| Research Article | ||
Open Vet. J.. 2025; 15(9): 4276-4285
Open Veterinary Journal, (2025), Vol. 15(9): 4276-4285 Research Article Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male ratsHawraa Mohammed Tareq* and Sawsan Kadhim MashiDepartment of Physiology, Pharmacology, and Biochemistry, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Hawraa Mohammed Tareq. Department of Physiology, Pharmacology, and Biochemistry, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: hawraa.tareq2306m [at] covm.uobaghdad.edu.iq Submitted: 19/07/2025 Revised: 18/08/2025 Accepted: 26/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Augmentin is a common antibiotic used to treat infections. However, it may cause liver damage. Toxicity often involves oxidative stress and inflammation. N-acetylcysteine (NAC) is known for its antioxidant and anti-inflammatory effects and may help protect the liver. Aim: This study aimed to assess whether NAC could reduce Augmentin-induced liver and bile duct injury. Methods: Forty adult male rats were divided into four groups (T1, T2, T3, and T4). Group 1 was the control group. Group 2 received Augmentin (30 mg/kg/day). Group 3 received 150 mg/kg/day NAC. Group 4 received both NAC and Augmentin. Treatments lasted for 35 days. Serum levels of tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), glutathione (GSH), and CYP7A1 were measured. Histopathology was also performed. Results: Augmentin alone caused a significant (p < 0.05) increase in TNF-α (13.82 ± 0.31), MDA (407.25 ± 10.65), and CYP7A1 (7.69 ± 0.48). GSH dropped to (9.10 ± 0.43). Liver tissues showed inflammation, sinusoidal venostasis, and bile duct damage. NAC-treated rats had significantly (p < 0.05) lower TNF-α (4.88–4.97), MDA (253.05–258.15), and CYP7A1 (4.30–4.38). GSH levels significantly (p < 0.05) increased to (15.58–17.02). Histology improved with NAC. Livers exhibited fewer cell injuries and a more normal architecture. Conclusion: NAC reduced the oxidative stress and inflammation caused by Augmentin. It also protected the liver structure. These findings suggest that NAC is a useful supplement for preventing drug-induced liver injury. Keywords: CYP7A1, Glutathione, Histopathology, Malondialdehyde, TNF-α. IntroductionDrug-induced liver injury (DILI) is a growing global health concern. It often leads to severe liver dysfunction and can mimic viral, autoimmune, or genetic liver diseases (Maris et al., 2025). Many cases go unrecognized due to nonspecific symptoms and a lack of specific diagnostic tools. Among pharmaceuticals, antibiotics are major contributors to DILI in both adults and children (Ma et al., 2024). The combination of amoxicillin–clavulanate is known to cause hepatocellular, cholestatic, or mixed types of liver injury depending on dose and duration. After antibiotic use, it may present as elevated liver enzymes, bile duct inflammation, or jaundice (Visentin et al., 2018). Hepatic damage can progress to fibrosis and rarely to acute liver failure. Although the incidence is low, the wide use of amoxicillin–clavulanate increases exposure and risk. The damage to the liver caused by this antibiotic does not follow a single, clear pathway. It may harm cells directly, but it can also trigger immune reactions that add to the injury (Sernoskie et al., 2021). The drug can trigger immune system responses that differ from one individual to another, and inherited traits may play a role. Researchers have also pointed to a connection between the gut and the liver in this process (Ghazi et al., 2024). Bacterial toxins can pass into the bloodstream through the portal vein and reach the liver when antibiotics disturb normal gut microbes (Fu et al., 2022). This triggers inflammation and increases the exposure of the organ to toxic drug by-products. In addition, antibiotics may generate oxidative stress in liver cells, disrupting mitochondrial function and reducing glutathione stores (Pham et al., 2021). With fewer antioxidants and more inflammatory messengers such as tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6, the injury can deepen and persist if nothing stops it. The bile ducts are another target for this study. Amoxicillin–clavulanate has been linked to cholangiopathy, in which the bile duct lining is damaged and bile flow slows down (Visentin et al., 2018). This often occurs with higher bilirubin and gamma-glutamyl transferase levels. The problem ties into bile acid regulation, which heavily depends on CYP7A1, the enzyme that starts bile acid production from cholesterol. Changes in CYP7A1 activity can upset the bile acid balance and interfere with liver metabolism (Low et al., 2020). Low glutathione (GSH) levels, ongoing inflammation, and altered gene activity can all contribute to this damage. Targeting these pathways might reduce the severity of the injury (Ghazi et al., 2024). N-acetylcysteine (NAC) is best known as an antidote for acetaminophen overdose; however, it also plays a broader role as an antioxidant and glutathione donor. NAC can restore the balance between oxidants and antioxidants, lower inflammation, and neutralize harmful free radicals (Daly, 2025). It also helps stabilize mitochondria and slows lipid peroxidation. In animal models, it has lowered serum markers of liver injury and improved tissue appearance after drug damage (Raghu et al., 2021). It may also reduce TNF-α and support healthy bile acid metabolism (Tenório et al., 2021). NAC acts on several different mechanisms and has a good safety record; therefore, it is considered a promising option for protecting the liver. In this study, we examined whether Augmentin could prevent or lessen liver and bile duct