluorescence immunoassay (Poway, CA 92064). Testosterone levels were assessed using the Boditechi-CHROMA™ Testosterone Test (1030, Brussels, Belgium). Blood samples were collected and processed to obtain serum. A 30 µl aliquot of the displacing reagent was added to 75 µl of serum and thoroughly mixed. The mixture was incubated for 3 minutes with gentle shaking. Then, 75 µl of the incubated mixture was transferred to a detection buffer tube. Then, 10 volumes of detection buffer were added, and the resulting solution was transferred to the sample well of the test device. Following a 10-minute incubation period, the test cartridge was inserted into the Boditechi-CHROMA™ Reader. The reader provided a quantitative measurement of testosterone levels in nanograms per milliliter (ng/ml). The Boditechi-CHROMA™ Testosterone Test has a sensitivity of 0.1 ng/ml and a detection range of 0.1–10 ng/ml. The intra- and inter-assay coefficients of variation were less than 6% and 9%, respectively, ensuring reliable reproducibility of the results. Histological procedureTesticular tissues were preserved in 10% neutral formalin for 48 hours. The histological protocol followed studies by Luna (1968), Al-Mahmodi et al. (2022), Almhanna et al. (2022), and Abid and Hamza (2024). The tissues were dehydrated using a graded series of ethanol solutions (70%, 80%, 90%, and 100%). Following this, the tissues were cleared with xylene. Subsequently, the tissues were embedded in paraffin wax and then sectioned into thin slices measuring 5–6 mm in thickness. Finally, the histological features of the testicular tissues from all experimental groups were visualized using hematoxylin and eosin staining. Tissue sections, including controls, were analyzed under a light microscope (Olympus CH-2 Phase Contrast model, Japan). Images were acquired using a Canon 7D digital camera (18 megapixels, Japan). RNA extraction and cDNA synthesisRNA was collected from testicular tissue using TRIzol Reagent. Tissue homogenates were treated with chloroform, isopropanol (ISA, UK), and ethanol to precipitate RNA. The RNA pellet was washed with RNase-free water, which was prepared by treating distilled water with 0.1% diethyl pyrocarbonate obtained from BKMAM Biotechnology Co. Ltd., China, followed by autoclaving to inactivate RNases. To eliminate DNA contamination, the RNA was treated with DNase I (Promega Company, Madison, WI). Reverse transcription was performed to generate cDNA from 1 µg RNA using the DiaStar™ OneStep RT-PCR Kit (BKMAM Biotechnology Co. Ltd, China). The concentration of the synthesized cDNA was then determined using a spectrophotometer. The cDNA samples were normalized to equal concentrations to ensure consistent input for subsequent RT-qPCR experiments. The concentration measurements were performed using a NanoDrop spectrophotometer (Thermo Scientific NanoDrop One Microvolume UV-Vis Spectrophotometer, USA). RT-qPCR techniqueGene expression levels of eosinophil cationic protein, tumor protein TP53 (TP53), and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were quantified in quail samples using real-time RT-qPCR. Specific primers were designed for TP53 (136 bp; GenBank accession: XM_015855944.1). Similarly, GAPDH primers (77 bp, GenBank accession: XM_015873412.2) were designed (Table 1). Primer design and synthesis were conducted by Scientific Researcher Co. Ltd. in Iraq. RT-qPCR reactions were performed using a SYBR Green-based qPCR master mix and a RT-qPCR system (Bio-Rad, Hercules, CA). Table 1. Primers used in this study.
