| Original Article | ||
Open Vet. J.. 2022; 12(3): 329-334 Open Veterinary Journal, (2022), Vol. 12(3): 329–334 Original Research Detection of Mycoplasma gallisepticum in broiler chickens by PCREbtehal N. Mahmmoud, Mohammad A. Hamad* and Zahraa N. KhudhurDepartment of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Mohammad A. Hamad. Department of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: mahmah1073 [at] uomosul.edu.iq Submitted: 07/02/2022 Accepted: 01/05/2022 Published: 21/05/2022 © 2022 Open Veterinary Journal
AbstractBackground: Mycoplasma is a significant microorganism of poultry, which can cause respiratory infections and synovial inflammation, bringing about huge financial misfortunes to poultry workmanship worldwide. Aim: The goal of existing research was to determine the infection rate of Mycoplasma gallisepticum (MG) from chronic respiratory disease cases among broilers fields in Mosul/ Iraq using the polymerase chain reaction (PCR) technique. Methods: All 92 lungs samples were collected from broilers with classical respiratory signs in different regions of the Nineveh governorate for 3 months from February to April 2021. Results: PCR tests were performed using two couple primers, one for the qualitative amplification of 16S rRNA genes (285 base pairs) in Mycoplasma spp. and the second couple for the detection of M. gallisepticum (580 base pairs). Among the samples obtained from broilers, 87 (94.7%) were positive for Mycoplasma and 79 (85.9%) were positive for M. gallisepticum. Conclusion: Our results showed that MG infection in broiler chickens leads to serious clinical symptoms and severe lesions. The rate of Mycoplasma isolation in this study is high despite the short lifespan of broiler chickens. Keywords: Mycoplasma gallisepticum, Broiler, Chronic respiratory disease, PCR. IntroductionMycoplasma belongs to the class Mollicutes, which contains more than 100 species, and is distinguished from bacteria by a phenotype of small size and complete lack of cell wall (Yassin et al., 2018). Mycoplasma spp. requires certain conditions to grow, and sometimes it takes up 3 weeks until the colonies appear clearly on the culture medium (Manimaran et al., 2019). The major pathogenic species of Mycoplasma in poultry are MG, M. meleagridis, M. synoviae, and M. iowae, and the most prevalent one is MG. Other types of birds are also infected by Mycoplasma spp. such as house finches, quails, guinea fowl, geese, starlings, etc. (Hamad et al., 2019a; Matucci et al., 2020). MG infection usually causes chronic respiratory disease (CRD) in chickens (Yadav et al. 2021). CRD clinical signs include nasal secretions, coughing, sneezing, tracheal thrombosis, and conjunctivitis, other less common diseases of MG are keratoconjunctivitis, arthritis, salpingitis, and encephalopathy (Ferguson-Noel et al., 2020). CRD is the predominant infection of broiler in Iraq and in the recent past, MG outbreaks took a heavy toll on poultry workmanship (Abed et al., 2021; Basit et al., 2021). There is a confusion in the differential diagnosis depending on clinical and autopsy findings with other infectious respiratory diseases. Isolation and identification of MG in vitro can be reliable, but because it is very delicate the results are not precise (Rauf et al., 2013). Accurate diagnosis based on cultural, biochemical, and serological tests is a routine but is time-consuming practice (Rauf et al., 2013). Recently, the detection of MG infection by PCR is recommended as a reliable test (Demirbilek et al., 2020). In comparing between conventional isolation techniques and PCR to identify the tracheal sample from the white leghorn layer infected with MG, it has shown