damage. Materials and MethodsExperimental animals and their groupingForty healthy adult male rats were used in this study. They were aged 10–12 weeks and weighed between 300 and 350 g. The animals were housed in the Animal House, Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Baghdad. Environmental conditions were maintained at a temperature of 20°C–25°C, a 12-hours light/dark cycle, and ad libitum access to tap water and pellet feed. The rats were given 10 days of acclimatization before the experiment began. All animals were housed in plastic cages with adequate ventilation. Sanitation was maintained daily, and the feed was standardized. The animals were randomly divided into four equal groups of 10 rats each. Group 1 (control) received only the basal diet. Group 2 (T1) was treated with oral Augmentin at a dose of 30 mg/kg/day. Group 3 (T2) received oral NAC at 150 mg/kg/day. Group 4 (T3) received both NAC (150 mg/kg) and Augmentin (30 mg/kg) by daily oral gavage. All treatments lasted for 35 consecutive days. The animals were anesthetized and euthanized for sample collection at the end of the experimental period. The selected doses of Augmentin (30 mg/kg/day) and NAC (150 mg/kg/day) were based on previous studies (Elrahman, 2023; Erigbali et al., 2023) Estimation of TNF-αBlood was collected via cardiac puncture after 35 days of treatment. Blood was transferred into gel tubes and centrifuged at 6,000 rpm for 10 minutes. Serum was separated and stored at −8°C until analysis. TNF-α levels were measured using a rat-specific enzyme-linked immunosorbent assay kit (ELISA) (SIMT, China). The assay followed the manufacturer’s protocol exactly. A microplate reader was used to measure the absorbance at 450 nm. All samples and standards were run in duplicate. The results are expressed in picograms per milliliter (pg/ml). Estimation of malondialdehyde (MDA)Blood samples were processed as described above, and the serum was stored at −8°C. MDA levels were measured using an ELISA kit specific for rat MDA (Cloud-Clone, USA). The procedure included binding of MDA to antibodies coated on the plate, followed by a color change reaction. The optical density was measured at 450 nm using a microplate reader. All samples were analyzed in duplicate. The standard curves ensured accurate quantification. The results were calculated using a curve-fitting software, and the concentrations were expressed in µmol/l. The assay was validated to detect changes in MDA caused by Augmentin and assess the antioxidant effect of NAC. Estimation of GSHBlood was collected and processed as described previously. Serum GSH levels were quantified using a rat-specific ELISA kit (Cloud-Clone, USA). A competitive binding format with absorbance detection at 450 nm was used. All samples were tested in duplicate. The GSH concentration was calculated using a standard curve generated with known concentrations. The ELISA method used in this study provided high specificity and sensitivity. All reagents were used according to the manufacturer’s instructions Aper the standard protocol to avoid variability. The results were recorded in mg/dl. Strict quality control measures were applied to ensure accurate and reproducible results. Estimation of CYP7A1 activityCYP7A1 enzyme levels were measured to assess bile acid metabolism. This enzyme plays a central role in the conversion of cholesterol to bile acids. Serum samples were analyzed using an ELISA kit specific for rat CYP7A1 (Linear Chemicals, Spain). The test followed the manufacturer’s protocol for sandwich ELISA. Samples and standards were pipetted into pre-coated wells, incubated, and then washed. The substrate solution was added, and the absorbance was read at 450 nm. The results are expressed in pmol/min/mg protein. Duplicate readings were obtained for all samples. The ELISA kit provided accurate and reproducible CYP7A1 measurement. To confirm assay validity, each run included positive and negative controls. This enzyme is a useful biomarker of bile flow disturbance. Histopathological examination of the liverAt the end of the experiment, the rats were euthanized under ethically approved anesthesia, and the liver was carefully removed. Tissue samples were washed in saline and fixed in 10% neutral buffered formalin neutral buffered formalin. After fixation, the samples were dehydrated in graded alcohols, cleared with xylene, and embedded in paraffin. Sections of 5 µm thickness were cut and stained with hematoxylin and eosin (H&E). Microscopic evaluation was performed using a light microscope (Olympus, Japan). Statistical analysisAll data were analyzed using one-way analysis of variance (ANOVA) to compare differences among the experimental groups. Tukey’s post hoc test was applied to identify pairwise significance. A p-value of 0.05 was considered statistically significant. Ethical approvalEthical approval was obtained from the Institutional Animal Ethics Committee at the College of Veterinary Medicine, University of Baghdad (approval no. 2476/30-12-2024). ResultsTNF-αThe level of TNF-α increased significantly (p < 0.05) in the augumentin-only group (T1) compared with all other groups. The average value was 13.82 ± 0.31 pg/ml, which was much higher than that of the control, T2, and T3 groups. Both NAC-treated groups showed significantly lower TNF-α levels. The values were 4.97 ± 0.29 and 4.88 ± 0.39 pg/mL for T2 and T3, respectively. There was no statistically significant difference between the control and NAC-treated groups, suggesting the strong anti-inflammatory effects of NAC. These results indicate that augmentin induces a proinflammatory state, and NAC effectively reduces this response (Fig. 1).
