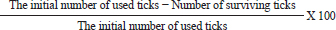| Original Article | ||
Open Vet. J.. 2022; 12(1): 44-60 Open Veterinary Journal, (2022), Vol. 12(1): 44–60 Original Research In-vivo and in-vitro effectiveness of three insecticides types for eradication of the tick Rhipicephalus sanguineus in dogsEman M. Aboelela1, Mohamed A. Sobieh2, Eman M. Abouelhassan3, Doaa S. Farid4 and Essam S. Soliman2*1Pet Animals Veterinary Medical Unit I, Directorate of Veterinary Medicine, Damietta, Egypt 2Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Animal, Poultry, and Environmental Hygiene Division, Suez Canal University, Ismailia, Egypt 3Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 4Department of Environmental Protection, Faculty of Environmental Agricultural Sciences, Arish University, Arish, Egypt *Corresponding Author: Essam S. Soliman. Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Animal, Poultry, and Environmental Hygiene Division, Suez Canal University, Ismailia, Egypt. Email: soliman.essam [at] vet.suez.edu.eg Submitted: 14/11/2021 Accepted: 03/01/2022 Published: 19/01/2022 © 2022 Open Veterinary Journal
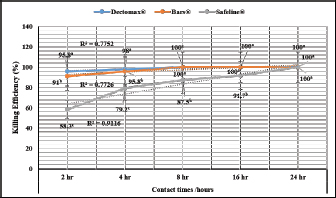
AbstractBackground: External parasites contribute to extensive harmful impacts on their hosts which is why control and eradication of external parasites have been included in all biosecurity plans of dog houses. Aim: To evaluate the in-vitro and in-vivo effectiveness of chemicals like Doramectin injectable and Fipronil 50 mg/ml drops and herbal mixes eco-friendly insecticides like phenylpyrazole–garlic–camphor mix spray for combating the external parasitism in dogs and their influence on the hematological, biochemical, and cortisol (CORT) profiles. Method: The in-vitro effectiveness of the insecticides was conducted by using a total of 216 developmental stage Rhipicephalus sanguineus (72 adults, 72 larvae, and 72 eggs) designed into three replicates of petri dishes (3 plates × 8 units × 3 stages/replicate); each replicate was exposed to 1 ml insecticide. The number of surviving ticks was recorded after 0, 2, 4, 8, 16, and 24 hours. Sixteen Rottweiler male dogs aged 1 year and 45.5 kg were divided into four groups. Three groups (G1, G2, and G3) were experimentally infested with R. sanguineus ticks 3–4 weeks post-dog arrival and kept under observation from zero-time of experimental infestation for 1–2 weeks. The three experimentally infested dog groups were treated with Doramectin injectable, Fipronil 50 mg/ml drops, and phenylpyrazole–garlic–camphor mix spray, respectively, and the fourth group was designed as a negative control. A total of 144 samples, including 48 ethylenediaminetetraacetic acid blood, 48 whole blood, and 48 sera samples, were collected. Results: The in-vitro efficacy revealed highly significant (p < 0.01) 100% killing efficacy that was achieved after 8 hours in Doramectin and Fipronil 50 mg/ml and 24 hours in phenylpyrazole–garlic–camphor mix. The in-vivo trials revealed highly significant (p < 0.01) improvements of red blood cells, hematocrit, mean corpuscular hemoglobin concentrations, platelets, total and differential leukocytic counts, erythrocyte sedimentation rates in the second hour, total protein, creatinine, alanine aminotransferase, urea, glucose, triglycerides, total cholesterol, and CORT levels in the 2-week (P1) and 4-week posttreatment (P2) samples in Dormectin, Fipronil 50 mg/ml, and phenylpyrazole–garlic–camphor mix-treated dogs with more pronounced recovery in phenylpyrazole–garlic–camphor mix spray-treated dogs. Conclusion: The insecticides were able to provide a high level of protection against experimental infestation with concern to the different modes of application. Phenylpyrazole–garlic–camphor mix spray (eco-friendly) achieved higher insecticidal action compared to the chemicals. Keywords: Dogs, Doramectin, Fipronil, Phenylpyrazole–garlic–camphor mix, Ticks. IntroductionA code of practice has to specify the minimum standards of accommodation, management, and care that are appropriate to meet the physical and behavioral demands of dogs (De Leeuw, 2003). These practices include monitoring the physical score of animals, proper housing, reporting of diseases, supervision of feeding and watering (National Research Council, 1996), satisfying the nutritional requirements, routine animal examination, strict hygienic measures, routine disinfection and sanitization, proper waste disposal, vaccination act against canine distemper, infectious canine hepatitis, canine parvovirus, canine cough, and rabies (Moore, 2003). Control and eradication of external parasites in dogs have been included in every single plan for biosecurity. The control actions depend on sealing with cement all the cracks in the walls and floors of the animals building, good housing practices, and design; rotational locations to encourage the death of the larvae from starvation; depopulation, if possible; washing of the animals routinely; and application of chemical or biological treatment means on the animals (Cochi et al., 1998). External parasites or ectoparasites can be defined as organisms that live and survive on the outer body surface at the expense of another organism (Rasouli et al., 2011). These external parasites might contribute to extensive harmful impacts on their hosts, including cardiovascular disorders such as congenital heart failure and anemia (Zendehfili et al., 2015). External parasites must suck their blood meals, first causing wounds in the skin of the host which can act as a second gate for the entrance of other microorganisms as bacterial and viral agents, whose toxins may enter into the bloodstream and contribute