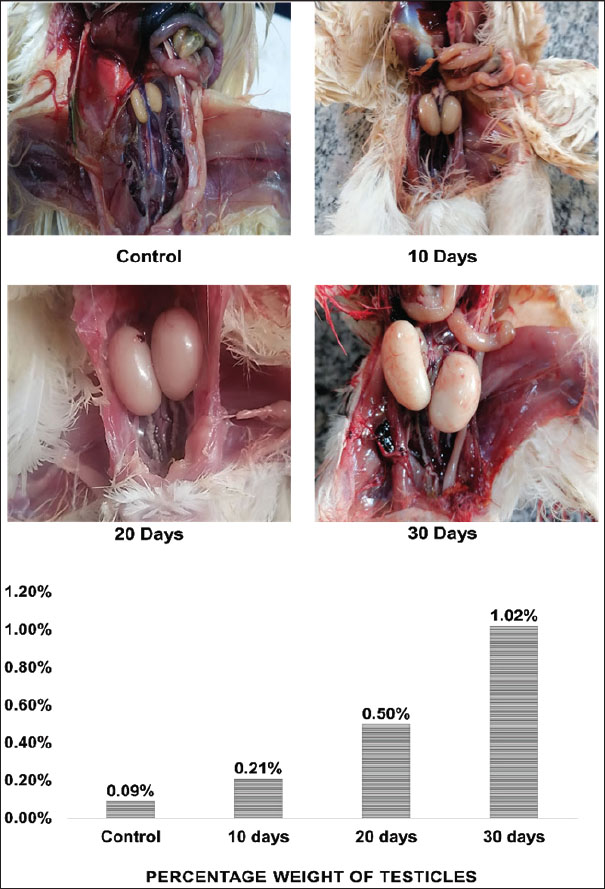
The thermal cycling conditions included an initial denaturation step at 50°C for 1 hour, followed by 45 cycles of denaturation at 95°C for 20 seconds and annealing/extension at 60°C for 30 seconds. A final melting curve analysis was performed to confirm the specificity of the PCR products, ranging from 60°C to 95°C with a 0.5°C increment (Almhanna et al., 2024). Statistical analysisGene expression levels were determined using the comparative Ct method (Livak and Schmittgen, 2001, Almhanna et al., 2022). Statistical significance between groups was assessed by one-way analysis of variance utilizing IBM SPSS Statistics 23.0. Differences were considered statistically significant at p ≤ 0.05. Data are presented as mean ± standard error (Ghareeb et al., 2024). Ethical approvalThis study followed the ethical protocols outlined by the College of Veterinary Medicine and the University of Al-Qadisiyah, approved under protocol number PG. Number 1899 in 2023. ResultsFormalin vapor exposure (5%) had detrimental effects on quails, as evidenced by behavioral and physiological changes. The testes’ weight percentages were also notably affected. The control group had a mean testicular weight of 2.125 ± 0.3 g, whereas the 10-, 20-, and 30-day groups exhibited weights of 3 ± 0.3, 4.165 ± 0.6, and 4.45 ± 0.3 g, respectively. Significant differences were observed between the groups (p < 0.05), with the most pronounced testicular enlargement observed in the 30-day group. In particular, the testicular weight gradually increased with the duration of exposure, as a percentage of total body weight (Fig. 1).
Fig. 1. Photographs and histograms showing changes in the size of quail testis after exposure to 5% formalin gas intrabdominal testes from the control and treated groups at 10–30 days. There was a clear increase in the size of both the right and left testes in the exposed groups compared with the control. Histogram showing a progressive increase in testicular weight and a decrease in body weight. The highest testis weight was recorded in the 30-day group (4.45 ± 0.34 g), whereas the body weight decreased to 130 ± 0.43 g. Significant differences (p < 0.05) were observed between the groups. Both the right and left testes exhibited a significant increase in size after 10, 20, and 30 days of exposure compared with the control and 10-day groups (Fig. 1). The 20-day group (1 ± 0.0 g) and 30-day group (2 ± 0.2 g) presented significant enlargement compared with the control and 10-day groups. However, no significant difference was observed between the control (0.44 ± 0.1 g) and 10-day (1 ± 0.0 g) groups (p < 0.05). Histological featuresThe testicular anatomy encompasses a three-layered capsule. From the outermost layer inwards, these layers are the tunica vaginalis, tunica albuginea, and tunica vasculosa. The tunica vaginalis comprises mesothelial cells that exhibit a direct association with the peritoneum of the coelomic cavity. Microscopic examination revealed the presence of peritubular tissue, characterized as delicate connective tissue septa, situated between adjacent seminiferous tubules (Fig. 2).
