| Research Article | ||
Open Vet. J.. 2025; 15(10): 4894-4903 Open Veterinary Journal, (2025), Vol. 15(10): 4894-4903 Research Article Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chickenAhmed I. Alajaji*Department of Veterinary Preventive Medicine, College of Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia *Corresponding Author: Ahmed I. Alajaji. Department of Veterinary Preventive Medicine, College of Veterinary Medicine, Qassim University, Buraydah, Saudi Arabia. Email: ahmedalajaji5 [at] gmail.com; ajajy [at] qu.edu.sa Submitted: 10/07/2025 Revised: 17/09/2025 Accepted: 25/09/2025 Published: 31/10/2025 © 2025 Open Veterinary Journal
AbstractBackground: The quality of drinking water affects antibiotic bioavailability in the broiler chickens. Aim: The current study highlights on the influence of water quality on the bioavailability of antibiotics in broilers. The water quality was determined based on the Zn, Cu, Fe, Mn, and Pb levels. Methods: The animal study involved 234 chickens divided into 6 broad groups. Chickens in subgroup A received water containing the maximum level of metals (low dose) while chickens in subgroup B received water containing 5 × of the A subgroup (high dose). The control group was given a bottled. The effects of metals in drinking water on the bioavailability of oxytetracycline and amoxicillin were evaluated for the various groups. Different concentrations of the metals cause different responses to the bioavailability of the antibiotics at different times. Results: Low levels of Zn, Pb, Mn, and Cu, and high levels of Zn and Pb resulted in a significant (p < 0.001) increase in the Cmax of oxytetracycline after 2 hours of treatment as compared with the control group. Low levels of Zn, Pb, Mn, Fe, and Cu, and high levels of Zn, Pb, and Mn also resulted in a significant (p < 0.001) increase in the Cmax of oxytetracycline after 4 hours of treatment as compared with the control group. Similarly, the water treated with low levels of Fe and Cu, and high levels of Zn and Fe resulted in a significant (p < 0.001) increase in the Cmax of amoxicillin after 2 hours of treatment as compared with the control group. Conclusion: The concentrations of the studied heavy metals significantly affect the bioavailability of the antibiotics and alter their effectiveness. Keywords: Antibiotics, Bioavailability, Broilers, Poultry, Saudi Arabia. IntroductionGroundwater resources remain the most important freshwater supply for various human activities. For the past 50 years, however, the susceptibility of aquifers to chemical pollution from different human activities has been unprecedented (Mallick et al., 2021; El-Rawy and Fathi, 2023). Other critical issues, like water resource scarcity and extensive groundwater exploitation, are also experienced by arid and semi-arid regions that suffer from multiple problems with water sources (El Osta et al., 2022; Aljassim et al., 2024). Saudi Arabia is an arid country, and the most essential natural resource for agriculture, domestic, and industrial uses is groundwater (Alshehri et al., 2023; Odnoletkova and Patzek, 2023). Water quality has become a major concern in most countries, especially in arid and semi-arid regions where water shortage is a common phenomenon (Morante-Carballo et al., 2022; Ingrao et al., 2023). However, the assessment of water quality has been given little attention. The wide use of groundwater for various agricultural, domestic, and industrial purposes makes it a valuable resource. On the other hand, Saudi Arabia has a scarce water resource, one of the driest world regions. Pollution from domestic sewerage, discharge of industrial effluent, and agricultural fertilizers is also affecting most shallow groundwater around Saudi Arabia's major cities (Mallick et al., 2021). Therefore, water quality and shortage are major issues in most places, especially in arid and semi-arid world regions, including Saudi Arabia (El Osta et al., 2022). Medication delivery through waterlines for poultry is a well-established procedure that can provide a more effective therapeutic dose. Medicine solubility in stock buckets and water lines may vary based on water quality and medicine makeup (Femke et al., 2020). Most drugs have electron-donor characteristic groups with affinity to bind with metallic ions. Furthermore, certain metals such as copper, iron, zinc, chromium, cobalt manganese, selenium, and molybdenum are nutrients and essential metal ions, thus playing an important role in maintaining normal healthy living in animals and humans. As a result, their excesses or deficiencies and abnormalities in their metabolism may cause serious diseases and mortality (Ahmed et al., 2020; Kontoghiorghes and Kontoghiorghe, 2020; Ahmed and Mikail, 2024). The important role of these essential metal ions is an integral part of enzymes and proteins, as well as that of transcription