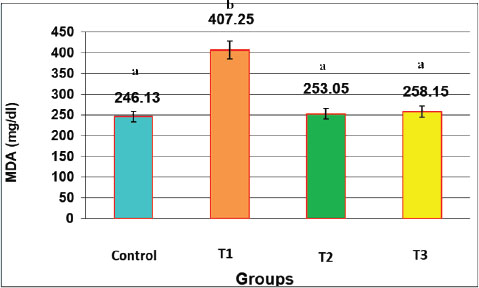
Fig. 1. Effect of oral administration of NAC and Augmentin on TNF-α Activity (mg/dl) in male rats LSD=0.880. Data are presented as mean ± SEM for each group (n=10). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. Different letters above the bars indicate significant differences between groups (p < 0.05). The graph (Fig. 1) shows the sharp rise in TNF-α in the T1 group and its reduction in the NAC-treated groups. The results demonstrate that NAC attenuates the inflammatory cytokine elevation caused by antibiotic toxicity. The consistency of TNF-α suppression in both the NAC and NAC+Augmentin groups confirms the protective role of NAC. These findings are consistent with the liver histology findings, where inflammation was more severe in T1. The values recorded in this experiment support that TNF-α is a reliable marker of inflammation due to Augmentin toxicity, and NAC can efficiently downregulate this pathway. MDAThere was a marked (p < 0.05) elevation in MDA levels was observed in the T1 group. The mean serum concentration was 407.25 ± 10.65 µmol/l, which was significantly higher than all other groups. In contrast, the control, T2, and T3 groups had much lower values: (246.13 ± 13.21), (253.05 ± 17.01), and (258.15 ± 5.34 µmol/l), respectively. These results confirm that augmentin caused significant oxidative stress. MDA is a lipid peroxidation product and a key indicator of oxidative membrane damage. The T2 and T3 groups showed a significant reduction in MDA levels compared with the T1 group, proving the antioxidant effect of NAC. This bar chart combines MDA and TNF-α values to show the total oxidative-inflammatory burden. T1 displays the highest combined stress. NAC administration drastically reduced this total load (Fig. 2).
Fig. 2. Effect of oral administration of NAC and augmentin on MDA activity (mg/dl) in male rats LSD=2.320. Summed levels of MDA and TNF-α show the cumulative oxidative-inflammatory burden in each group. Data are presented as mean ± SEM for each group (n=10). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. Different letters above the bars indicate significant differences between groups (p < 0.05). The graphical representation of MDA in Fig. 2 shows that NAC treatment prevented the rise in lipid peroxidation. The T2 group receiving only NAC had MDA levels similar to the control group. The T3 group, which received both NAC and Augmentin, also had low levels of MDA. This confirms that NAC protects the liver from oxidative stress even when co-administered with a hepatotoxic agent. The high MDA level in the T1 group was consistent with the histological signs of membrane disruption and fat droplet formation. Together, these biochemical data and histological changes support that MDA is a strong indicator of liver damage, and NAC has potent protective effects. GSHA significant (p < 0.05) drop in GSH levels was observed in the T1 group treated with augmentin alone. The recorded value was 9.10 ± 0.43 mg/dl, which was the lowest among all groups. In contrast, the GSH concentration was the highest in the T3 group (17.02 ± 0.42) and the T2 group (15.58 ± 0.47). The level of the control group was (15.17 ± 1.34). These findings demonstrate that Augmentin induced the depletion of antioxidant defenses, while NAC restored GSH to near-normal levels (Fig. 3).