Fig. 2. (A) Histological sections of testicular tissue in quails stained with hematoxylin and eosin (H&E) at 100 magnification. The control group exhibited normal seminiferous tubules with clear germ cell layers and an organized structure. The formalin-exposed group exhibited severe tissue damage, including necrosis, germ cell loss, degeneration, and inflammation. (B) Bar chart showing the serum testosterone levels in quails. The control group had the highest level (2.22 ± 0.30 ng/ml). Levels decreased significantly in the exposed groups: 1.57 ± 0.35 ng/ml (10 days), 1.1 ± 0.27 ng/ml (20 days), and 0.9 ± 0.07 ng/ml (30 days). Asterisks (*) indicate significant differences (p < 0.05) compared with the control. The seminiferous tubules exhibited a convoluted morphology and were encircled by a delicate sheath. This sheath comprised smooth muscle fibers and multiple layers of myoid cells. Spermatogonia cells appeared as a single layer of cells supported by Sertoli cells and extended with spermatozoa. The diameter of the straight tubules (tubuli recti) was smaller than that of the seminiferous tubules. They were lined only by Sertoli cells and showed a high degree of anastomosis (Fig. 2). Our histological analysis revealed widespread damage across all testicular tissues. The main findings included tissue shrinkage, degeneration, and a significant loss of germinal cells. Seminiferous tubules exhibited poorly defined cellular details and structural integrity loss. Extensive damage was observed in many tissue sections, characterized by large areas of necrosis, inflammation, and a loss of cellular detail. Additionally, blood vessels appeared congested and exhibited potential signs of endothelial damage (Fig. 2). Biochemical analysis of blood samplesThe testosterone levels in quails exhibited a statistically significant decrease between the experimental groups (p < 0.05). In contrast, the control group demonstrated a notable increase in serum testosterone concentration, reaching 2.2 ± 0.3 for all groups. In contrast, the groups exposed to formalin gas showed a clear decrease in testosterone serum concentration at 10, 20, and 30 days, with levels of 1.6 ± 0.4, 1.1 ± 0.3, and 0.9 ± 0.1, respectively. Analysis of the data revealed significant differences (p < 0.05) among the four groups. The group exposed to formalin gas for 30 days demonstrated the most substantial decline in testosterone concentration relative to the other groups (Fig. 2). Analysis of blood samples revealed statistically significant variations in WBC counts between the formalin gas-exposed and control groups. Notably, all three treatment groups exhibited a significant increase in WBC percentage compared with the control group, with a statistical significance level of p < 0.05. The WBC counts were as follows: 81.5 ± 51.3 in the control group, and 125.3 ± 57.8, 143.6 ± 78.3, and 145.3 ± 83.5 in the treated groups, respectively. However, the fourth group (30 days) exhibited the highest percentage compared to the other groups and the control. Moreover, exposure to 5% formalin gas in quails led to a significant reduction in the percentage of circulating lymphocytes in their blood. This decrease in lymphocyte count appears to be associated with the exposure duration or concentration of formalin (Fig. 3).
Fig. 3. Bar graphs showing blood parameters in quails exposed to 5% formalin gas for 10–30 days compared with the control group. WBC count increased from 81.5 ± 51.3 (control) to 145.3 ± 83.5 (30-day group). The lymphocyte percentage decreased with longer exposure. The RBC count increased from 3.2 ± 1.8 (control) to 4.3 ± 2.2 (30-day group). HGB levels increased from 17.9 ± 9.2 (control) to 22.2 ± 11.5 (30-day group). All changes were significant (p < 0.05). A statistically significant increase (p < 0.05) in RBC counts was observed in the formalin-treated groups compared with the control group. The control group exhibited an RBC count of 3.2 ± 1.8, whereas the first, second, and third treated groups displayed counts of 3.3 ± 2.1, 4.0 ± 1.8, and 4.3 ± 2.2, respectively. Analysis revealed significant differences among the four groups (p < 0.05). Notably, all three treatment groups demonstrated a higher percentage of RBCs compared with the control group, with the third group exhibiting the most pronounced elevation (Fig. 3). Analysis of HGB levels revealed a significant elevation in Hb concentration in groups exposed to formalin gas compared with the control group. The mean Hb values for the exposed groups were 18.0 ± 9.2, 19.7 ± 10.4, 20.7 ± 10.9, and 22.2 ± 11.5, respectively, with statistically significant differences (p < 0.05) observed between groups. Compared with the control, a notable increase in HGB percentage was observed across all three groups, with the fourth group demonstrating the most pronounced elevation (Fig. 3). Real-time quantitative polymerase chain reaction This study also examined how 5% formalin gas affects TP53 gene expression in the testes of quails. The analysis showed a clear increase in TP53 expression in the formalin-exposed groups compared with the control (p < 0.05). The threshold cycle (Ct) values were lower in the treated (24.5) groups, indicating higher gene expression. The mean Ct value for TP53 expression in the control group was approximately 30.2. The increase in TP53 expression was dose-dependent and became more pronounced with longer exposure. Compared with the control group, the 10-day, 20-day, and 30-day groups showed a 2.3-, 4.7-, and 8.1-fold increase in TP53 expression (p < 0.05), based on the ΔΔCt method. The melting curve analysis confirmed the specificity of the TP53 primers. No primer-dimers or non-specific products were detected in this study. The melting temperatures ranged from 70°C to 80°C. The PCR amplification efficiency for TP53 was 96.4%, which supports the accuracy and reliability of the expression data (Fig. 4).