that the molecular diagnosis was more accurate (80.51%) than isolation technique (39.28%) (Rauf et al., 2013). Therefore, the existing study aimed to detect MG in broiler chickens in Mosul city, Iraq using PCR as molecular tools. Materials and MethodsBroilers’ specimensNinety-two lung specimens were summed from diseased broilers that showed classical signs and P.M. lesions for CRD during the period between February to April 2021 from fields in Mosul city. The specimens were collected aseptically and subjected to DNA extraction. DNA extractionDNA has extracted from broilers’ specimens (Kilic et al., 2013; Hamad et al., 2019a, 2019b), for that 25 mg of each lung was cut and summed in the disposable container and kept at −80°C to be used later (Santos et al., 2010). DNA extraction kit was supplied by gSYNC™ Geneaid extraction kit, Korea. According to the kit instructions, the specimen was ground in a 1.5 ml tube, then GST buffer (200 µl) and proteinase-K (20 µl) were supplemented. Samples were swirled fully for 15 seconds then incubated at 60°C nightlong. Digested specimens were centrifugated at 16,000 × g for 120 seconds, the floating was gathered in a novel 1.5 ml tube, and 200 µl of GSB was supplemented to the floating, then swirled again for 10 seconds and blended utterly with 200 µl of absolute ethanol by a vortex. The blend was moved to the GS column and centrifugated at 16,000 × g for 1 minutes, after that 400 and 600 µl of W1 and W2 buffers were appended, respectively, to the GS column with centrifugation, and lasting, 100 µl of warmed eluted buffer was appended to the tubes after thoroughly dehydrating for eluting the purified DNA. The resulting product was kept at −20°C till used up. DNA amplificationFor the diagnoses of MG by PCR technique, two pairs of PCR primers were used Table 1 and consisted of universal primer pair for the genus of Mycoplasma depending on 16S rRNA sequences and the second pair was specific for MG according to (Aghabalaei and Hedaiati, 2012; Malekhoseini et al., 2017), and the efficacy of these primers was confirmed by (Hamad et al., 2019a, 2019b; Al-dabbagh et al., 2021). primers were synthesized by Bioneer Co., Korea. PCR reaction was operated in whole size 25 µl as in Kit and consisted of: 5 µl from extracting DNA (template), 1 µl of every primer, 2 µl of MgCl2, 6 µl of PCR water, and 10 µl of 2.5× prepared Mastermix solution. The control positive in both PCR runs was supplied by Hamad et al. (2019a and 2019b). Thermocycler programs were explained in Table 2. Electrophoresis was done using 10 µl of the amplified DNA in a 2% agarose gel. The bands were distinguished at 245–312 nm through the UV transilluminator (Biometra, Germany). Ethical approvalNot needed for this study. Table 1. Primers are used to detect MG.
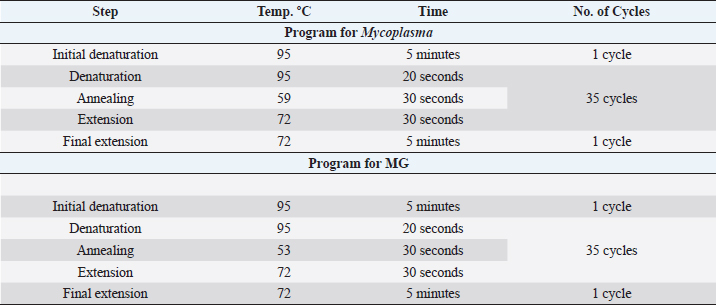
Table 2. PCR thermocycler program for Mycoplasma (genus) and MG.
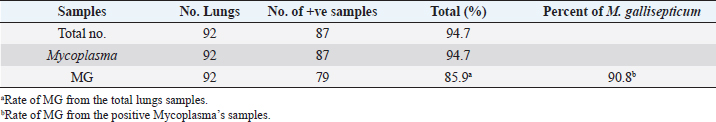
Table 3. The percentage of Mycoplasma and MG in broiler lungs.
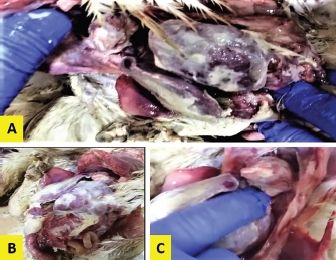
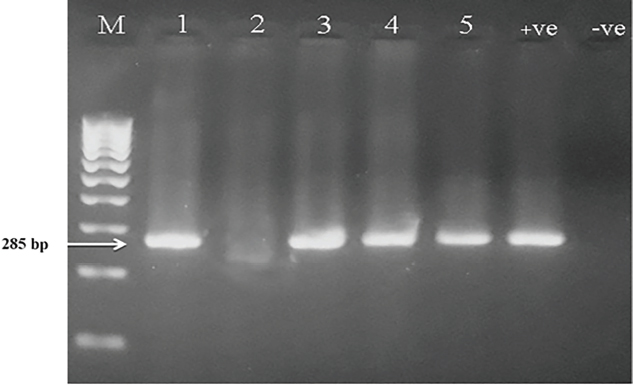
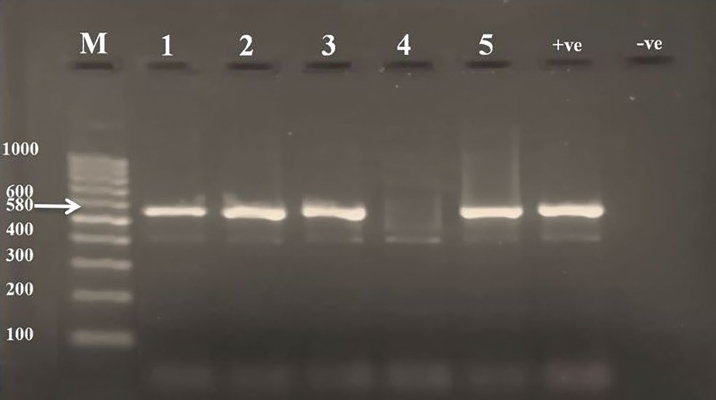
ResultsThe clinical signs of the diseased broilers were recorded and included gauntness, sternutation, coughing, meager growing, mouth-gasping, diminished feed ingesting, and other miscellaneous signs. When necropsy was carried out on diseased broilers, revealed the deposit of caseous materials on air sacs, heart, liver, and other organs (Fig. 1). The PCR results demonstrated that 87 out of 92 samples were definite as Mycoplasma (94.7%), and 79 samples were assured as MG (85.9%), which that MG exist in (90.8%) of Mycoplasma’s positive samples (Table 3 and Figs 2 and 3).