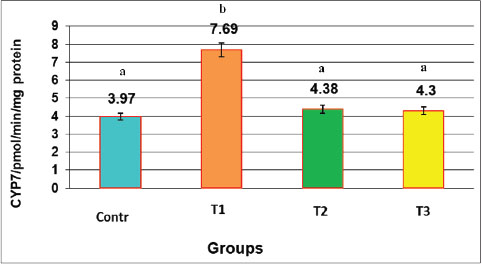
Fig. 3. Effect of the oral administration of NAC and Augmentin on the GSH activity (mg/dl) in male rats LSD=2.320. Data are presented as mean ± SEM for each group (n=10). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. Different letters above the bars indicate significant differences between groups (p < 0.05). CYP7A1CYP7A1 levels significantly increased in the T1 group. The average value was (7.69 ± 0.48) pmol/min/mg protein, which was markedly higher than that of the control (3.97 ± 0.19), T2 (4.38 ± 0.42), and T3 (4.30 ± 0.22) groups. NAC treatment brought the values close to normal, indicating its role in restoring metabolic balance. This analysis visualizes the percentage change in CYP7A1 activity from the control group. The T1 group showed a sharp increase, whereas the NAC groups maintained near-normal levels (Fig. 4).
Fig. 4. Effect of oral administration of NAC and Augmentin on CYP7 expression (pmol/min/mg protein) in male rats LSD=1.057. Percent change in CYP7A1 activity relative to the control group. Significant increase in T1, whereas NAC normalized this effect. Data are presented as mean ± SEM for each group (n=10). Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test. Different letters above the bars indicate significant differences between groups (p < 0.05). Fig. 4 shows that NAC significantly suppressed CYP7A1 activity. Elevated enzyme levels in the T1 group reflect a compensatory response to bile flow disturbance or liver injury. The NAC-only and NA plus augmentin groups had normal enzyme levels, indicating metabolic protection. The reduction of CYP7A1 by NAC could be due to its antioxidant effects or nuclear receptor modulation. These findings, along with histopathological changes, show that NAC effectively preserved bile canaliculi structure and regulated bile acid synthesis. Histopathological examinationHistological sections from the control group revealed normal liver structure. Hepatic lobules had well-defined central veins, uniform hepatocytes, and clear sinusoids. No signs of inflammation or damage were observed. In contrast, liver sections from the T1 group exhibited severe histopathological alterations. These included portal and central vein dilation and venostasis, and intravascular hemolysis. The other slides showed microvesicular steatosis, sinusoidal venostasis, and bile duct dilation (Fig. 5).
Fig. 5. Cross-section of the liver. A. Control group shows: hepatic lobules show normal central vein (V), with normal sinusoids and normal appearance of hepatic cords (arrows). H&E.100×. B. The T1 group shows severe congestion with portal vein dilation (P) with cholangiocyte proliferation. Severe dilation with sinusoid congestion (Arrows) (Arrows).H&E.100×. C. T1 group shows severe portal vein dilation with intravascular hemolysis (P) and cholangiocyte proliferation (Black arrow), severe sinusoid congestion (S) and bile canaliculi dilation (Red arrows).H&E.400×. T2 exhibited near-normal tissue appearance, similar to the control group. Some mild venostasis of the sinusoids and central veins was noted, but the hepatocyte architecture remained intact. In the NAC plus augmentin group (T3), the liver sections showed mild cellular swelling, mild sinusoidal venostasis, and preserved bile canaliculi. NAC reduced inflammation, oxidative damage, and structural alterations in the liver tissue, confirming its protective role (Fig. 6).