Fig. 4. (A) RT-qPCR amplification curves for the GAPDH housekeeping gene in quail testicular tissue. (B) Amplification curves for TP53 in the control and formalin-exposed groups. Blue lines show Group 1, and red lines show the control. (C) Bar graph showing TP53 gene expression levels. Expression increased with longer exposure time. The highest expression was observed in the 30-day group. Statistical differences were observed: 30-day group and Group 1 (p=0.042), Group 2 (p=0.072), and the control group (p=0.018). Asterisks (*) indicate statistically significant differences between groups. DiscussionThis study focused on the harmful effects of exposure to 5% formalin gas on the reproductive system of male quails. Quails that inhaled formalin gas developed visible symptoms, such as rapid breathing, restlessness, and stress. These behavioral changes suggest respiratory tract irritation and possible effects on the central nervous system. Gas may cause discomfort, anxiety, and breathing difficulty, leading to increased movement, vocalization, and avoidance behavior. Such symptoms reflect a stress response commonly observed in birds exposed to inhaled irritants. These findings support earlier observations where female quails fed with formalin showed similar toxic responses (Khan et al., 2005), indicating that both formalin ingestion and inhalation can trigger behavioral distress in quails. The body weight of the treated quails dropped with time. The 30-day group lost the most weight. This weight loss might be linked to the effects of gas exposure or toxic damage to internal organs (Kou et al., 2022). Microscopic examination of the testes revealed severe tissue damage. Changes included interstitial tissue enlargement, blood vessel congestion, and seminiferous tubule destruction. These findings are consistent with earlier reports on chemical-induced damage to reproductive organs (Duong et al., 2011). Inhaled formaldehyde is known to increase the expression of heat shock protein 70 (Hsp70), a stress marker, which contributes to cellular damage in the testes (Ortega-Atienza et al., 2016). Previous studies on rats have shown that exposure to formaldehyde vapors leads to poor sperm production, altered sperm shapes, and low sperm counts, which can result in infertility (Razi et al., 2013). Our findings in quails support this, as severe testicular changes were observed. These effects also included a significant decrease in testosterone levels. This hormone is produced by Leydig cells that were damaged in the exposed groups. These results agree with those of Leisegang and Henkel (2018), who showed that formaldehyde harms steroid-producing cells. The blood test results also revealed strong changes. WBC and RBC counts, as well as HGB levels, were increased in the treated birds. This may reflect inflammation or stress in response to formalin. These changes were contrary to earlier findings, where oral formalin exposure led to decreased blood values and increased liver enzymes (Khan et al., 2006). This suggests that inhaled formalin may cause a different type of body response than ingested formalin (Kou et al., 2022). Formalin exposure may impair fertility by directly damaging testis germ cells. This damage can disrupt the seminiferous tubule structure and reduce sperm production. When DNA damage occurs in these cells, the TP53 gene activates as a defense mechanism. TP53 controls cell division and can trigger apoptosis to remove damaged cells. As a result, increased TP53 expression may lead to spermatogenic cell death and reduced sperm output. This process helps protect against mutations but also limits fertility by stopping normal spermatogenesis. Interestingly, another study found no major changes in blood values among workers exposed to formaldehyde (La Torre et al., 2024). The difference may be due to the form, dose, or exposure duration. Long-term gas exposure in quails may create stronger internal stress than occasional occupational contact. TP53 helps protect the body from cancer. It works by checking for cell damage. When cells get damaged, this gene turns on to stop them from growing and dividing (Aubrey et al., 2016; Benitez et al., 2024). In our study, TP53 levels increased in birds exposed to formalin. The increase was strongest in the group exposed for 30 days. TP53 can slow cell growth. It also helps fix DNA or causes the cell to die if the damage is severe (Khan et al., 2024; Mir, 2024). The high levels suggest that formalin damaged the testis cells. Consequently, the TP53 gene became more active. The RT-qPCR results supported this hypothesis. Gene expression increased with longer exposure. The 30-day group had the highest TP53 level. Thus, long exposure to formalin can trigger the body’s cancer defense response in the testis. ConclusionThis study demonstrated that 5% formalin gas harmed the testes of male quails. The birds that breathed the gas looked stressed. They lost weight and had breathing problems. When we examined their testis tissue under the microscope, we observed clear damage. Blood tests showed changes in cell counts and hormone levels, especially a sharp drop in testosterone levels. These findings confirm the toxic effects of formalin gas on reproductive health. Furthermore, the strong increase in TP53 gene expression suggests that formalin may cause DNA damage that could lead to cancer. These risks may also apply to other animals and possibly humans exposed to formalin over time. AcknowledgmentsThe Department of Anatomy, Histology, and Embryology within the College of Veterinary Medicine, University of Al-Qadisiyah, Iraq, supported this research. Conflict of interestThe authors declare no competing interests regarding the authorship or publication of this article. FundingThe authors self-funded the study. No external funding source is available. Authors’ contributionsAll authors participated equally in the study. Data availabilityData are available when requested by the corresponding author. ReferencesAbid, A. and Hamza, A. 2024. Histomorphological development study of the heart of local Awassi sheep (Ovis aris) at postnatal stages. J. Anim. Health Prod. 12(s1), 9–14. Agarwal, S., Saini, S., Parashar, D., Verma, A., Sinha, A., Jagadish, N., Batra, A., Suri, S., Gupta, A. and Ansari, A.S. 2013. The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. Oncoimmunology 2(5), e24270. Al-Mahmodi, A.M.M., Almhanna, H., Abd Murad, A.-M.N., Yassin, A. and Hassan, W.R. 2022. Morphometric and histological study of the papillary muscles and tendinous chords of the heart of local breed bulls (Bos taurus). Rev. Electron. Vet. 23, 415–428. Almhanna, H., Al-Mamoori, N.A.M. and Naser, H.H. 2022. mRNA expression of the severe acute respiratory syndrome-coronavirus 2 angiotensin-converting enzyme 2 receptor in the lung tissue of Wistar rats according to age. Vet. World 15(2), 427. Almhanna, H., Yassin, A., Al-Mamoori, N., Kilroy, D. and Kumar, A. 2024. Structural and network analysis of ADAM9 and ADAM10 and its transcript expression in the male reproductive tract of Bos taurus. Ger. J. Vet. Res. 4(3), 126–139. American Veterinary Medical Association. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg, IL: American Veterinary Medical Association. Available via https://www.avma.org/sites/default/files/2023-01/Guidelines-on-Euthanasia-2020.pdf Aubrey, B.J., Strasser, A. and Kelly, G.L. 2016. Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb. Perspect. Med. 6(5), a026062. Barszcz, M., Tuśnio, A. and Taciak, M. 2024. Poultry nutrition. Phy. Sci. Rev. 9(2), 611–650. Baugh, E.H., Ke, H., Levine, A.J., Bonneau, R.A. and Chan, C.S. 2018. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 25(1), 154–160. Benitez, D.A., Cumplido-Laso, G., Olivera-Gómez, M., Del Valle-Del Pino, N., Díaz-Pizarro, A., Mulero-Navarro, S., Román-García, A. and Carvajal-Gonzalez, J.M. 2024. TP53 genetics and biology in lung carcinomas: insights, implications and clinical applications. Biomedicines 12(7), 1453. Bernardini, L., Barbosa, E., Charão, M.F. and Brucker, N. 2022. Formaldehyde toxicity reports from in vitro and in vivo studies: a review and updated data. Drug Chem. Toxicol. 45(3), 972–984; doi:10.1080/01480545.2020.1795190 Duong, V.-A., Park, J.-M., Lim, H.