Fig. 1. Fibrin deposits on the internal organs of the diseased broilers. DiscussionCRD is considered one of the main illnesses that affect poultry workmanship and cause great economic losses, especially if they are accompanied by secondary infections (Chandhar et al., 2018). MG is responsible for this disease, and the annual global economic losses incurred by these organisms to the poultry industry have been estimated at more than $780 million (Basit et al. 2021). Economic losses in poultry are represented by reduced weight and feed conversion efficiency and may lead to decreased egg production and increased fetal mortality (Ali et al., 2020). The clinical signs that appeared on the infected chickens under this study, were upper and lower respiratory tract infections because the MG mostly colonized the mucosal surface of the host respiratory tract and proliferated in the lung, trachea, and air sacs (Manimaran et al., 2019). The observed signs were limited to coughing, mouth-gasping, nasal secretions, and general gauntness. Such signs were described by researchers (Islam et al., 2011; Feizi et al., 2013; Karthik et al., 2018). Several researchers have described lesions seen during postmortem examination (Chandhar et al. 2018; Basit et al. 2021), which are hemorrhagic secretions in the trachea and bronchi, as well as cheesy secretions in the air sacs, heart, liver, and lungs, and congestion in these organs, the same observations were observed in the current study. The samples that were used to isolate MG were taken from the lungs because it was found to be feasible and beneficial according to the study (Hamad et al., 2019a). This is inconsistent with the findings of Mukhtar et al. (2012), who isolated MG from the air sacs and trachea of commercial laying chickens, but they could not perform the isolation from the lungs, so they considered the trachea to be an important organ for detecting MG.
Fig. 2. PCR results of genus Mycoplasma on 2% agarose gel. (M): Ladder; (Lanes 1, 3, 4, 5): positive for Mycoplasma at 285 bp; (Lane 2): negative sample; (+ve): positive control for Mycoplasma; (−ve): negative control.
Fig. 3. PCR results of M. gallisepticum on 2% agarose gel. (M): Ladder; (Lanes 1, 2, 3, 5): positive samples of MG at 580 bp molecular weight; (Lane 4): negative sample; (+ve): positive control for MG; (−ve): negative control. Mycoplasma culture techniques are tiring, costly, time-consuming (Demirbilek et al., 2020), and because Mycoplasma spp. belongs to the group of fastidious organisms, it needs special nutritional requirements (Prajapati et al., 2018). Its growth may take 3 weeks or more, during this time, Mycoplasma saprophytic species may grow, which are characterized by fast growing, such as M. gallinarum and M. gallinaceum (Bibak et al., 2013). In serological techniques, the probability of obtaining nonspecific outcomes is increasing due to M. synoviae and MG cross-reaction (Manimaran et al., 2019), so the serological methods are used for flock monitoring in MG control programs (Qasem et al., 2015). The diagnosis of Mycoplasma need a fast, more specific, and sensitive method such as polymerase chain reaction (Khalifa et al., 2013 , Rauf et al., 2013), in addition to the above reasons, this pathogen has many strains; therefore, diagnosing diseases of these organisms by conventional methods is ineffective (Qasem et al., 2015). Based on the PCR results, Mycoplasma was recorded in 94.7% of the examined samples from broiler (Table 3 and Fig. 2). This percentage was much higher than previous studies recorded in