factors and other co-factors that secure the normal growth and development of the body. These metal ions, and especially iron, play a central role in important physiological processes such as oxygen transport and utilization, as well as respiration, and also other processes involving the metabolism of proteins, lipids, carbohydrates, and nucleic acids (Saleh et al., 2023). Heavy metals (HMs) in groundwater occur in particulate, colloidal, and dissolved phases (Aldaddou et al., 2022; Ammar Aldaddou et al., 2023; Zhu et al., 2023). Their natural sources are possibly volcanism-extruded products leaching and weathering rocks, while their anthropogenic sources are mostly from domestic and industrial effluents and the disposal of solid wastes (Briffa et al., 2020; Mitra et al., 2022). The water used with antibiotics should be of good quality to get the most effect for most antibacterial drugs (Hanna et al., 2020). For instance, quinolones are common agents of complexation for various metallic ions, as well as transition and alkaline earth metallic ions. Quinolones’ coordination with metallic ions, including Mg2+, Cu2+, and Ca2+, is very important for quinolone antibiotic activity (Uivarosi, 2013). Increasing concentrations of HMs are also responsible for antibiotic resistance decline (Zhu et al., 2023). There have been several reports about how the interactions between HMs and tetracycline modify the desorption and sorption behaviors (Wang et al., 2008). Therefore, the current study was aimed at investigating the influence of pollution and water quality used in some poultry farms located in the Qassim region on the bioavailability of antibiotics in broiler chickens. Materials and MethodsWater sample collectionsThirty water samples were collected from 30 poultry farms located in the Qassim region for 1 year (August 2019 and July 2020). The collection, preservation, and transportation of water samples were carried out according to the American Public Health Association (APHA, 2005). Chemical analysis of water samplesThe chemical analysis of water samples was performed at the laboratory of the General Directorate of Water Resources and Rationalization at Qassim region belonging to the Ministry of Environment, Water and Agriculture, KSA. Water samples analysis was carried out for Zn, Pb, Mn, Fe, and Cu using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)-Thermo Fisher Scientific. Water sample preparationThe stock solution was prepared for each chemical using the following equation: (1) The aqueous stock solution was prepared by dissolving 6 g Zn sulfate, 3.66 g Lead acetate, 4.47 g Manganese oxide, 6.71 Ferric sulfate, and 6.15 g Copper sulfate, in 2 l of bottled water from Hana Food Industries Company at the Qassim region for each heavy metal to obtain 1,000 mg concentration of Zn, Pb, Mn, Fe, and Cu, respectively. The prepared water samples were stored in plastic gallons at 5°C. Then, the stock solutions were analyzed by ICP-OES to make sure that the required concentration was achieved. Animal studiesThe rearing of chickens was done at the experimental poultry houses at the College of Agriculture and Veterinary Medicine, Qassim University. Alwatania Poultry Company supplied 468 one-day-old chickens (Cobb 500)®. Chickens were separated into 13 wooden partitions (36 chickens/partition) in a completely randomized design at 1 day old. The experimental chickens were raised from day one till 6 weeks of age on deep litter with a stocking density of 10 chickens/m². Electrical heaters were used to maintain house temperature at 35°C throughout the first week, and then the temperature was reduced gradually by 3°C weekly until the temperature reached 24°C and was fixed till the end of the rearing period. Artificial lighting was provided constantly during the experimental period, whereas chickens were kept under 23 hours of light and 1 hour of darkness. Watering of chickens inside the house was done with manual plastic bell-shaped drinkers. Water and feed were given ad libitum during the experiment to all chickens, where chickens received a balanced commercial poultry ration that was formulated to meet the requirements of broiler chickens (Table 1). Table 1. Feed additives of trace minerals used for broilers.
Animal groupingThe animal study involved 234 chickens divided into 6 broad groups (each group containing subgroups A and B as low dose and high dose, respectively). There were 18 Chickens in each subgroup. Chickens in subgroup A received water containing the maximum level of heavy metals (low dose) while chickens in group B received water containing 5 × of the A subgroup (high dose). The control group was given bottled water from a water company in the Qassim region without adding heavy metals. The description of experimental groups is shown in Table 2. Table 2. Description of the experimental groups of reared broiler chickens.