-J. and Lee, H. 2021. Proteomics in forensic analysis: applications for human samples. Appl. Sci. 11(8), 3393. Duong, A., Steinmaus, C., McHale, C.M., Vaughan, C.P. and Zhang, L. 2011. Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat. Res. Rev. Mutat. Res. 728(3), 118–138. Egbuniwe, I.C., Akogwu, M.S. and Obetta, T.U. 2024. Mechanisms underlying reproductive responses of Japanese quails to heat stress conditions. Int. J. Biometeorol. 68, 1–12. Eladl, A.H., Farag, V.M., El-Shafei, R.A., Aziza, A.E., Awadin, W.F. and Arafat, N. 2022. Immunological, biochemical and pathological effects of vitamin C and Arabic gum co-administration on H9N2 avian influenza virus vaccinated and challenged laying Japanese quails. BMC Vet. Res. 18(1), 408. Ghareeb, O., Abdulla, G. and Sheikhzadeh, S. 2024. Evaluation of proinflammatory cytokines in arsenic induced hepatotoxicity in vivo. J. Anim. Health Prod. 12(s1), 196–201. Grillo, F., Pigozzi, S., Ceriolo, P., Calamaro, P., Fiocca, R. and Mastracci, L. 2015. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem. Cell Biol. 144(1), 93–99. Gupta, A., Shah, K., Oza, M.J. and Behl, T. 2019. Reactivation of TP53 gene by MDM2 inhibitors: a novel therapy for cancer treatment. Biomed Pharmacother. 109, 484–492. Han, S.P., Zhou, D.X., Lin, P., Qin, Z., An, L., Zheng, L.R. and Lei, L. 2015. Formaldehyde exposure induces autophagy in testicular tissues of adult male rats. Environ. Toxicol. 30(3), 323–331. Hanel, W. and Moll, U.M. 2012. Links between mutant TP53 and genomic instability. J. Cell. Biochem. 113(2), 433–439. Hasan, I., Pervin, M., Kobir, M.A., Sagor, S.H. and Karim, M.R. 2021. Effect of formaldehyde and urea contaminated feed exposure into the liver of young and adult pigeons (Columba livia). Vet. World 14(3), 769–774. Kadhim, A.B., Almhanna, H.K., Sharoot, H.A., Abid Al-Redah, S.A. and Almamoori, N.A. 2023. Anatomical and histological study of the kidney of the one-humped camel. Iraqi J. Vet. Sci. 37, 73–79. Kastan, M.B. and Bartek, J. 2004. Cell-cycle checkpoints and cancer. Nature 432(7015), 316–323. Khan, A., Bachaya, H., Khan, M. and Mahmood, F. 2005. Pathological effects of formalin (37% formaldehyde) feeding in female Japanese quails (Coturnix coturnix japonica). Hum. Exp. Toxicol. 24(8), 415–422. Khan, A., Hussain, S. and Khan, M. 2006. Effects of formalin feeding or administering into the crops of white leghorn cockerels on hematological and biochemical parameters. Poult. Sci. 85(9), 1513–1519. Khan, R., Pari, B. and Puszynski, K. 2024. Comprehensive bioinformatic investigation of TP53 dysregulation in diverse cancer landscapes. Genes 15(5), 577. Kim, K.-H., Jahan, S.A. and Lee, J.-T. 2011. Exposure to formaldehyde and its potential human health hazards. J. Environ. Sci. Health Part C 29(4), 277–299. Kou, Y., Zhao, H., Cui, D., Han, H. and Tong, Z. 2022. Formaldehyde toxicity in age-related neurological dementia. Ageing Res. Rev. 73, 101512; doi:10.1016/j.arr.2021.101512 Kuethe, D.O. 1988. Fluid mechanical valving of air flow in bird lungs. J. Exp. Biol. 136(1), 1–12. Kumar, M., Lu, Z., Takwi, A.A.L., Chen, W., Callander, N.S., Ramos, K.S., Young, K.H. and Li, Y. 2011. Negative regulation of the tumor suppressor TP53 gene by microRNAs. Oncogene 30(7), 843–853. Lansford, R. and Cheng, K.M. 2024. The Japanese quail. In The UFAW handbook on the care and management of laboratory and other research animals . Chichester, UK: Wiley-Blackwell, pp: 762–786. La Torre, G., Manai, M.V., Moretti, L., Vezza, F., Breccia, M., Cafolla, A., Bernardinetti, G., Leo, G., Stefanantoni, K. and Pavan, A. 2024. Does occupational exposure to chemicals/carcinogens affect the hematological parameters of workers? J. Clin. Med. 13(21), 6317. La Torre, G., Vitello, T., Cocchiara, R.A. and Della Rocca, C. 2023. Relationship between formaldehyde exposure, respiratory irritant effects and cancers: a review of reviews. Public Health 218, 186–196; doi:10.1016/j.puhe.2023.03.009 Leisegang, K. and Henkel, R. 2018. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 16, 1–11. Leroy, B., Girard, L., Hollestelle, A., Minna, J.D., Gazdar, A.F. and Soussi, T. 2014. Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum. Mutat. 