Iraq and other countries including 58% in Kuwait (Qasem et al., 2015), 75% in northern Pakistan (Abbas et al., 2018), 36.6% in Iraq (Jafar and Noomi, 2019), and higher than in other birds like starlings 78.8 % (Hamad et al., 2019a) and in turkeys 64.3% (Al-dabbagh et al., 2021). The increase in the percentage may be due to the time of samples collection, the differences in weather, or it may be due to a lack of biosafety and biosecurity in the respective study area, as well as the professionalism in samples collection. The infection rate of MG (85.9%) in the running study had disagreed with the outcomes of other researchers (Ching et al., 2016; Michiels et al., 2016; Chandhar et al., 2018; Jafar and Noomi, 2019; Marouf et al., 2020) who reported less than what was recorded in the existing study on (Table 3 and Fig. 3). The ratios were, respectively: 2.7%, 63.5%, 13.33%, 78.4%, and 50%. These differences in MG infection ratios might be due to sampling size and type, stage of infection (chronic or acute), and age of birds since MG affects younger birds more seriously than adult birds (Chandhar et al. 2018). According to the species diagnosis, several samples (15.1%) appeared negative for MG, while manifested positive for the genus of Mycoplasma, and that revealed possibility of the existence of other Mycoplasma spp. employed as the causative agent of CRD (Hamad et al., 2019a; Ferguson-Noel et al., 2020). ConclusionThe results of the current study showed that the MG infection in broilers leads to occasional serious clinical symptoms and gross lesions and can lead to decreased performance of broiler breeds. The rate of MG isolation in this study is high despite the short lifespan of broiler chickens. This leads to the suggestion that the area is constantly vulnerable to infection with these organisms. AcknowledgmentsThe authors are very grateful to the Department of Microbiology, College of Veterinary Medicine, University of Mosul, for facilitating the carryout of the current study. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbbas, N., Suleman, M., Khan, N.A., Ali, I., Rauf, M. and Rahman, S. 2018. Prevalence of Mycoplasma gallisepticum in poultry and wild life birds suspected of chronic respiratory disease in Northern Pakistan. Pakistan J. Zool. 50(3), 1071–1077. Abed, A.A., Al-Iedani, A.A. and Neamah, A.J. 2021. Sequencing based phylogenetic analysis of local Mycoplasma gallisepticum of broiler chickens in Al-Dewaniyah Province / Iraq. Ann. Rom. Soc. Cell Bio. 25(5), 2719–2738. Aghabalaei, E. and Hedaiati, M.H. 2012. Detection of urogenital mycoplasmas using culture and PCR: a descriptive pilot study. J. Anim. Vet. Adv. 11(16), 2905–2909. Al-dabbagh, S.Y.A., Rasheed, B.Y. and AL-Jumaa, Z.M. 2021. Molecular diagnosis of Mycoplasma gallisepticum in Turkey in Mosul city. Vet. Pract. 22(1), 1–3. Ali, B.H., Ali, A.J. and Yosif, E.H. 2020. Isolation and molecular characterization of Mycoplasma synoviae from infected chickens with respiratory signs. Iraqi J. Agri. Sci. 51(5), 1466–1473. Basit, Md.Sh., Al Mamun, M., Rahman, Md.M. and Noor, M. 2021. Isolation and molecular detection of Mycoplasma gallisepticum in commercial layer chickens in Sylhet, Bangladesh. World Vet. J. 11(4), 614–620. Bibak, F., Kalidari, G.H.A., Razmyar, J. and Rad, M. 2013. Isolation of Mycoplasma spp. from broiler flocks with the respiratory syndrome in Mashhad, Iran. Iranian J. Vet. Sci. Tech. 5(1), 11–18. Chandhar, P.I., Swamy, M., Verma, Y. and Dubey, A. 2018. Prevalence and pathology of chronic respiratory disease in broilers. Asian J. Sci. Technol. 