Collection of blood samplesOxytetracycline hydrochloride soluble powder was administered in the drinking water of chickens at a level of 1g/l of drinking water, while amoxicillin trihydrate was administered at 1g /10 kg of BW. Clock-watch timing was used to achieve a near timing interval between drug administration and sampling of the blood from the wing vein, thus, time differences between targeted and actual blood sampling times were at most 2 minutes (Sumano et al., 2004). On the 25th and 32nd days of the examined broiler's age, approximately 2–3 ml blood samples from three randomly selected chickens per group were drawn from the wing vein in a clean plastic centrifuge tube. Blood samples for oxytetracycline analysis were collected on the 25th day of age at 2 hours and 4 hours intervals after drug administration while amoxicillin sample analysis was collected on day 32nd of age at 2 hours and 4 hours intervals after drug administration (Table 3) where, these interval times at which the antibiotics could reach their maximum serum concentration Cmax in the blood according to (Vancutsem et al., 1990). Blood samples were left at room temperature for 2 hours to clot. The samples were centrifuged at 3,000 rpm for 10 minutes (Centrifuge – CL008, CYAN), serum was collected in clean Eppendorf tubes, then 200 μl will be pooled from each serum sample/ the same group and placed together in one Eppendorf tube to represent one serum sample of 600 μl volume/each group. Finally, a total of 156 serum samples were obtained, labeled, and stored at −20°C for subsequent evaluation of the efficiency of the antibiotics (oxytetracycline and amoxicillin) in the laboratory using (HPLC). Table 3. Time of antibiotics administration and collection of samples.
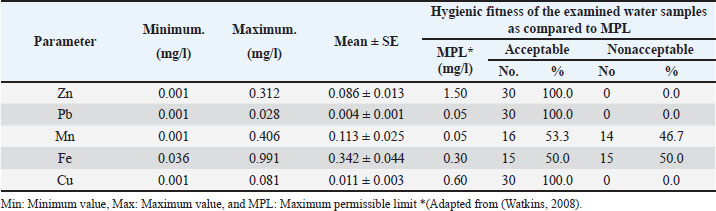
Evaluation of the efficiency of oxytetracycline and amoxicillinReagents and chemicalsAcetonitrile and acetic acid were obtained from (Merck®, Nogent-Sur-Marne, France), methanol) Sigma® -Aldrich, Co. Steinheim, Germany), HPLC system grade water. Reference standardsThe active pharmaceutical ingredients (API) including oxytetracycline (Bio-Basic, Canada Inc. USA) and amoxicillin (Merck Sharp and Dohme, Rahway, N.J.) were used as reference standards without further purification with concentrations levels of (100, 250, 500, 1,000, 5,000, and 10,000 ppb) for oxytetracycline and (250, 500, 1,000, 2,500, 5,000, and 10,000 ppb) for amoxicillin. Apparatus and instrumentsHigh-performance liquid chromatographic systemHPLC system comprised an iso pump, a water solvent delivery system, and a manual injector (rheodyne) attached with a 20 μl loop, equipped with an injection valve (Rheodyne® model 7125) with a 20 μl fixed loop and a variable ultraviolet detector (Varian® model 4000) as well as a Waters fluorescent detector (model 420). The system was controlled by the ChemStation software (Hewlett-Packard, Les Ulis, Germany). 0.45 μm pore size Millipore Millex®—HV filter units, Saint Quentin Yvelines, France, a reversed-phase column (C18, Zorbax column, 250 mm, 4.6 mm i.d., 5 µm—Agilent Co., USA), Bond Elute C18 (500 mg, 3 or 6 ml; Varian, Les Ulis, France. It was carried out in the Residue Analysis Unit, Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, Egypt, using HPLC to measure the maximum or peak serum concentration Cmax of antibiotics in the samples as described in the literature (Morton et al., 1986). Briefly, HPLC analysis was performed using an ultraviolet detector at 280 wavelengths and adjusted its flow rate at 1 ml/minute during sample injection with a rheodyne syringe loading sample injector at an Injection volume of 25 μl. The run time for each sample is ± 25 minutes. All the analyses were carried out at room temperature, where a reversed-phase column was used. The mobile phase for HPLC analysis was prepared by adding 50 ml acetic acid 5% to 950 ml deionized HPLC-grade water to form a volume of 1 l, then removing 100 ml from the solution and adding a mixture of 50 ml methanol and 50 ml acetonitrile were added. The mobile phase was filtered using a membrane filter (Saint Quentin Yvelines, France) and then degassed before its use. It was allowed to stabilize for at least 2 days and stored at 4°C. All solutions and solvents were filtered using the membrane filter and degassed before use. Standard and