| Research Article | ||
Open Vet. J.. 2025; 15(9): 4286-4294
Open Veterinary Journal, (2025), Vol. 15(9): 4286-4294 Research Article Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconateManal E. Shafi*Department of Biological Sciences, Zoology, Sustainable Agriculture Production Research Group, King Abdulaziz University, Jeddah, Saudi Arabia *Corresponding Author: Manal E. Shafi. Department of Biological Sciences, Zoology, Sustainable Agriculture Production Research Group, King Abdulaziz University, Jeddah, Saudi Arabia. Email: meshaf [at] kau.edu.sa Submitted: 27/06/2025 Revised: 22/08/2025 Accepted: 30/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
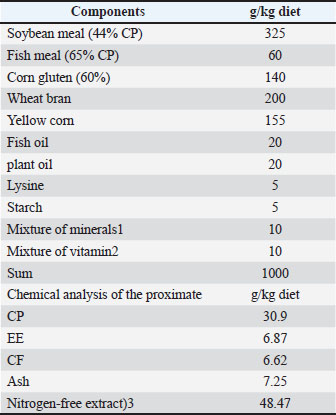
AbstractBackground: The aquaculture industry is actively seeking antibiotic-based alternatives to improve fish growth, immunity, and overall health. Natural products contain specific metabolites with immunomodulatory properties. Aim: The main aim of this experiment was to estimate the effects of nutrition dimethyl itaconate (DMIC) on feed utilization, growth traits, blood hematology and metabolites, antioxidant capacity, and inflammation biomarkers of Nile tilapia following an Aeromonas hydrophila infection. Methods: Over 56 days, the fish (weight 20.97 ± 0.33 g, n=200) were fed a main diet supplemented with different DMIC doses: zero, 50 mg/kg, 100 mg/kg, and 200 mg/kg. Results: All the fish receiving DMIC showed enhanced growth traits (final body weight, Specific growth rate, weight gain) and feed intake compared with the untreated section (p < 0.05), while feed conversion ratio had no significant impact (p > 0.05). In addition, the fish fed with DMIC showed significantly improved levels of glutathione, red blood cells, neutrophils, hemoglobin, and hematocrit in their blood (p < 0.001). Interestingly, the groups fed with DMIC also showed a dose-based increase in serum amounts of IgM (p < 0.05), glucose, total protein, albumin, nitric oxide, and lysosome activity. All the DMIC-intake groups showed greater phagocytic impact, while serum amounts of malondialdehyde, creatinine, alkaline phosphatase, uric acid, aspartate aminotransferase, and alanine aminotransferase were significantly reduced compared to the control treatment (p < 0.01). The fish intake fed with DMIC also appeared to significantly reduce serum amounts of Tumor necrosis factor alpha, interferon-gamma, and interleukin-4 (p < 0.05). Furthermore, fish infected by A. hydrophila and fed with DMIC treatments for 15 days showed a higher survival percentage compared with the untreated groups. Conclusion: Dietary addition with DMIC at doses of 100 or 200 mg/kg may be a novel strategy to enhance the health, immunity, blood profile, antioxidant status, and resistance to A. hydrophila in Nile tilapia fish. Keywords: Dimethyl itaconate, Fish, Performance, Health, Immunity, and inflammation. IntroductionIn recent decades, the aquaculture sector has diversified rapidly, significantly contributing to global animal protein production. According to the Food and Agriculture Organization (FAO) (2020) report, aquaculture and fisheries together provide 17% of the world’s animal protein for human consumption. Many strategies have been implemented to promote aquatic fish productivity, overall health, and growth under extensive systems (Farag et al., 2021; Rossignoli et al., 2023). Antibiotics have been widely used for many years to prevent illnesses and as growth promoters (Saeed et al., 2017; Alagawany et al., 2018; El-Shall et al., 2019; Ghanima et al., 2020; Swelum et al., 2020; El-Saadony et al., 2021; Kamboh et al., 2023), but their excessive use has contributed to the development and widespread resistance of bacteria to antibiotics. Consequently, there is increasing interest in alternative therapies. Metabolites can improve the systemic immune system, as shown in previous studies on immune metabolism (Mauro et al., 2022; Kumar et al., 2023). Therefore, natural metabolites are likely to be promising antibiotic alternatives (Kim, 2018; Alagawany et al., 2021a; Naiel et al., 2023). Itaconate, an important Krebs cycle metabolite, has recently garnered concern for its immunomodulatory, antiinflammatory, and antioxidant effects. Dimethyl itaconate (DMIC) is a promising compound that illustrates how metabolism changes the immune response (Ferreira et al., 2023). Itaconate, a main Krebs cycle metabolite with immunomodulatory and anti-inflammatory effects, is made from cis-aconitate by the enzyme cis-aconitate decarboxylase. Ferreira et al. (2023)encoded this enzyme by the IRG1 gene (immune-responsive gene 1). On the other hand, mounting evidence suggests that DMIC has multiple biological benefits, including antioxidant, antiviral, and anti-inflammatory traits (Zhang et al., 2021; Li et al., 2023; Wang et al., 2023). In addition, DMIC has been used to prevent certain diseases, such as colitis-linked colorectal tumor (Wang et al., 2020). In addition, itaconate has been reported to promote the Nrf2/ heme oxygenase-1 (HO-1) pathway (Fu et al., 2021), which enhances antioxidant and antiinflammatory traits. Dietary supplementation with itaconate (0.1%) may be a novel approach to avoid enteritis and intestinal inflammation-triggered hepatopancreatic damage in gibel carp (Carassius gibelio) (Fu, et al., 2023). The antimicrobial activity of DMIC was also tested by several authors (Sendra et al., 2020; Fu, et al., 2023). It was previously developed in a TNBS-induced enteritis example in gibel carp fish (Fu et al., 2021). Itaconate supplementation tempered soybean-triggered liver inflammation and syndromes of glycolipid metabolism (Fu et al., 2021). The effects of DMIC on feed utilization, fish growth, immunity, redox status, and inflammatory responses in Nile tilapia fish infected with Aeromonas hydrophila have been limited. Based on the biological activity of DMIC, the dietary administration of DMIC is hypothesized to improve the health status and growth, reduce the inflammatory response, and enhance the resistance of Nile tilapia fish to the challenges of A. hydrophila. Materials and MethodsFish management and experiment designJuvenile Nile tilapia with a mean weight of 20.97 ± 0.33 g were presented to the Fish Farm of KAU at the Faculty of Marine Sciences, Saudi Arabia. Dimethyl itaconate (592498, DMIC) was obtained from Sigma-Aldrich (St. Louis, MO, USA). The trial was conducted in high-density polyethylene (HDPE)-lined ponds at the KAU Fish Farm in Obhur, Jeddah, for eight weeks. A total of 200 Nile tilapia were placed in 20 glass aquaria (35 cm × 40 cm × 70 cm) (85 L) and mixed with an air stone for aeration. The fish were allocated into four groups, each comprising 10 fish (5 polyethylene-lined ponds). The treated groups were given the main diet fortified with DMIC at amounts of zero (DMIC0), 50 mg (DMIC50), 100 mg (DMIC100), and 200 mg (DMIC200) of DMIC kg-1 diet. Fish were acclimated in lined HDPE ponds for 1 week. The light regime system in this farm was maintained at 12 hours light/12 hour dark. Aerated water was pumped into the ponds using air pumps. Throughout the experiment, the fish were fed a well-balanced main diet with a protein ratio of 30.1% and a lipid ratio of 6.3%. The fish were fed on the designed experimental diets at 6% of their live body weight (BW) for the first 4 weeks and then at 5% of their BW for the last 4 weeks. The fish were hand-fed three times per day (till visible satiation). The research lasted for approximately 8 weeks during winter. The water temperature was maintained throughout this period between 19°C and 23°C. The ponds were purified by siphoning to remove garbage and leftover food particles during the acclimation and testing stages. Following cleaning, healthy, dechlorinated water from tanks for storage with the same temperature range was used to replace approximately 25% of the rearing water. The water quality parameters, including temperature (averaging 26.42°C ± 0.36°C), pH (averaging 6.67 ± 0.12), and dissolved oxygen (averaging 6.62 ± 0.37 mg/l), were monitored daily using a multiparameter meter probe (HI9829-03042-HANNA® instruments, available at https://www.hannainst.com). The level of total ammonia nitrogen (averaging 0.26 ± 0.04 mg/l) was determined using a portable colorimeter (Martini Instrument MI 405, Romania). Table 1 shows the basal diet’s ingredients and its analytical chemical constituents. Table 1. Proximate chemical makeup, feed ingredients, and main diet (dry weight, percentage) of the current experiment.
Growth performanceAt the end of the research, the fish were anesthetized with sulfonate tricaine methane (MS222, 25 mg/l, Argent Laboratories, Washington) following the results and conclusions of El-Wafai et al. (2022) to acquire the single weight and length (Ln, cm) of every fish. In addition to the feed intake (FI, g), which was modified based on the overall fish biomass in each pond, tilapia fish were independently measured at the beginning of the experiment (IBW, g) and every 2 weeks thereafter. The efficiency of the feed and growth variables was assessed according to the following equations: WG=FBW, IBW, where WG=weight gain (each fish, g) and FBW=final body weight (each fish, g). The total FI was recorded over the entire experimental period. FCR=FI/WG, where FCR=feed conversion ratio (g feed/g gain). Specific growth rate (SGR) (%/day)=100 [(Ln FBW Ln IBW)/T] (T is the feeding period). Blood samplingBefore blood sampling, the fish were fasted for 12 hours. Anesthetize the fish to minimize stress and minimize movement through blood collection. The popular anesthetic method, tricaine methane sulfonate (MS-222, 50 mg/l of water), was used in this experiment. Blood samples (n=6/group) were collected from the caudal vein of the fish using sterile disposable injection. The collected blood samples were divided into 2 parts. One part was mixed with ethylenediaminetetraacetic acid as an anticoagulant for blood hematological analysis. The other subsample stayed in a temperature-controlled room for serum collection. The samples