9(10), 8854–8859. Ching, G.T., Mahadevan, J., Aini, I., Sheikh, O., Abdul, R., Abdul, R.M. and Nadzri, S. 2016. Prevalence of Mycoplasma gallisepticum in commercial chickens and free-flying birds. J. Agri. Vet. Sci. 9, 89–95. Demirbilek, S.K., Ardicli, O. and Carli, K.T. 2020. Comparison of Mycoplasma gallisepticum infection in different samples and ages of Chicken breeder flocks. Barazilian J. Poult. Sci. 22(2), 1–6. Feizi, A., Khakpour, M., Nikpiran, H., Kaboli, K., Moggadam, A.R.J. Bijanzad, P. and Hosseini, H. 2013. Study on clinical signs and gross lesions of Mycoplasma gallisepticum in broiler breeder farms. Euro. J. Exper. Bio. 3(2), 387–390. Ferguson-Noel, N., Armour, N.K., Noormohammadi, A.H., El-Gazzar, M. and Bradbury, J.M. 2020. Mycoplasmosis. In Diseases of poultry. Eds., Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., Prajitno, T.Y., Rubinoff, I., and Zavala, G. Hoboken, NJ: Wiley, pp: 907–965. Hamad, M.A., Al-Aalim, A.M. and Alchalaby, A.Y.H. 2019a. Diagnosis of Mycoplasma from starlings lungs. J. Pure Appl. Microbiol. 13(4), 2273–2279. Hamad, M.A., AL-Jumaa, Z.M., Al-Aalim, A.M. and Mayahi, M.T.J. 2019b. Detection of Mycoplasma bovis in pneumonic calves. J. Pure Appl. Microbiol. 13(4), 2437–2443. Islam, A., Aslam, A., Chaudhry, Z.I., Ahmed, M.U.D., Rehman, H.U., Saeed, K. and Ahmad, I. 2011. Pathology of Mycoplasma gallisepticum in naturally infected broilers and its diagnosis through PCR. Int. J. Agric. Biol. 13, 835–837. Jafar, N.A. and Noomi, B.S. 2019. Detection of Mycoplasma gallisepticum and Mycoplasma synoviae by using of cultural and PCR techniques. Iraqi J. Vet. Sci. 33(2), 469–473. Karthik, K., Bharathi, R., Mahaprabhu, R., Manimaran, K. and Shoba, K. 2018. Chronic respiratory disease outbreak in an organized native chicken farm. J. Dairy Vet. Anim. Res. 7(3), 79–82. Khalifa, K.A., Abdelrahim E.S., Badwi, M. and Mohamed, A.M. 2013. Isolation and molecular characterization of Mycoplasma gallisepticum and Mycoplasma synoviae in chickens in Sudan. J. Vet. Med. 2013, Article ID: 208026. https://doi.org/10.1155/2013/208026. Kilic, A., Kalender, H., Eroksuz, H., Muz, A. and Tasdemir, B. 2013. Identification by culture, PCR, and immunohistochemistry of Mycoplasmas and their molecular typing in sheep and lamp lungs with pneumonia in Eastern Turkey. Trop. Anim. Health Prod. 45, 1525–1531. Malekhoseini, G., Pourbakhsh, S.A., Homayounimehr, A.R., Zolfeghari, M.R., Ashtari, A. and Abtn, A.R. 2017. Simultaneous identification of Mycoplasma gallisepticum and Mycoplasma synoviae by duplex PCR assay. Immunol. Case Rep. 1(1), 12–16. Manimaran, K., Mishra, A., Hemalatha, S., Karthik, K. and Ganesan, P.I. 2019. Detection of Mycoplasma gallisepticum infection in chickens from Tamil Nadu State of India. Indian J. Anim. Res. 53(1), 115–118. Marouf, S., Moussa, I.M., Salem, H., Sedeik, M., Elbestawy, A., Hemeg, H.A., Dawoud, T.M., Mubarak, A.S., Mahmoud, H., Alsubki, R.A. and Bahkali, A.H. 2020. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud. Univ. Sci. 32(3), 2263–2268. Matucci, A., Stefani, E., Gastaldelli, M., Rossi, I., Grandi, G. De, Gyuranecz, M. and Catania, S. 2020. Molecular Differentiation of Mycoplasma gallisepticum Outbreaks: A last Decade Study on Italian Farms Using GTS and MLST. Vaccines 8, 665–680. Michiels, T., Welby, S., Vanrobaeys, M., Quinet, C., Lieze Rouffaer, L., Lens, L., Martel, A. and Butaye, P. 2016. Prevalence of Mycoplasma gallisepticum and Mycoplasma synoviae in commercial poultry, racing pigeons, and wild birds in Belgium. Avian Pathol. 