spike solutions preparationThe API, including oxytetracycline (Bio-Basic, Canada Inc. USA) and amoxicillin (Merck Sharp and Dohme, Rahway, N.J.) were used as reference standards without further purification at different concentration levels (100, 250, 500, 1,000, 5,000, and 10,000 ppb for oxytetracycline & 250, 500, 1,000, 2,500, 5,000, and 10,000 ppb for amoxicillin). Spike solutions were prepared by dissolving oxytetracycline or amoxicillin in sterile deionized water. The solutions were diluted in heat-inactivated pooled serum of normal chickens for standards with concentrations of (100, 250, 500, 1,000, 5,000, and 10,000 ppb) for oxytetracycline and (250, 500, 1,000, 2,500, 5,000, and 10,000 ppb) for amoxicillin. Sample preparationSample preparation for HPLC required the addition of 0.2 ml of methanol for the precipitation of protein, followed by 30 seconds of vortex mixing and centrifuging at 5.500 × g for 10 minutes. The supernatants were taken out and filtered through a membrane filter. The filtrates were employed for the analysis. Standards, spikes, and samples were preserved at −70°C till analysis. Calibration curvesSix different concentration levels (100, 250, 500, 1,000, 5,000, and 10,000 ppb) and (250, 500, 1,000, 2,500, 5,000, and 10,000 ppb) were obtained from oxytetracycline and amoxicillin standard solution, respectively, and diluted with the mobile phase. Each of the solutions was injected into the chromatographic system. The mean values of the peak areas were plotted against their concentrations. The curves were adjusted using linear regression with the method of least mean square (Hewlett-Packard, Les Ulis, Germany). Also, three different concentration levels (75, 750, and 1,500 ppb) and (2,000) of oxytetracycline and amoxicillin spikes solution, respectively, were obtained and diluted with the mobile phase. Each of the solutions was injected into the chromatographic system. The mean values of the peak areas were plotted against their concentrations. QuantificationQuantification of antibiotics (oxytetracycline and amoxicillin) in the samples was obtained and calculated from the area under the curves extrapolated automatically by the software. Formula: y=mx + b m: 3.93337 e-2 x: Amount b: 4.68869 e-1 y: Height of the peak Linearity and rangeThe evaluation of the linearity of the proposed method was done by preparing at a minimum of 5 different concentrations of the antibiotic standard and using calibration curves to estimate the intercept and coefficient of correlation values. Where linearity was defined using the squared correlation coefficient (r2). According to the International Council for Harmonization (ICH), it should be 0.99. Method precisionSix replicates of the standard solutions (oxytetracycline amoxicillin) were used. Acceptance criteria: RSD ≤ 1%, according to ICH. Criteria: RSD ≤ 1%, according to ICH. Accuracy and recoveryThe chickens’ serum samples were spiked with the addition of known quantities of each standard. The samples were analyzed against similar concentrations of the standard solutions. The accuracy was estimated from the results as a percentage (%) recovery. Statistical analysisData are presented at mean ± standard error (SD). The obtained data were statistically analyzed according to (SAS Institute Inc, 2002). The results were analyzed using one-way ANOVA. Values with different superscript letters (a, b, c, and so on) in the same column are significantly different (p < 0.001), as measured by Tukey’s HSD Test. Ethical approvalThe protocol was approved by the Committee of Research Ethics, Deanship of Scientific Research, Qassim University (on july 18, 2019), SA, which is regulated by the National Committee of BioEthics (NCBE), and the study was conducted in accordance with approved guidelines. ResultsChemical analysis of water samplesThe results of the chemical examination of water samples used for poultry farms (n=30) are shown in Table 4 as well as Figures 1 and 2. The mean values of the Zn, Pb, Mn, Fe, and Cu were 0.086, 0.004, 0.113, 0.342, and 0.011 mg/l, respectively. Samples of water from 30 poultry farms were fully acceptable (100%) regarding Zn, Pb, and Cu contents, while 53.3% and 50.0 % of water samples were acceptable for Mn and Fe levels, respectively. Table 4. Chemical examination of water samples used for poultry.