Fig. 6. Cross-section of hepatic lobules. A. The T2 group shows normal central vein (V), with normal sinusoids and normal hepatic cord appearance (arrows). H&E.100×. B. The T2 group shows: normal sinusoids (S) and normal appearance of hepatocytes (H) and bile canaliculus (c) .H&E.400×. C. The T3 group shows: mild venostasis of the central vein and normal hepatocytes. H&E.100×. D. The T3 group shows mild venostasis of the central vein with normal hepatocytes and normal sinusoid and canaliculi. H&E.400×. DiscussionAugmentin caused clear harmful effects on liver tissue, increasing TNF-α levels due to strong inflammation. This inflammation damages the tissue and triggers oxidative stress. As a result, MDA levels increased because of increased lipid peroxidation. Simultaneously, GSH levels dropped because antioxidants were used to fight the stress. Augmentin causes both inflammation and oxidative damage (Abdul Latif and Yousif, 2025; AL-Anbagi and Allawe, 2025; Elmanama et al., 2025; Jafer and Kassab, 2025). NAC is known for its antioxidant and protective properties in many organs, including the liver. In this study, NAC reduced TNF-α levels and restored GSH concentrations, which explains the improved antioxidant capacity observed in the treated groups (Nejati et al., 2022). These changes indicate reduced inflammation and improved redox balance. Similar findings have been reported in other models of oxidative injury, where NAC significantly restored antioxidant defenses and limited damage (Faghfouri et al., 2020). The observed changes in MDA further support the role of NAC in reducing lipid peroxidation (Al-Doskey, 2024; Jihad and Yousif, 2024; Kadhim et al., 2024; Mohamed et al., 2024). This supports earlier evidence showing how NAC decreases oxidative stress markers in both experimental and clinical settings (Pedre et al., 2021). NAC influenced CYP7A1 enzyme levels beyond redox modulation. The reduction in CYP7A1 expression after NAC treatment suggests improved cholesterol metabolism and less bile acid-related hepatotoxicity. This enzyme is often elevated in hepatobiliary stress, especially in DILI. The effects of NAC on liver enzymes have been previously linked to its ability to regulate inflammation, metabolism, and cellular repair (Jiang et al., 2022). Our study shows that NAC maintains normal enzyme levels, which might explain the histological improvements observed in liver tissue. NAC has also been considered for conditions where inflammation affects metabolic organs, and similar effects on enzymes have been reported in polycystic ovary syndrome models (Liu et al., 2023). Enzyme normalization might be an indirect result of lowered oxidative and inflammatory burden. In the control group, the liver sections appeared normal. The lobular structure was well organized, with clear central veins, uniform hepatocytes, and open sinusoids. No signs of inflammation or tissue damage were observed. These features are similar to those described by Ghafarizadeh et al. (2021), who showed intact hepatocyte cords and unobstructed sinusoids in healthy rat livers. Nejati et al. (2022) also reported that untreated rats maintained stable vascular and parenchymal patterns. The lack of congestion, necrosis, or bile duct changes in the control group confirms that the experimental setting did not cause injury. As in earlier studies, the stable morphology in this study contrasts with the marked alterations seen in the treated groups. In rats given Augmentin, the liver showed severe changes. Sinusoids were congested, portal veins were dilated, and cholangiocyte proliferation was visible. The bile canaliculi were also expanded. These lesions resemble the cholestatic and vascular injury described by Visentin et al. (2018), where antibiotic exposure led to bile duct epithelial growth and vascular congestion. Low et al. (2020) also linked similar patterns to disruptions in BA metabolism. The findings of this study on intravascular hemolysis and heavy congestion agree with those of Ma et al. (2024), who documented both hepatocellular and cholestatic injury after amoxicillin–clavulanate treatment. Compared with the normal control tissue, the Augmentin group showed extensive structural damage affecting both the blood vessels and bile ducts, consistent with reports that oxidative stress and inflammation drive such damage. Livers from the NAC-only group appeared mostly normal. The hepatocyte arrangement was intact, and only mild venostasis was noted. This aligns with the