were centrifuged at 1,200 g for 10 minutes at 4°C. Serum was gathered and stored at −20ºC until the evaluation of serum biochemicals. Blood biochemical and hematological analysesAll erythrocytes, such as red blood cells (RBCs), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), and leukocytes, such as white blood cells, lymphocytes, neutrophils, and monocytes, were estimated using a Veterinary Hematology Analyzer (HEMA-A3-35, Infitek Co., Ltd.). Shandong, China) (Fazio, 2019). Serum was used to assess the biochemical variables; albumin (ABL) and total proteins were evaluated using a commercial colorimetric kit (Sigma-Aldrich, St. Louis, MO, USA) (Doumas et al., 1997). Simultaneously, the globulin (GLU) was evaluated mathematically (GLU=TP-ABL). The amounts of creatinine and UA were evaluated using the colorimetric technique (Inker and Perrone, 2014). The production and status of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) were evaluated by colorimetric analysis using specific diagnostic kits (Sigma-Aldrich, St. Louis, USA). The method of Huang et al. (2006)is then followed. Immune, oxidative stress, and inflammatory responsesenzyme-linked immunosorbent assay (ELISA) kits made by Cusabio (Houston, TX 77054, USA) were used to detect serum immunoglobulin M (IgM) and G (IgG) levels in tilapia fish. The phagocytic index and activity were assessed according to Kumar et al. (2014), where phagocytic activity=phagocytic cell containing yeast/total number of phagocytic cells × 100 and phagocytic index=number of cells phagocytized/number of phagocytic cells. The serum activity of lysozyme was assessed by ELISA (Demers and Bayne, 1997). Nitric oxide levels were assessed (Eddy, 2005). Superoxide dismutase (SOD), glutathione, catalase, and malondialdehyde (MDA) levels were determined using ELISA kits (Inova Biotechnology, China) using a microplate ELISA reader. The interferon-gamma (IFNγ) levels in serum fish (MBS702530) were determined using assay kits made by MyBiosource.com. TNF-α (Tumor necrosis factor alpha (TNF-), myeloperoxidase, and IL-4 were assessed using assay kits according to the method of Takizawa et al. (2011). The experimental bacterial challengeAfter finalizing an eighth week feeding study, 15 fish from every treatment group (5 fish per pound) were challenged with A. hydrophila (ATCC-13037; provided by the Faculty of Science, King Abdulaziz University). The fish were injected intraperitoneally (IP) with 0.2 ml of a hang containing 1 × 108 CFU/ml of A. hydrophila, as described in the study by Moustafa et al. (2020). The mortality rate was recorded throughout the 14-day monitoring period following the outlined methodology in the experiment by El-Wafai et al. (2022). During this observation period, the fish were fed their respective diets according to the nutritional trial. The following procedure considered the cumulative survival rate: Survival rate=(Number of fish remaining at the end of the bacterial challenge ÷ Number of fish at the beginning of the bacterial challenge) × 100%. Statistical analysisShapiro–Wilk normality examination was used to check the normality of the data. The data were analyzed using the one-way analysis of variance test by the SPSS program (version 21). Figures were drawn using GraphPad Prism 9 (GraphPad Prism v9.0, San Diego, CA, USA). The data are presented as Mean ± SE. Values of p < 0.05 were considered statistically significant. Ethical approvalThe current research and aquatic animal techniques procedure were approved according to the guidelines of the Research Ethical Committee, Faculty of Marine Sciences, KAU, Saudi Arabia. ResultsEffects on growth performanceTable 2 shows the changes in growth performance indices and feed intake of Oreochromis niloticus fed diets supplemented with various levels of DMIC for 8 weeks. FBW, FI, SGR, and WG were significantly enhanced in all DMIC-treated groups compared with the control one (p < 0.001). FCR did not change significantly between the DMIC treatments (p > 0.05) and the group control. Both groups (DMIC100 and DMIC200) had greater RGR than the others. RGR was similar in the control and DMIC50 groups (p > 0.05). Table 2. Growth parameters of Nile tilapia (O. niloticus) fed diets supplemented with varying concentrations of dimethyl itaconate (0, 50, 100, and 200 mg/kg food).
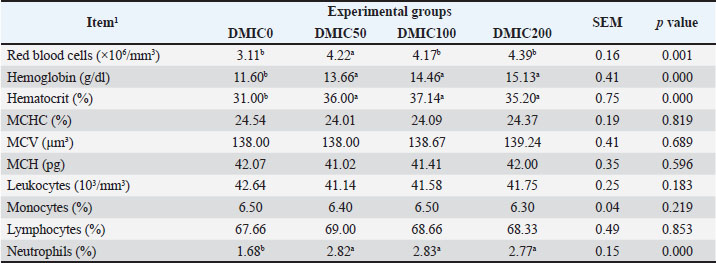
Effects on hematological bloodTable 3 shows the impact of supplementing diets with different DMIC levels on the blood hematology of O. niloticus Nile tilapia. RBCs, hemoglobin, and hematocrit were significantly increased in the DMIC-treated groups (p < 0.001). Conversely, the MCHC, MCV, MCH, WBCs, monocytes, and lymphocytes of O. niloticus Nile tilapia were not significantly affected by dietary DMIC inclusion. DMIC dietary supplementation significantly increased the neutrophils in the blood of O. niloticus Nile tilapia (p < 0.001). Table 3. Hematological parameters of the blood of Nile tilapia (O. niloticus) fed with varying concentrations of dimethyl itaconate.