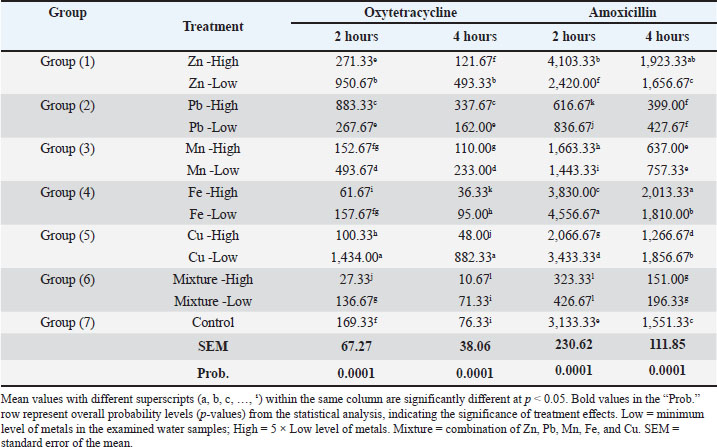
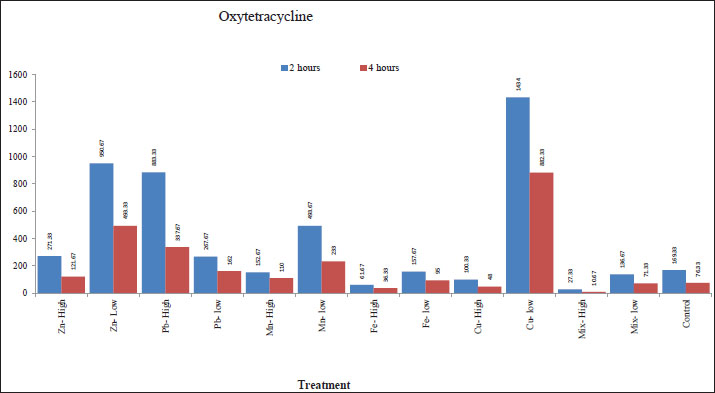
Effect of metals in drinking water on the bioavailability of oxytetracycline and amoxicillinThe results (Table 5 and Fig. 1) showed that the water treated with low levels of Zn, Pb, Mn, and Cu, and high levels of Zn and Pb resulted in a significant (p < 0.001) increase in the Cmax of oxytetracycline after 2 hours of treatment as compared with the control group. In addition, a low level of the mixture and high levels of Fe, Cu, and mixture resulted in a significant (p < 0.001) decrease in the Cmax of oxytetracycline after 2 hours of treatment in comparison with the control group. On the other hand, no significant differences were found among the control and low level of Fe and high level of Mn. Table 5. Effect of metals on the bioavailability of oxytetracycline and amoxicillin.
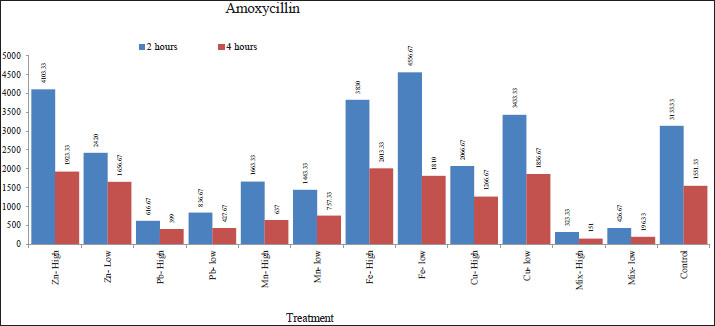
Fig. 1. Effect of metals in drinking water on the bioavailability of oxytetracycline. The results also showed that the water treated with low levels of Zn, Pb, Mn, Fe, and Cu, and high levels of Zn, Pb, and Mn resulted in a significant (p < 0.001) increase in the Cmax of oxytetracycline after 4 hours of treatment as compared with the control group. In addition, high levels of Fe, Cu, and the mixture resulted in a significant (p < 0.001) decrease in the Cmax of oxytetracycline after 4 hours of treatment as compared with the control group. There were no significant changes found among the control group and low levels of the mixture. Similarly, the water treated with low levels of Fe and Cu, and high levels of Zn and Fe resulted in a significant (p < 0.001) increase in the Cmax of amoxicillin after 2 hours of treatment as compared with the control group (Table 5 and Fig. 2). In addition, low levels of Zn, Pb, Mn, and the mixture as well as high levels of Pb, Mn, Cu, and the mixture resulted in a significant (p < 0.001) decrease in the Cmax of amoxicillin after 2 hours of treatment as compared with the control group.
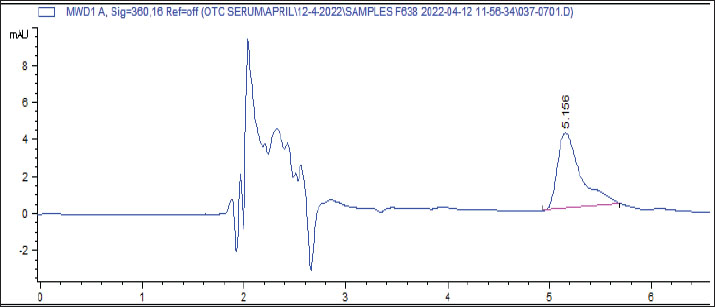
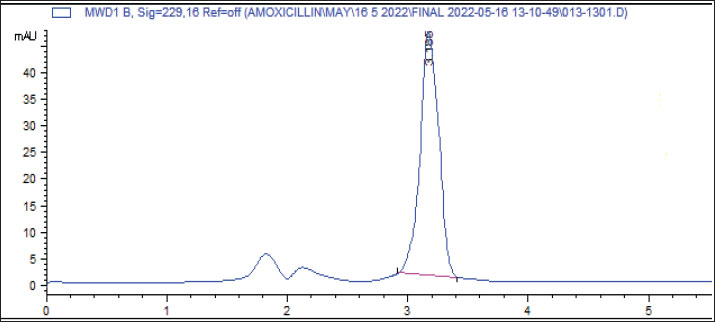
Fig. 2. Effect of metals in drinking water on the bioavailability of amoxicillin. The results also showed that the water treated with low levels of Fe and Cu, as well as high levels of Zn and Fe resulted in a significant (p < 0.001) increase in the Cmax of amoxicillin after 4 hours of treatment in comparison with the control group. Furthermore, low levels of Pb, Mn, and the mixture as well as high levels of Pb, Mn, Cu, and the mixture resulted in a significant (p < 0.001) decrease in Cmax of amoxicillin after 4 hours of treatment as compared with the control group, while no significant differences were found among control and low level of Zn. Spikes HPLC systemThe results of the quantification of different concentrations of oxytetracycline and amoxicillin spikes in the HPLC system are illustrated in Figures 3 and 4.