findings of Faghfouri et al. (2020), where NAC preserved the liver architecture under oxidative stress. Ghafarizadeh et al. (2021) also found minimal tissue changes in NAC-treated animals, supporting its non-toxic and protective profile. The mild venostasis was not linked to cell degeneration, which is consistent with the findings of Nejati et al. (2022), who observed that NAC supplementation kept sinusoidal patterns normal and prevented bile duct injury. Compared with the augmentin group, NAC alone offered clear structural protection, suggesting its role in membrane stability and vascular support. When NAC was given with Augmentin, the liver showed clear improvement. The damage was less than in the Augmentin group. Only mild swelling and slight venostasis appeared. Hepatocyte cords and bile canaliculi stayed intact. Similar results were reported by Jiang et al. (2022), who found that NAC reduced necrosis and congestion in drug-related liver injury. Akakpo et al. (2022) also showed that NAC reduced vascular and biliary damage from oxidative stress. In this study, congestion was reduced and there was no cholangiocyte proliferation, unlike the severe damage in the Augmentin group. Mild changes were still seen compared with NAC alone. This means NAC gives strong protection but cannot fully reverse the effects of Augmentin. Other studies also support this finding. Ghafarizadeh et al. (2021) showed that NAC reversed structural damage caused by toxic agents. NAC reduces necrosis and helps keep normal liver structure. Its action is linked to glutathione production, membrane protection, and lowering inflammation (Smaga et al., 2021). Animals treated with NAC had less vascular congestion and less hepatocyte ballooning (Akakpo et al., 2022). NAC is widely used in medicine. It is approved for acetaminophen overdose and chronic lung disease (Mokra et al., 2023). Its use has spread to psychiatric, endocrine, and metabolic conditions because of low toxicity and many benefits (Lee et al., 2021). In this study, the NAC-only group showed no tissue damage and normal biochemical markers. This proves NAC does not harm the liver (Shahveghar Asl et al., 2023). Some cancer studies suggest NAC may interfere with ROS-based drugs. Still, current data supports its protective role in non-cancer models (Kwon, 2021). Both animal and human work show NAC helps recovery and tissue repair when given in proper doses (Nejati et al., 2022). Our results add to this evidence and confirm that NAC protects the liver against Augmentin injury. This study also shows that Augmentin alone causes severe hepatobiliary injury. The damage comes from oxidative stress, inflammation, and changes in bile acid metabolism. High TNF-α and MDA levels with low GSH confirm this stress response. These findings agree with Faghfouri et al. (2020) and Pedre et al. (2021). CYP7A1 was increased in the Augmentin group, showing a reaction to bile flow problems, as noted by Low et al. (2020). NAC treatment reversed these changes. It lowered TNF-α and MDA, restored GSH, and normalized CYP7A1. Histologically, NAC preserved the liver architecture and prevented steatosis and vascular stasis, in agreement with the findings of Ghafarizadeh et al. (2021). Unlike previous studies, this study integrates biochemical, enzymatic, and histological endpoints to demonstrate the multi-level protection of NAC. These findings provide new evidence that NAC may serve as a targeted supplement in preventing antibiotic-induced liver injury, especially when bile duct involvement is suspected. This study has some limitations. The study did not assess post-treatment recovery beyond 35 days, and the long-term reversibility or progression of liver damage remains unknown. Future studies should include follow-up periods to evaluate tissue recovery or relapse. ConclusionNAC effectively protects the liver against Augmentin-induced damage. It reduces inflammation, oxidative stress, and enzyme alterations. NAC also improves liver structure and function. These findings support its potential as a safe hepatoprotective agent. AcknowledgmentThe authors thank the College of Veterinary Medicine, University of Baghdad, for their support in this study. Conflict of interestThe authors declare no conflicts of interest. FundingThe authors self-funded the study. No external funding source is available. Authors’ contributionsAll authors participated equally in the study. Data availabilityData are available when requested by the corresponding author. ReferencesAbdul Latif, S.A.K. and Yousif, A. 2025. Clinical, bacteriological, and molecular study of Streptococcus equi isolated from horses in Baghdad, Iraq. Iraqi J. Vet. Med. 49(1), 1–7; doi:10.30539/fk326b45 Akakpo, J.Y., Ramachandran, A., Curry, S.C., Rumack, B.H. and Jaeschke, H. 2022. Comparing N-acetylcysteine and 4-methylpyrazole as antidotes for acetaminophen overdose. Arch. Toxicol. 