35(6), 756–765. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4), 402–408. Luna, L. 1968. Methods for carbohydrates and mucoproteins. In Manual of histologic staining methods of the Armed Forces Institute of Pathology, pp: 153–173. Mallette, F.A. and Ferbeyre, G. 2007. The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle 6(15), 1831–1836. Mir, M.A. 2024. TP53 in breast cancer: molecular mechanisms, clinical implications, and therapeutic targets. Boca Raton, FL: CRC Press. Ortega-Atienza, S., Rubis, B., McCarthy, C. and Zhitkovich, A. 2016. Formaldehyde is a potent proteotoxic stressor causing rapid heat shock transcription factor 1 activation and Lys48-linked polyubiquitination of proteins. Am. J. Pathol. 186(11), 2857–2868. Razi, M., Malekinejad, H., Sayrafi, R., Hosseinchi, M.R., Feyzi, S., Moshtagion, S.M. and Janbaz, H. 2013. Adverse effects of long-time exposure to formaldehyde vapour on testicular tissue and sperm parameters in rats. Vet. Res. Forum 4(3), 213–221. Snow, A.N., Stence, A.A., Pruessner, J.A., Bossler, A.D. and Ma, D. 2014. A simple and cost-effective method of DNA extraction from small formalin-fixed paraffin-embedded tissue for molecular oncologic testing. BMC Clin. Pathol. 14, 1–10. Söderberg-Nauclér, C. 2006. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Inter. Med. 259(3), 219–246. Van Agteren, M.H., Keuning, S. and Oosterhaven, J. 1998. Handbook on biodegradation and biological treatment of hazardous organic compounds. Springer Science & Business Media. Xiong, X., Zhang, S., Liao, X., Du, J., Zheng, W., Hu, S., Wei, Q. and Yang, L. 2024. An umbrella review of the evidence associating occupational carcinogens and cancer risk at 19 anatomical sites. Environ. Pollut. 345, 123531; doi:10.1016/j.envpol.2024.123531 Zhan, Q. 2005. Gadd45a, a TP53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res. Fund. Mol. Mech. Mutagen. 569(1–2), 133–143. | ||
| How to Cite this Article |
| Pubmed Style Hacham IIA, Albaghdady EF, Kadhim AB, Sharoot HA, Almhanna H. Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Vet. J.. 2025; 15(9): 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 Web Style Hacham IIA, Albaghdady EF, Kadhim AB, Sharoot HA, Almhanna H. Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). https://www.openveterinaryjournal.com/?mno=269091 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.33 AMA (American Medical Association) Style Hacham IIA, Albaghdady EF, Kadhim AB, Sharoot HA, Almhanna H. Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Vet. J.. 2025; 15(9): 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 Vancouver/ICMJE Style Hacham IIA, Albaghdady EF, Kadhim AB, Sharoot HA, Almhanna H. Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 Harvard Style Hacham, I. I. A., Albaghdady, . E. F., Kadhim, . A. B., Sharoot, . H. A. & Almhanna, . H. (2025) Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Vet. J., 15 (9), 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 Turabian Style Hacham, Iman Ibrahim Al, Eman F. Albaghdady, Abdulrazzaq Baqer Kadhim, Hassaneen A. Sharoot, and Hazem Almhanna. 2025. Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Veterinary Journal, 15 (9), 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 Chicago Style Hacham, Iman Ibrahim Al, Eman F. Albaghdady, Abdulrazzaq Baqer Kadhim, Hassaneen A. Sharoot, and Hazem Almhanna. "Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix)." Open Veterinary Journal 15 (2025), 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 MLA (The Modern Language Association) Style Hacham, Iman Ibrahim Al, Eman F. Albaghdady, Abdulrazzaq Baqer Kadhim, Hassaneen A. Sharoot, and Hazem Almhanna. "Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix)." Open Veterinary Journal 15.9 (2025), 4265-4275. Print. doi:10.5455/OVJ.2025.v15.i9.33 APA (American Psychological Association) Style Hacham, I. I. A., Albaghdady, . E. F., Kadhim, . A. B., Sharoot, . H. A. & Almhanna, . H. (2025) Impact of formalin gas exposure on quail testicular health in quail (Coturnix coturnix). Open Veterinary Journal, 15 (9), 4265-4275. doi:10.5455/OVJ.2025.v15.i9.33 |