Fig. 3. The results of the quantification of different concentrations of oxytetracycline spikes, HPLC system.
Fig. 4. The results of the quantification of different concentrations of amoxicillin spikes, HPLC system. DiscussionThe use of antibiotics is relevant in the poultry industry owing to the need to enhance the production of meat through disease prevention, feed conversion, and growth rate. In poultry production, antibiotics are generally used at sub-therapeutic doses for the promotion of growth and birds' health protection (Mehdi et al., 2018) because they also follow hermetic responses (Ahmed et al., 2020; Ahmed et al., 2022). The quality of drinking water generally affects both the absorption and antibiotics bioavailability. (Kotb et al., 2019). Different concentrations of the metals cause different responses to the bioavailability of the antibiotics at different times. For instance, low levels of Zn, Pb, Mn, and Cu, and high levels of Zn and Pb resulted in a significant (p < 0.001) increase in the Cmax of oxytetracycline after 2 hours of treatment while a low level of the mixture and high level of Fe, Cu and mixture resulted in a significant (p < 0.001) decrease in the Cmax of after 2 hours of treatment in comparison with the control group while low levels of Fe and Cu, and high levels of Zn and Fe resulted in a significant (p < 0.001) increase in the Cmax of amoxicillin after 2 hours of treatment. The obtained results indicated that the metals affected the bioavailability of antibiotics for broiler chicken to oxytetracycline and amoxicillin. These results agree with the results recorded by other studies (Wang et al., 2008; Zhao et al., 2013; Yao et al., 2020). According to the literature, when the ratio of concentration between Cu and oxytetracycline was 1:1, the adsorption of oxytetracycline was enhanced significantly, while oxytetracycline had a weak effect on the adsorption of Cu (Xue et al., 2021). Similarly, certain metals enhanced antibiotics' adsorption capacities, while antibiotics had only a slight enhancement in some metal's adsorption capacities (Yao et al., 2020) The quality of drinking water affects both antibiotic absorption and bioavailability in the bodies of animals. Thus, poor quality of water influences the bioavailability and stability of tetracycline. However, there is a non-significant statistical correlation between HMs correlation in water and the decrease of doxycycline therapeutic concentration in vitro (Kotb et al., 2019). However, the poor quality of water in developing countries is responsible for the use of higher doses of antibiotics to attain the required optimum concentration in treated animals (Dosoky et al., 2015; Okocha et al., 2018). ConclusionThe quality of drinking water affects both antibiotic absorption and bioavailability in the bodies of broiler chickens. Thus, poor quality of water negatively affects the bioavailability and stability of antibiotics. Different concentrations of the metals cause different responses to the bioavailability of the antibiotics at different times in the experimental broiler chickens. AcknowledgmentsThe Researcher would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025) Conflict of interestThe author declares that he has no conflict of interest. FundingNone. Authors' contributionA.I.A. Study design, Sample and data collection, Methodology, result analysis and interpretation, Writing—original draft, and Writing–review & editing. A.I.A. has read and agreed to the published version of the manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAhmed, I.A. and Mikail, M.A. 2024. Diet and skin health: the good and the bad. Nutrition 119, 112350. Ahmed, I.A., Mikail, M.A., Zamakshshari, N. and Abdullah, A.S.H. 2020. Natural anti-aging skincare: role and potential. Biogerontology 21(3), 293–310. Ahmed, I.A., Mikail, M.A., Zamakshshari, N.H., Mustafa, M.R., Hashim, N.M. and Othman, R. 2022. Trends and challenges in phytotherapy and phytocosmetics for skin aging. Saudi J. Biol. Sci. 29(8), 103363. Aldaddou, W.A., Aljohani, A.S.M., Ahmed, I.A., Al-Wabel, N.A. and El- Ashmawy, I.M. 2022. Ameliorative effect of methanolic extract of Tribulus terrestris L. on nicotine