96(2), 453–465; doi:10.1007/s00204-021-03211-z Al-Anbagi, G.K. and B Allawe, A. 2025. Isolation of SAT2 foot and mouth disease virus in Iraq. Iraqi J. Vet. Med. 49(1), 16–22; doi:10.30539/pnc4zf32 Al-Doskey, Z. 2024. Taxonomic study on Paeonia L.,1753 (Paeoniaceae) from Iraq. Bull. Iraq Natural Hist. Museum 18(2), 611–631; doi:10.26842/binhm.7.2024.18.2.0373 Daly, A.K. 2025. Genetic and genomic approaches to the study of drug-induced liver injury. Liver Int. 45(1), e16191; doi:10.1111/liv.16191 Elmanama, A.A., Tayyem, N.E.A., Winkler, M.S., Abu-Dan, R.I. and El Hindi, M.W. 2025. Knowledge, attitude and practices of antimicrobial use among veterinarians and para-veterinarians in Gaza Strip, Palestine. Iraqi J. Vet. Med. 49(1), 23–30; doi:10.30539/2jb8ab31 Elrahman, M. 2023. The possible protective role of N-acetyl cysteine against tamoxifen-induced hepatotoxicity in the adult female albino rats: biochemical and histological study. Zagazig Univ. Med. J. 29(3), 841–851. Erigbali, P.P., Egbejimi, A.M., Sule, O.J. and Nordee, J.V. 2023. Hepato protective and antioxidant function of Indian almond (Terminalia catappa) leaf extract in augmentin-induced wistar rats experimental model of n-acetyl cysteine in the prophylaxis of “meconium ileus equivalent. J. Pediatrics 71(6), 887–889. Faghfouri, A.H., Zarezadeh, M., Tavakoli-Rouzbehani, O.M., Radkhah, N., Faghfuri, E., Kord-Varkaneh, H., Tan, S.C. and Ostadrahimi, A. 2020. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: a systematic review and meta-analysis of controlled clinical trials. Eur. J. Pharmacol. 884, 173368; doi:10.1016/j.ejphar.2020.173368 Fu, L., Qian, Y., Shang, Z., Sun, X., Kong, X. and Gao, Y. 2022. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf causality assessment method: emerging role of gut microbiota dysbiosis. Front. Med. 9, 972518; doi:10.3389/fmed.2022.972518 Ghafarizadeh, A., Malmir, M., Naderi Noreini, S. and Faraji, T. 2021. Antioxidant effects of N-acetylcysteine on the male reproductive system: a systematic review. Andrologia 53(1), e13898; doi:10.1111/and.13898 Ghazi, A.M., Ali Al-bayati, M.A. and Janabi, A.H. 2024. Metabolomics-detected alterations generated by phytosomal propolis and phytosomal lycopene in male rats with induced benign prostatic hyperplasia. Iraqi J. Vet. Sci. 38(Suppl I–IV), 7–15; doi:10.33899/ijvs.2024.147764.3531 Jafer, R.S. and J Kassab, H. 2025. Development and characterization of lornoxicam-infused ocular gel for effective treatment of ocular inflammation in domestic cats. Iraqi J. Vet. Med. 49(1), 8–15; doi:10.30539/pw6vsy73 Jiang, S.X., Hussaini, T. and Yoshida, E.M. 2022. N-acetylcysteine for non-acetaminophen induced acute liver failure: a review. Saudi J. Gastroenterol. 28(2), 85–91; doi:10.4103/sjg.sjg_406_21 Jihad, H. and Yousif, N. 2024. A survey of gastropods in the greenhouses of Iraq. Bull. Iraq Natural Hist. Museum 18(2), 565–576; doi:10.26842/binhm.7.2024.18.2.0371 Kadhim, Z., Al-Warid, H. and Al-Zaidawi, J. 2024. A new record of two nematodes, Mesorhabditis franseni Fuchs, 1933 (Mesorhabditidae) and Pratylenchus goodeyi Sher and Allen, 1953 (Pratylenchidae) with molecular description from Iraq. Bull. Iraq Natural Hist. Museum 18(2), 545–563; doi:10.26842/binhm.7.2024.18.2.0370 Kwon, Y. 2021. Possible beneficial effects of N-acetylcysteine for treatment of triple-negative breast cancer. Antioxidants 10(2), 169; doi:10.3390/antiox10020169 Lee, T.M., Lee, K.M., Lee, C.Y., Lee, H.C., Tam, K.W. and Loh, E.W. 2021. Effectiveness of N-acetylcysteine in autism spectrum disorders: a meta-analysis of randomized controlled trials. Aust N Z J Psychiatry 55(2), 196–206; doi:10.1177/0004867420952540 Liu, J., Su, H., Jin, X., Wang, L. and Huang, J. 2023. The effects of N-acetylcysteine supplement on metabolic parameters in women with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Nutr. 10, 1209614; doi:10.3389/fnut.2023.1209614 Low, E.X.S., Zheng, Q., Chan, E. and Lim, S.G. 2020. Drug induced liver injury: east versus West – A systematic review and meta-analysis. Clin. Mol. Hepatol. 