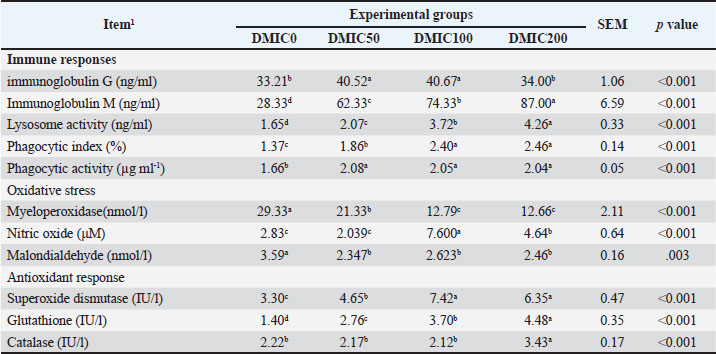
Effects on immunity and redox statusInterestingly, the groups fed with DMIC significantly augmented the serum amounts of immunoglobulin M (p < 0.05), nitric oxide, and lysosome activity in a dose-based manner (Table 4). The best finding for IgG was detected in the DMIC50 and DMIC100 groups compared with the other groups (p < 0.01). Both DMIC0 and DMIC200 exhibited similar IgG results (p > 0.05). All DMIC-treated groups had a greater phagocytic status, while serum MDA levels were significantly reduced compared with the control group one (p < 0.01). The phagocytic index (%) and SOD were higher in the DMIC100 and DMIC200 groups than in the other groups (p < 0.01). Glutathione was dose-dependently improved in all DMIC groups in a dose-based manner (p < 0.01). The DMIC200 group recorded the highest catalase activity levels among other groups (p < 0.01). Table 4. Serum immunoglobulins, oxidative stress, and antioxidant biomarkers of Nile tilapia (O. niloticus) fed diets mixed with different rates of dimethyl itaconate.
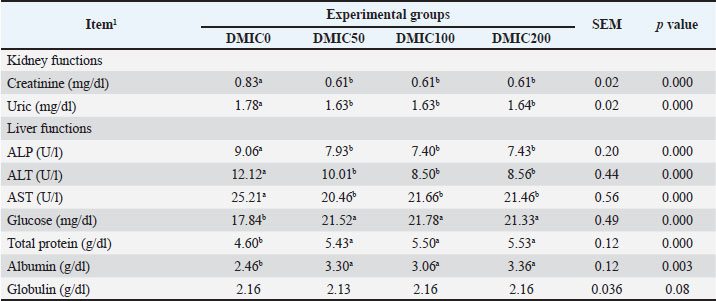
Blood metabolitesThe kidney and liver functions of Nile tilapia fed with various levels of DMIC are presented in Table 5. Both uric acid and creatinine were noticeably reduced by DMIC supplementation in Nile tilapia fish's diets (p < 0.01). DMIC-treated groups had significantly improved serum levels of glucose, total protein, and albumin, whereas no significant effect for globulin was noticed among all groups (p=0.08). ALP, ALT, and AST activities were significantly decreased in all DMIC treatments compared with DMIC0 (p < 0.05). Table 5. Kidney and liver parameters of Nile tilapia (O. niloticus) fed diets mixed with different rates of dimethyl itaconate.
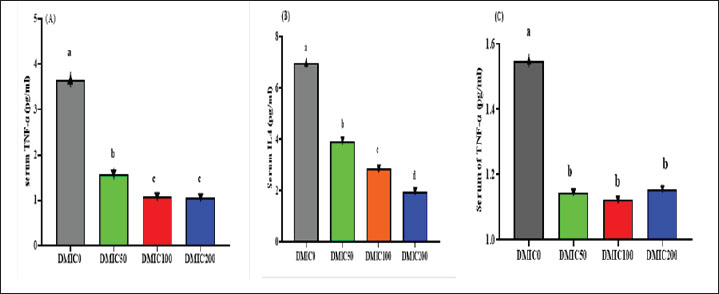
Effects on the inflammatory responseFigure 1 shows the impact of additional diets with varying DMIC ranges on the serum profiling of inflammatory-related biomarkers. Dietary DMIC administration significantly decreased serum levels of Tumor necrosis factor alpha (TNF-α) (Fig. 1C). The serum levels of IFNγ (Fig. 1A) and IL-4 (Fig. 1B) were significantly lower after feeding fish with DMIC in a dose-dependent manner.
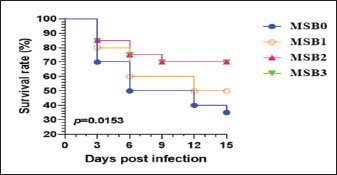
Fig. 1. Inflammatory response of Nile tilapia (O. niloticus) fed diets supplemented with varying concentrations of dimethyl itaconate, including IFNγ (Fig. 1A; p < 0.001), IL4 (Fig. 1B; p < 0.001), and TNF-α (Fig. 1C, p < 0.001). Mean ± SEM was used to present the data. Significant differences exist between means with different letters (p < 0.05). At DMIC kg-1 diet levels of 0.0 (DMIC0), 50 (DMIC50), 100 (DMIC100), and 200 (DMIC200), fish were given a basic diet supplemented with dimethyl itaconate. Bacterial infection trailThe survival rates of A. hydrophila-infected fish fed DMIC for 15 days were higher than those of the untreated groups (Fig. 2). The survival rates of the DMIC0, DMIC1, DMIC2, and DMIC3 groups were 35%, 50%, 70%, and 70%, respectively.