and lead-induced degeneration of sperm quality in male rats. J. Ethnopharmacology 295, 115337. Aljassim, M.T., Almulla, A.A., Berekaa, M.M. and Alsaif, A.S. 2024. Evaluating the influence of reverse osmosis on lakes using water quality indices: a case study in Saudi Arabia. Water 16(10), 1351. Alshehri, F., El-Sorogy, A.S., Almadani, S. and Aldossari, M. 2023. Groundwater quality assessment in western Saudi Arabia using GIS and multivariate analysis. J. King Saud Univ. - Sci. 35(4), 102586. Ammar Aldaddou, W., Aljohani, A.S.M., Adewale Ahmed, I., Al-Wabel, N.A. and El-Ashmawy, I.M. 2023. Salvia officinalis L. methanolic extract reduces lead and nicotine-induced sperm quality degeneration in male rats. Chem. &. Biodivers. 20(7), e202300115. APHA. 2005. Standard Methods for the Examination of Water and Wastewater. 21st Edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC. Briffa, J., Sinagra, E. and Blundell, R. 2020. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9), 4691. Dosoky, R., Kotb, S. and Farghali, M. 2015. Efficiency of silver nanoparticles against bacterial contaminants isolated from surface and ground water in Egypt. J. Adv. Vet. Anim. Res. 2(2), 175–184. El Osta, M., Masoud, M., Alqarawy, A., Elsayed, S. and Gad, M. 2022. Groundwater suitability for drinking and irrigation using water quality indices and multivariate modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 14(3), 483. El-Rawy, M. and Fathi, H. 2023. Groundwater pollution sources and its quality in the kingdom of Saudi Arabia: State of the Art. Femke, V., de Carvalho Ferreira, H.C., Devreese, M., Dewulf, J., Daeseleire, E., Eeckhout, M. and Croubels, S. 2020. Stability, homogeneity and carry-over of amoxicillin, doxycycline, florfenicol and flubendazole in medicated feed and drinking water on 24 pig farms. Antibiotics 9, 563. doi:10.3390/antibiotics9090563 Hanna, N., Purohit, M., Diwan, V., Chandran, S.P., Riggi, E., Parashar, V., Tamhankar, A.J. and Lundborg, C.S. 2020. Monitoring of water quality, antibiotic residues, and antibiotic-resistant Escherichia coli in the Kshipra River in India over a 3-year period. Int. J. Environ. Res. Public Health 17(21), 17. Ingrao, C., Strippoli, R., Lagioia, G. and Huisingh, D. 2023. Water scarcity in agriculture: an overview of causes, impacts and approaches for reducing the risks. Heliyon 9(8), e18507. Kontoghiorghes, G. and Kontoghiorghe, C. 2020. Iron and chelation in biochemistry and medicine: new approaches to controlling iron metabolism and treating related diseases. Cells 9(6), 9. Kotb, S., Ahmed, M., Hassan, D. and Soltan, E. 2019. Stability of antibiotics in drinking water: an advanced approach towards the impacts of water quality parameters on doxycycline bioavailability. J. Adv. Vet. Anim. Res. 6(4), 438. Mallick, J., Singh, C.K., Almesfer, M.K., Singh, V.P. and Alsubih, M. 2021. Groundwater Quality Studies in the Kingdom of Saudi Arabia: prevalent Research and Management Dimensions. Water 13(9), 1266. Mehdi, Y., Létourneau-Montminy, M.P., Gaucher, M.L., Chorfi, Y., Suresh, G., Rouissi, T., Brar, S.K., Côté, C., Ramirez, A.A. and Godbout, S. 2018. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 4(2), 170–178. Mitra, S., Chakraborty, A.J., Tareq, A.M., Emran, T.B., Nainu, F., Khusro, A., Idris, A.M., Khandaker, M.U., Osman, H., Alhumaydhi, F.A. and Simal-Gandara, J. 2022. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J. King Saud Univ. - Sci. 34(3), 101865. Morante-Carballo, F., Montalván-Burbano, N., Quiñonez-Barzola, X., Jaya-Montalvo, M. and Carrión-Mero, P. 2022. What do we know about water scarcity in semi-arid zones? a global analysis and research trends. Water 14(17), 2685. Morton, S.J., Shull, V.H. and Dick, J.D. 1986. Determination of norfloxacin and ciprofloxacin concentrations in serum and urine by high-pressure liquid chromatography. Antimicrob. Agents Chemotherapy 30(2), 325–327. Odnoletkova, N. and Patzek, T.W. 2023. Water resources in Saudi Arabia: trends in rainfall, water consumption, and analysis of agricultural water footprint. Npj Sustain. Agriculture 1(1), 7. Okocha, R.C., Olatoye, I.O. and Adedeji, O.B. 2018. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 39(1), 1–22. Saleh, N., Ayoub, M., Nossair, M., Alqhtani, A., Swelum, A., Khojah, H., Gamal, M., Imam, M., Khafaga, A., Arif, M., Abd El-hack, M., Saleh, N. and El-Hack, A. 2023. Influence of water quality and pollution on broiler's performance, vaccine and antibiotic efficiencies. Ann. Anim. Sci. 23(4), 1021–1036; doi: 10.2478/aoas-2023-0023 SAS, USG. 2002. Statistics, version 9. Statistical Analysis System. SAS Inst. Inc., Cary, NC. Sumano, L.H., Gutierrez, O.L., Aguilera, R., Rosiles, M.R., Bernard, B.M.J. and Gracia, M.J. 2004. Influence of hard water on the bioavailability of enrofloxacin in broilers. Poultry Sci. 83(5), 726–731. Uivarosi, V. 2013. Metal complexes of quinolone antibiotics and their applications: an update. Molecules 18(9), 11153–11197. Vancutsem, P.M., Babish, J.G. and Schwark, W.S. 1990. The fluoroquinolone antimicrobials: structure, antimicrobial activity, pharmacokinetics, clinical use in domestic animals and toxicity. Cornell Vet. 80(2), 173–186. Vandael, F., Helena Cardoso de Carvalho Ferreira., Devreese, M., Dewulf, J., Daeseleire, E., Eeckhout, M. and Croubels, S. 2020. Stability, homogeneity and carry-over of amoxicillin, doxycycline, florfenicol and flubendazole in medicated feed and drinking water on 24 pig farms. Antibiotics 2020. 9, 563. Wang, Y.J., Jia, D.A., Sun, R.J., Zhu, H.W. and Zhou, D.M. 2008. Adsorption and cosorption of tetracycline and copper (II) on montmorillonite as affected by solution pH. Environ. Sci. &. Technol. 42(9), 3254–3259. Watkins. 2008. Water: identifying and correcting challenges. Avian Advice 10(3, 10–15.). Xue, X.D., Fang, C.R. and Zhuang, H.F. 2021. Adsorption behaviors of the pristine and aged thermoplastic polyurethane microplastics in Cu (II)-OTC coexisting system. J. Hazardous Mater. 407, 124835. Yao, N., Li, C., Yu, J., Xu, Q., Wei, S., Tian, Z., Yang, Z., Yang, W. and Shen, J. 2020. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Separat. Purification Technol. 236, 116278. Zhao, Y., Tan, Y., Guo, Y., Gu, X., Wang, X. and Zhang, Y. 2013. Interactions of tetracycline with Cd (II), Cu (II) and Pb (II) and their coadsorption behavior in soils. Environ. Pollut. 180, 206–213. Zhu, H.S., Liang, X., Liu, J.C., Zhong, H.Y., Yang, Y.H., Guan, W.P., Du, Z.J. and Ye, M.Q. 2023. Antibiotic and Heavy Metal Co-Resistant Strain Isolated from Enrichment Culture of Marine Sediments, with Potential for Environmental Bioremediation Applications. Antibiotics (Basel). 12(9), 12. | ||
| How to Cite this Article |
| Pubmed Style Ahmed I. Alajaji. Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Vet. J.. 2025; 15(10): 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 Web Style Ahmed I. Alajaji. Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. https://www.openveterinaryjournal.com/?mno=276490 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i10.9 AMA (American Medical Association) Style Ahmed I. Alajaji. Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Vet. J.. 2025; 15(10): 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 Vancouver/ICMJE Style Ahmed I. Alajaji. Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Vet. J.. (2025), [cited January 25, 2026]; 15(10): 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 Harvard Style Ahmed I. Alajaji (2025) Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Vet. J., 15 (10), 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 Turabian Style Ahmed I. Alajaji. 2025. Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Veterinary Journal, 15 (10), 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 Chicago Style Ahmed I. Alajaji. "Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken." Open Veterinary Journal 15 (2025), 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 MLA (The Modern Language Association) Style Ahmed I. Alajaji. "Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken." Open Veterinary Journal 15.10 (2025), 4894-4903. Print. doi:10.5455/OVJ.2025.v15.i10.9 APA (American Psychological Association) Style Ahmed I. Alajaji (2025) Impact of heavy metals pollution on antibiotic bioavailability in drinking water of broiler chicken. Open Veterinary Journal, 15 (10), 4894-4903. doi:10.5455/OVJ.2025.v15.i10.9 |