45(2), 244–252. Mukhtar, M., Awais, M.M., Anwar, M.I., Hussain, Z., Bhatti, N. and Ali, S. 2012. Seroprevalence of Mycoplasma gallisepticum Among Commercial Layers in Faisalabad, Pakistan. J. Bas. Appl. Sci. 8, 183–186. Prajapati, A., Subhashree, N., Susan, J.S., Reddy, M.G.B., Yogisharadhya, R. and Patil, S.S. 2018. Prevalence of Mycoplasma gallisepticum and Mycoplasma synoviae in Poultry- India perspective. Int. J. Curr. Microbiol. App. Sci. 7(5), 2213–2220. Qasem, J.A., Al-Mouqati, S.A., Al-Ali, E.M. and Ben-Haji, A. 2015. Application of molecular and serological methods for rapid detection of Mycoplasma gallisepticum infection (Avian Mycoplasmosis). Pakistan J. Biol. Sci. 18, 81–87. Rauf, M., Chaudhary, Z.I., Younus, M., Anjum, A.A., Ali, M.A., Ahmad, A.N. and Khan, M.U.R. 2013. Identification of Mycoplasma gallisepticum by a polymerase chain reaction and conventional diagnostics from white leghorn layer flocks. J. Anim. Plant Sci. 23(2), 393–397. Santos, E.M., Paula, J.F.R., Motta, P.M.C., Heinemann, M.B., Leite, R.C., Haddad, J.P.A., Del Puerto, H.L. and Reis, J.K.P. 2010. Comparison of three methods of DNA extraction from peripheral blood mononuclear cells and lung fragments of equines. Gene Mol. Res. 9(3), 1591–1598. Yadav, J.P., Tomar, P., Singh, Y. and Khurana, S.K. 2021. Insights on Mycoplasma gallisepticum and Mycoplasma synoviae infection in poultry: a systematic review. Anim. Biotechnol. 10, 1–10. doi: 10.1080/10495398.2021.1908316. Yassin, M.H., Mohamed, A.A., Hassan, M.M., Baiomy, A.A. and Ibrahim, A.M. 2018. Molecular characterization of two new Mycoplasma species isolated from chickens in Saudi Arabia. Biotechnol. 17, 142–150. | ||
| How to Cite this Article |
| Pubmed Style Mahmmoud EN, Hamad MA, Khudhur ZN. Detection of Mycoplasma gallisepticum in broiler by PCR. Open Vet. J.. 2022; 12(3): 329-334. doi:10.5455/OVJ.2022.v12.i3.4 Web Style Mahmmoud EN, Hamad MA, Khudhur ZN. Detection of Mycoplasma gallisepticum in broiler by PCR. https://www.openveterinaryjournal.com/?mno=29985 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i3.4 AMA (American Medical Association) Style Mahmmoud EN, Hamad MA, Khudhur ZN. Detection of Mycoplasma gallisepticum in broiler by PCR. Open Vet. J.. 2022; 12(3): 329-334. doi:10.5455/OVJ.2022.v12.i3.4 Vancouver/ICMJE Style Mahmmoud EN, Hamad MA, Khudhur ZN. Detection of Mycoplasma gallisepticum in broiler by PCR. Open Vet. J.. (2022), [cited January 25, 2026]; 12(3): 329-334. doi:10.5455/OVJ.2022.v12.i3.4 Harvard Style Mahmmoud, E. N., Hamad, . M. A. & Khudhur, . Z. N. (2022) Detection of Mycoplasma gallisepticum in broiler by PCR. Open Vet. J., 12 (3), 329-334. doi:10.5455/OVJ.2022.v12.i3.4 Turabian Style Mahmmoud, Ebtehal N., Mohammad A. Hamad, and Zahraa N. Khudhur. 2022. Detection of Mycoplasma gallisepticum in broiler by PCR. Open Veterinary Journal, 12 (3), 329-334. doi:10.5455/OVJ.2022.v12.i3.4 Chicago Style Mahmmoud, Ebtehal N., Mohammad A. Hamad, and Zahraa N. Khudhur. "Detection of Mycoplasma gallisepticum in broiler by PCR." Open Veterinary Journal 12 (2022), 329-334. doi:10.5455/OVJ.2022.v12.i3.4 MLA (The Modern Language Association) Style Mahmmoud, Ebtehal N., Mohammad A. Hamad, and Zahraa N. Khudhur. "Detection of Mycoplasma gallisepticum in broiler by PCR." Open Veterinary Journal 12.3 (2022), 329-334. Print. doi:10.5455/OVJ.2022.v12.i3.4 APA (American Psychological Association) Style Mahmmoud, E. N., Hamad, . M. A. & Khudhur, . Z. N. (2022) Detection of Mycoplasma gallisepticum in broiler by PCR. Open Veterinary Journal, 12 (3), 329-334. doi:10.5455/OVJ.2022.v12.i3.4 |