Fig. 2. Survival levels of Nile tilapia after infection with A. hydrophila for 15 days and feeding with varying amounts of dimethyl itaconate DMIC was added to fish’s basic meal at doses of 0.0 (DMIC0), 50 (DMIC50), 100 (DMIC100), and 200 (DMIC200) mg of DMIC kg-1 diet. DiscussionAquaculture requires viable substitutes for antibiotics to manage intestinal and hepatic inflammation and metabolic disturbances resulting from many environmental issues (Alagawany et al., 2021b). Immunometabolism, a branch of study focused on the relationship between the immune system and metabolism, has demonstrated the immunomodulatory properties of certain metabolites. Dimethyl itaconate, a subunit of metabolites in the Krebs cycle, has attracted considerable attention as a promising antiinflammatory medication. In the present experiment, the possible impacts of nutrition dimethyl itaconate inclusion on Nile tilapia fish growth, blood hematology, feed utilization, oxidative stress, immune responses, serum metabolites, and inflammation biomarkers were evaluated. The survival rate of A. hydrophila-infected fish was also assessed. The findings indicate that dimethyl itaconate improves growth indices (FBW, FI, SGR, and WG), erythron variables, and neutrophils and modulates kidney and liver activity in Nile tilapia fish. Dietary dimethyl itaconate boosts immune function and antioxidant status by reducing inflammatory signals and oxidative-related biomarkers in fish. Our results confirmed the immune-modulatory, anti-inflammatory, and antioxidant activities of itaconate. This study proposed that itaconate metabolite could be used as a potential treatment approach to improve fish health and resistance to A. hydrophila infestation. This study demonstrates that dimethyl itaconate improves growth performance and feed intake in tilapia fish. These results are consistent with previous studies on juvenile gibel carp (Fu, et al., 2023) and stressed boilers (Li et al., 2023; Wang et al., 2023). Fu et al. (2021) reported that the addition of 4-octyl itaconate significantly enhanced the growth of juvenile carp gibel. This may be related to 4-octyl itaconate’s ability to improve carbohydrate metabolism (Fu et al., 2021). Enhancing nutrient metabolism by itaconate could support fish growth and WG (Fu et al., 2021; Fu, et al., 2023). Another study (Fu, et al., 2023) found that 4-octyl itaconate dietary inclusion improved the growth performance of juvenile gibel carp. Several previous studies have clarified that dietary fortification with dimethyl itaconate enhanced the growth traits of boilers under hot conditions (Li et al., 2023; Wang et al., 2023). Researchers attributed this enhancement to the regulatory impact of 4-octyl itaconate in increasing the abundance of beneficial intestinal microorganisms (Fu, et al., 2023). It has been clarified that 4-octyl itaconate can regulate the ppar-γ (peroxisome proliferator-activated receptor gamma (ppar-) and its objective genes (glut2 and gk) for improving liver glycolysis and de novo lipogenesis of lipids (Fu, et al., 2023). In this regard, improving the glycolysis of the liver may enhance more energy for supporting growth, as evidenced in the current study. Blood profile may reflect aquatic fish health conditions after feeding on feed additives. In this study, a considerable improvement in the RBCs, hemoglobin, neutrophils, and hematocrit in response to feeding diets with itaconate was found. Wang et al. (2023) reported that DMIC (200 mg/kg diet) significantly improved the levels of antioxidant-related bio-indicators (SOD, catalase, and TAC) and significantly decreased the levels of oxidative-linked biomarkers (MDA, hydroxyl free radicals, and superoxide) in the serum of broilers with stress. Broilers stressed feeding diets including DMIC (150 or 200 mg/kg diet) remarkably upregulated the antioxidant-linked genes like HO-1, nuclear factor (erythroid-derived 2)-like 2 (NRF-2), SOD2, NADPH quinone acceptor oxidoreductase 1, or glutathione-S-transferases. Itaconate, a metabolic substance produced by the enzyme encoded by IRG1, has recently emerged as a macrophage organizer (Kuo et al., 2020). DMIC can motivate the NRF-2/ Nrf2 routes and considerably decrease the expression of genes related to HO-1 and NQO-1 (Zhang et al., 2021). Itaconate also exhibited antiinflammatory traits by suppressing the impact of succinate dehydrogenase, a key pro-inflammatory regulator. The powerful anti-inflammatory action of itaconate appears to release antioxidant factors and activate the antioxidant Nrf2 transcription factor. This motivation occurs through the alkylation of cysteine remnants on its suppressor KEAP1 and the repression of IL-1β repression in M. galloprovincialis (Sendra et al., 2020). The amount of itaconic acid could be used as a biomarker of bacterial infections in marine bivalves. Recently, natural immunomodulatory