26(2), 142–154; doi:10.3350/cmh.2019.1003 Ma, J., Björnsson, E.S. and Chalasani, N. 2024. Hepatotoxicity of antibiotics and antifungals and their safe use in hepatic impairment. Seminars Liver Dis. 44(2), 239–257; doi:10.1055/s-0044-1787062 Maris, B.R., Grama, A. and Pop, T.L. 2025. Drug-induced liver injury–pharmacological spectrum among children. Int. J. Mol. Sci. 26(5), 2006; doi:10.3390/ijms26052006 Mohamed, F., Hamdy, R. and Abd-Elkhader, E. 2024. Taxonomic revision of the family Casuarinaceae R. Br., 1814 (Order, Fagales) in Egypt. Bull. Iraq Natural Hist. Museum 18(2), 577–610; doi:10.26842/binhm.7.2024.18.2.0372 Mokra, D., Mokry, J., Barosova, R. and Hanusrichterova, J. 2023. Advances in the use of N-acetylcysteine in chronic respiratory diseases. Antioxidants 12(9), 1713; doi:10.3390/antiox12091713 Nejati, M., Dehghan, P., Jamilian, P. and Zarezadeh, M. 2022. The effects of N-acetylcysteine on recovery biomarkers: a systematic review and meta-analysis of controlled trials. J. Food Biochem. 46(7), e14116; doi:10.1111/jfbc.14116 Pedre, B., Barayeu, U., Ezeriņa, D. and Dick, T.P. 2021. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H(2)S and sulfane sulfur species. Pharmacol. Therapeutics 228, 107916; doi:10.1016/j.pharmthera.2021.107916 Pham, T.P., Alou, M.T., Golden, M.H., Million, M. and Raoult, D. 2021. Difference between kwashiorkor and marasmus: comparative meta-analysis of pathogenic characteristics and implications for treatment. Microbial Pathogenesis 150, 104702; doi:10.1016/j.micpath.2020.104702 Raghu, G., Berk, M., Campochiaro, P.A., Jaeschke, H., Marenzi, G., Richeldi, L., Wen, F.Q., Nicoletti, F. and Calverley, P.M.A. 2021. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr. Neuropharmacol. 19(8), 1202–1224; doi:10.2174/1570159X19666201230144109 Sernoskie, S.C., Jee, A. and Uetrecht, J.P. 2021. The emerging role of the innate immune response in idiosyncratic drug reactions. Pharmacol. Rev. 73(3), 861–896; doi:10.1124/pharmrev.120.000090 Shahveghar Asl, Z., Parastouei, K. and Eskandari, E. 2023. The effects of N-acetylcysteine on ovulation and sex hormones profile in women with polycystic ovary syndrome: a systematic review and meta-analysis. Br. J. Nutr. 130(2), 202–210; doi:10.1017/S0007114522003270 Smaga, I., Frankowska, M. and Filip, M. 2021. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 178(13), 2569–2594; doi:10.1111/bph.15456 Tenório, M.C.D.S., Graciliano, N.G., Moura, F.A., Oliveira, A.C.M.D. and Goulart, M.O.F. 2021. N-acetylcysteine (NAC): impacts on human health. Antioxidants 10(6), 967; doi:10.3390/antiox10060967 Visentin, M., Lenggenhager, D., Gai, Z. and Kullak-Ublick, G.A. 2018. Drug-induced bile duct injury. Biochimica Et Biophysica Acta (BBA). - Mol. Basis Dis. 1864(4 Pt B), 1498–1506; doi:10.1016/j.bbadis.2017.08.033 | ||
| How to Cite this Article |
| Pubmed Style Tareq HM, Mashi SK. Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Vet. J.. 2025; 15(9): 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 Web Style Tareq HM, Mashi SK. Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. https://www.openveterinaryjournal.com/?mno=271889 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.34 AMA (American Medical Association) Style Tareq HM, Mashi SK. Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Vet. J.. 2025; 15(9): 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 Vancouver/ICMJE Style Tareq HM, Mashi SK. Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 Harvard Style Tareq, H. M. & Mashi, . S. K. (2025) Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Vet. J., 15 (9), 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 Turabian Style Tareq, Hawraa Mohammed, and Sawsan Kadhim Mashi. 2025. Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Veterinary Journal, 15 (9), 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 Chicago Style Tareq, Hawraa Mohammed, and Sawsan Kadhim Mashi. "Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats." Open Veterinary Journal 15 (2025), 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 MLA (The Modern Language Association) Style Tareq, Hawraa Mohammed, and Sawsan Kadhim Mashi. "Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats." Open Veterinary Journal 15.9 (2025), 4276-4285. Print. doi:10.5455/OVJ.2025.v15.i9.34 APA (American Psychological Association) Style Tareq, H. M. & Mashi, . S. K. (2025) Investigation of augmentin-induced hepatobiliary damage and its modulation by N-acetylcysteine in male rats. Open Veterinary Journal, 15 (9), 4276-4285. doi:10.5455/OVJ.2025.v15.i9.34 |