agents in aquaculture, instead of antibiotics, have received more attention due to their safety, environmental, and effectiveness. Various techniques, including the use of nutraceuticals, are being tested and emerging to enhance fish immunity and counteract infections (Alagawany et al., 2021a; Farag et al., 2021). Fish fed with different amounts of DMIC showed higher levels of IgG and IgM. According to recent reports, DMIC can enhance the trained immunity feature (Ferreira et al., 2023). According to this earlier research, DMIC can trigger innate immunological memory (Ferreira et al., 2023). Additionally, a recent study showed that the addition of DMIC can have regulatory effects on immunological responses by activating the Nrf2/HO-1 pathway (Yang et al., 2021; Ferreira et al., 2023). Numerous authors have reported the immunomodulatory effects of DMICs (Kuo et al., 2020; Yang et al., 2021; Ferreira et al., 2023). By activating Nrf2, feeding young gibel carp fish with 0.1% 4-octyl itaconate dramatically decreased inflammatory biomarkers (Fu, et al., 2023). The mRNA levels of Bcl-2-associated X, caspase 3, and caspase 9 in chickens were considerably reduced (p < 0.01) by adding 150 and 200 mg/kg of DMIC to the diet (Wang et al., 2023). DMIC decreased broiler Bcl-2 mRNA levels (Wang et al., 2023). Furthermore, DMIC has been used as an anti-inflammatory agent in cancer cells by preventing intestinal epithelial cells from secreting the cytokine IL-1β, which in turn reduces the recruitment of macrophages into the tumor microenvironment (Wang et al., 2020). Itaconate can reduce inflammation of the intestine in juvenile gibel carp and lessen the damage caused by enteritis to the hepatopancreas by increasing anti-inflammatory cytokines in the intestinal cells and limiting glycolysis both in vitro and in vivo (Fu, et al., 2023). When DMIC was administered to septic mice, their lung function improved, their survival rate increased, and their blood concentrations of TNF-α and IL-6 decreased (Zhang et al., 2021). Furthermore, DMIC can inhibit bone marrow–derived macrophages' production of IL-6, TNF-α, and NOS2 in response to LPS (Zhang et al., 2021). A modern experiment by Ferreira et al. (2023) reported that itaconate rates in human plasma correlate with enhanced ex vivo proinflammatory cytokine production. Mice treated with MDMIC demonstrated improved survival rates when infected with Staphylococcus aureus (Ferreira et al., 2023). MDMIC exhibits direct antimicrobial action by targeting isocitrate lyase, an enzyme essential for the survival of various pathogens, including S. aureus, C. albicans, and M. tuberculosis (Tomlinson et al., 2021). DMIC is a promising feed additive for fish, with the potential to improve fish health and growth traits. Nevertheless, more investigation is needed to fully comprehend the ways in which DMIC works and to maximize its application in aquaculture. ConclusionDMIC shows promising potential as a novel dietary additive in Nile tilapia aquaculture, significantly improving growth performance, hematological and biochemical indices, antioxidant defenses, immune status, and resistance to A. hydrophila infection. Supplementation with 100 or 200 mg/kg DMIC yielded the most favorable outcomes in terms of both physiological and disease resistance parameters. These findings highlight DMIC as a viable strategy for enhancing fish health and productivity under aquaculture conditions. However, further research is warranted to explore its efficacy across other fish species and to elucidate its molecular and proteomic mechanisms of action. AcknowledgmentsNone. FundingNone. Author's contributionsConceptualization, Investigation, Data curation, Methodology, Validation, Original Draft Preparation, Review and Editing, M.E.S. The author read and agreed to the final version of the manuscript. Conflict of interestThe author has no conflicts of interest to declare. Data availabilityData will be available upon request. ReferencesAlagawany, M., Abd El-hack, M.E., Farag, M.R., Elnesr, S.S., El-Kholy, M.S., Saadeldin, I.M. and Swelum, A.A. 2018. Dietary supplementation with Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci. 97, 3126–3137. Alagawany, M., Farag, M.R., Abdelnour, S.A. and Elnesr, S.S. 2021. A review on the beneficial effect of thymol on fish health and production. Rev. Aquacult. 13(1), 632–641; doi:10.1111/raq.12490 Alagawany, M., Farag, M.R., Abdelnour, S.A., Dawood, M.A., Elnesr, S.S. and Dhama, K. 2021a. Curcumin and its different forms: a review of fish nutrition. Aquaculture 532, 736030; doi:10.1016/j.aquaculture.2020.736030 Demers, N.E. and Bayne, C.J. 1997. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 21, 363–373. Doumas, B.T., West, W. and Biggs, H.G. 1997. Albumin standards and serum albumin measurement with bromcresol green. Clinica. Chirica. Acta 258(1), 21–30; doi:10.1016/S0009-8981(96)06447-9 Eddy, F.B. 2005. Role of nitric oxide in larval and juvenile fish. Comparative Biochem. Physiol. Part A. Mol. Integr. Physiol. 142(2), 221–230; doi:10.1016/j.cbpb.2005.05.038 El-Saadony, M.T., Abd El-hack, M.E., Swelum, A.A., Al-Sultan, S.I., El-Ghareeb, W.R., Hussein, E.O.S. and Nader, M.M. 2021. Enhancing the quality and safety of raw buffalo meat using pea and red kidney bean bioactive peptides under refrigeration conditions. Italian J. Anim. Sci. 20(1), 762–776. El-Shall, N.A., Awad, A.M., Abd El-hack, M.E., Naiel, M.A.E., Othman, S.I., Allam, A.A. and Sedeik, M.E. 2019. Simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces bacterial fecal shedding in Salmonella-challenged broilers. Animals 10, 70. El-Wafai, N.A., Alharbi, N.K., Ahmed, A.E., El-Zamik, F.I., Mahgoub, S.A., Atia, A.M. and Abdel-Hamid, E.A. 2022. Controlling multidrug-resistant A. hydrophila infection in Nile tilapia (Oreochromis niloticus) using Ah01 and Ah02 virulent bacteriophage isolates. Saudi J. Biol. Sci., doi:10.1016/j.sjbs.2022.02.050 Farag, M.R., Abdelnour, S.A., Patra, A.K., Dhama, K., Dawood, M.A.O., Elnesr, S.S. and Alagawany, M. 2021. Propolis: properties and composition, health benefits, and applications in fish nutrition. Fish Shellfish Immunol. 115, 179–188; doi:10.1016/j.fsi.2021.06.010 Fazio, F. 2019. Fish hematology analysis as an important aquaculture tool: a review. Aquaculture 500, 237–242; doi:10.1016/j.aquaculture.2018.10.030 Ferreira, A.V., Kostidis, S., Groh, L.A., Koeken, V.A., Bruno, M., Baydemir, I., Kilic, G., Bulut., Andriopoulou, T. and Spanou, V. 2023. Dimethyl itaconate induces long-term innate immune responses and protects against infection. Cell Rep. 42(6), 112658; doi:10.1016/j.celrep.2023.112658 Fu L Cai., Liu, W., Hooft, H., Øverland, M., Han D Zhu., Yang, X., Jin, Y. and Xie, J. 2023. Effects of 4-octyl itaconate and dimethyl fumarate on growth performance, intestinal microbiota, and intestinal and hepatopancreatic health of juvenile gibel carp (Carassius gibelio). Aquaculture, 569; doi:10.1016/j.aquaculture.2023.739376 Fu, L., Liu, H., Cai, W., Han, D., Zhu, X., Yang, Y. and Xie, S. 2021. 4-octyl itaconate supplementation relieves soybean diet-induced liver inflammation and glycolipid metabolic disorders by activating the nrf2-pparγ Pathway in juvenile gibel carp. J. Biol. Chem. Biotechnol. 86(2), 520–531; doi:10.1021/acs.jafc.1c05783 Ghanima, M.M.A., Abd El-hack, M.E., Othman, S.I., Taha, A.E., Allam, A.A. and Abdel-Moneim, A.M.E. 2020. Impact of different rearing systems on broiler growth, carcass traits, oxidative stress biomarkers, and humoral immunity. Poult. Sci. 99(6), 3070–3078. Huang, X.J., Choi, Y.K., Im H South., Yarimaga, O., Yoon, E. and Kim H South. 2006. Detection of aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) Sensors. Sensors 6(7), 756–782; doi:10.3390/s6070756 Inker, L.A. and Perrone, R.D. 2014. Kidney function assessment UpToDate, Waltham, MA. Available via https://www.wolterskluwer.com/en/know/clinical-effectiveness-terms (Accessed 9 XXX XX). Kamboh, A.A., Leghari, R.A. and North, K. 2023. Introduction: antibiotics and probiotics in animal food: impact and regulation. London, UK: IntechOpen; doi: 10.5772/intechopen.108682 Kim, C.H. 2018. Immune regulation by microbiome metabolites. Immunology 154(2), 220–229; doi:10.1111/imm.12930 Kumar, S., Korra, T., Thakur, R., Arutselvan, R., Kashyap, A.S., Nehela, Y., Chaplygin, V., Minkina, T. and Keswani, C. 2023. Role of plant secondary metabolites in defense and transcriptional regulation in response to biotic stress. Plant Stress 8, 100154; doi:10.1016/j.stress.2023.100154 Kumar, S., Sharma, G., Sidiq, T., Khajuria, A., Jain, M., Bhagwat, D. and Dhar, K.L. 2014. Immunomodulatory potential of a bioactive fraction from the leaves of Phyllostachys bambusoides (Bamboo) in BALB/C mice. EXCLI. J. 13, 137; Kuo, P.C., Weng, W.T., Scofield, B.A., Paraiso, H.C., Brown, D.A., Wang, P.Y., Wang, P.Y., Yu, I.C. and Yen, J.H. 2020. Dimethyl itaconate, an itaconate derivative, exhibits immunomodulatory effects on neuroinflammation in experimental AE. J. Neuroinflamm. 17(1), 138; doi:10.1186/s12974-020-01768-7 Li, L., Cui H Wang., Huang. and Ma. 2023. Dietary supplementation with dimethyl itaconate protects against chronic heat stress–induced growth performance impairment and lipid metabolism disorder in broiler chickens. J. Anim. Sci. 1010, 120; doi:10.1093/jas/skad120 Mauro, C., Diana, M. and Nicholas, J. 2022. Metabolites: fueling the immune response. Clin. Experimen. Immunol. 208(2), 129–131; doi:10.1093/cei/uxac053 Moustafa, E.M., Dawood, M.A., Assar, D.H., Omar, A.A., Elbialy, Z.I., Farrag, F.A., Shukry, M. and Zayed, M.M. 2020. Modulatory effects of fenugreek seed powder on histopathology, oxidative status, and immune-related gene expression in Nile tilapia (Oreochromis niloticus) infected with A. hydrophila. Aquaculture 515, 734589; doi:10.1016/j.aquaculture.2019.734589 Naiel, M.A., Negm, S.S., Ghazanfar, S., Shukry, M. and Abdelnour, S.A. 2023. Risk assessment of high‐fat diet in farmed fish and its mitigation approaches: a review. J. Anim. Physiol. Anim. Nutr. 107, 948–969; doi:10.1111/imm.12930 Rossignoli, C.M., Lozano Lazo, D.P., Barman, B.K., Dompreh, E.B., Manyise, T., Wang, Q., Dam Lam, R., Moruzzo, R., Paz Mendez, A. and Gasparatos, A. 2023. Multistakeholder perception analysis of status, characteristics, and factors affecting small-scale carp aquaculture systems in Bangladesh. Front. Sustain. Food Syst. 7, 1121434; doi:10.3389/fsufs.2023.1121434 Saeed, M., Naveed, M., Arain, M.A., Arif, M., Abd El-hack, M.E., Alagawany, M., Siyal, F.A., Soomro, R.N. and Sun, C. 2017. Quercetin: nutritional and beneficial effects in poultry. World’s. Poult. Sci. J. 73(2), 355–364. Sendra, M., Saco, A., Rey-Campos, M., Novoa, B. and Figueras, A. 2020. Immune-responsive gene 1 (IRG1) and dimethyl itaconate are involved in the immune response of mussels. Fish Shellfish Immunol. 106, 645–655; doi:10.1016/j.fsi.2020.07.034 Swelum, A.A., Shafi, M.E., Albaqami, N.M., El-Saadony, M.T., Elsify, A., Abdo, M., Taha, A.E., Abdel-Moneim, A.M.E., Al-Gabri, N.A., Almaiman, A.A., Al-Wajeeh, A.S., Tufarelli, V., Staffa, V.N., Abd El-Hack, M.E. 2020. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 7, 578. Takizawa, F., Koppang, E.O., Ohtani, M., Nakanishi, T., Hashimoto, K., Fischer, U. and Dijkstra, J.M. 2011. The constitutive high expression of interleukin-4/13A and GATA-3 in the gill and skin of salmonid fish suggests that these tissues form Th2-skewed immune environments. Mol. Immunol. 48(12-13), 1360–1368; doi:10.1016/j.molimm.2011.02.014 Tomlinson, K.L., Lung, T.W.F., Dach, F., Annavajhala, M.K., Gabryszewski, S.J., Groves, R.A., Drikic, M., Francoeur, N.J., Sridhar, S.H., Smith, M.L., Khanal, S., Britto, C.J., Sebra, R., Lewis, I., Uhlemann, A.C., Kahl, B.C., Prince, A.S. and Riquelme, S.A. 2021. Staphylococcus aureus induces an itaconate-dominated immunometabolic response that drives biofilm formation. Nature Commun. 12(1), 1399; doi:10.1038/s41467-021-21718-y Wang, H., Yang, Y., Huang, B., Cui, Z. and Li, L. 2023. Dietary dimethyl itaconate supplementation has protective effects on oxidative stress, inflammation, and apoptosis in broilers under long-term heat stress. J. Anim. Sci. 1010, 1010; doi:10.1093/jas/skad356 Wang, Q., Li, X.L., Mei, Y., Ye, J.C., Fan, W., Cheng, G.H., Zeng, M.S. and Feng, G.K. 2020. Dimethyl itaconate, an anti-inflammatory drug, protects against colitis-associated colorectal cancer. J. Mol. Med. 98(10), 1457–1466; doi:10.1007/s00109-020-01963-2 Yang, S., Zhang, X., Zhang, H., Lin, X., Chen, X., Zhang, Y., Lin, X., Huang, L. and Zhuge, Q. 2021. Dimethyl itaconate inhibits lipopolysaccharide-induced microglia inflammation and inflammasome-mediated pyroptosis by inducing autophagy and regulating the Nrf2/HO1 signaling pathway. Mol. Med. Rep. 24(3), 672–614; doi:10.3892/mmr.2021.12311 Zhang, S., Jiao, Y., Li, C., Liang, X., Jia, H., Nie, Z. and Zhang, Y. 2021. Dimethyl itaconate alleviates macrophage inflammatory responses in sepsis. Inflammation 44(2), 549–557; doi:10.1007/s10753-020-01352-4 | ||
| How to Cite this Article |
| Pubmed Style Manal E. Shafi. Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Vet. J.. 2025; 15(9): 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 Web Style Manal E. Shafi. Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. https://www.openveterinaryjournal.com/?mno=273660 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.35 AMA (American Medical Association) Style Manal E. Shafi. Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Vet. J.. 2025; 15(9): 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 Vancouver/ICMJE Style Manal E. Shafi. Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 Harvard Style Manal E. Shafi (2025) Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Vet. J., 15 (9), 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 Turabian Style Manal E. Shafi. 2025. Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Veterinary Journal, 15 (9), 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 Chicago Style Manal E. Shafi. "Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate." Open Veterinary Journal 15 (2025), 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 MLA (The Modern Language Association) Style Manal E. Shafi. "Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate." Open Veterinary Journal 15.9 (2025), 4286-4294. Print. doi:10.5455/OVJ.2025.v15.i9.35 APA (American Psychological Association) Style Manal E. Shafi (2025) Improving the growth, health status, immune function, and resistance against Aeromonas hydrophila in Nile Tilapia supplemented with dimethyl itaconate. Open Veterinary Journal, 15 (9), 4286-4294. doi:10.5455/OVJ.2025.v15.i9.35 |