to intoxication in the animal host (Mansour et al., 2017). The presence of the external parasites all over the body of their host can contribute to irritation and sometimes allergy. In light of the external parasitism, parasites suck their blood meal from the animals, and as a consequence; feeding the animals become a waste of money, as they suffer from severe bodyweight loss, reduced strength, and reduced ability to work, contributing to great economic losses (Tong et al., 2019). The greater danger of external parasitism might be caused by the mechanical transmission of enteric microorganisms like Escherichia coli species, Salmonella species, or some pyogenic microorganisms like Corynebacteria species, Staphylococcus species, or Streptococcus species (Apanaskevich and Apanaskevich, 2016; Kwak et al., 2021). They also can biologically transmit some pathogenic blood protozoa, such as Babesia spp. (red water), Thileria spp., and Trypanosoma species; bacterial pathogens, such as Pasteurella pestis; and viral pathogens, such as yellow fever (Shirazi et al., 2013; Apanaskevich et al., 2019). Insecticides are substances that are designed to kill external parasites and insects. They act mainly as ovicidal or larvicidal agents (Lucia and Guzmán, 2021). The insecticides can act directly on the nervous system of the external parasites through many means: as an acetylcholinesterase inhibitor, sodium channel blockers, GABA-gated chloride channel blockers, voltage-dependent sodium channel modulators, glutamate-gated chloride (GluCl) channel allosteric modulators, juvenile hormone mimics, chordotonal organ channel modulators, microbial disruptors of insect midgut membranes, inhibitors of mitochondrial adenosine triphosphate synthase, uncouplers of oxidative phosphorylation, inhibitors of chitin biosynthesis, mitochondrial complex electron transport inhibitors, and inhibitors of acetyl CoA carboxylase (Metcalf, 2002). The use of insecticides has been problematic in affecting nontarget species, runoff and percolation, pollinator decline, and bird decline. The development and spread of insecticide resistance can be considered a threat to the biosecurity, prevention, and control strategies applied to animal farms, as well as for human beings (Hafez and Abbas, 2021). The higher the resistance of the external parasites to the commercially available insecticides, the higher the chances for the spread of infectious and contagious vector-borne diseases that might contribute to epidemics in such cases (Minetti et al., 2020). A fact that deliberates a necessity for the use of some alternatives categorized as natural eco-friendly compounds or green chemistry has been investigated to reduce the use of chemical insecticides and is associated with higher and extended efficacy on animals and humans, like garlic (González-Macedo et al., 2021; Chen et al., 2022), camphor (Elbrense et al., 2022), potato extract (Khorrami and Soleyman, 2021), pomegranate (Saad et al., 2021), and clay (Oliveira et al., 2022). We aimed in this study to evaluate the in-vitro and in-vivo effectiveness of some commercial chemical insecticides like Doramectin injectable (Dectomax®) and Fipronil 50 mg/ml drops (Bars®), and herbal-based (Eco-friendly) insecticides like phenylpyrazole–garlic–camphor mix spray (Safeline®) in the recommended dose and concentration for combating the external parasitism in dogs and for their influence on the hematological and biochemical profile, as well as on the cortisol (CORT) stress marker. Materials and MethodsStudy period and locationThe in-vitro study was conducted for four successive weeks from April 1, 2021 to May 1, 2021 in the Animal, Poultry, and Environmental Hygiene laboratory. The in-vivo study was conducted from June 1, 2021 to August 1, 2021 in the laboratory animal units of the Faculty of Veterinary Medicine at Suez Canal University in Ismailia, Egypt. The in-vivo study period was divided into three stages: the first 2 weeks for acclimatization of dogs; the second 2 weeks for experimental infestation; and the third stage was 1 month for zero-sampling, treatment application, and posttreatment sampling (P1 at 2 weeks and P2 at 4 weeks posttreatment). Biochemical profile was conducted in the Animal, Poultry, and Environmental Hygiene laboratory. Hematological and hormonal assays were conducted in the Clinical Pathology laboratories at Suez Canal University Hospital. Commercial insecticidesThree insecticides were chosen were Dectomax® (Dramectin), Bars® (Fipronil 50 mg/ml), and Safeline® (Phenylpyrazole–garlic–camphor mix). Dectomax® (Dramectin) is an injectable solution of Doramectin (1 g) in the form of an oily excipient for the treatment. It is indicated for the treatment and control of gastrointestinal roundworms, lungworms, eye worms, grubs, sucking lice, and mange mites. It is administered as a subcutaneous or intramuscular single injection at a dosage of 200 µg of Doramectin per kg (1 ml/ 50 kg) body weight. Bars® (Fipronil 50 mg/ml) drops used against fleas and mites for dogs contain Fipronil 50 mg/ml, diflubenzuron 1 mg/ml, and dicarboximide (MGC-264) 5 mg/ml. It is appropriate for dogs from the age of 8 weeks for the treatment and prevention of entomoses (lice, fleas, and withers) and sarcoptic disease. It is applied as 1 dropper pipette (1.4 ml) per 10 kg of the animal weight. Safeline® (phenylpyrazole–garlic–camphor mix) spray (1 l) is a fast-acting, long-lasting, waterproof treatment. Herbal mix eco-friendly Safeline® spray is used for the control of fleas, ticks, and chewing lice in dogs. It is composed of phenylpyrazole (Fipronil), garlic oil, camphor oil, and vehicle. In-vitro effectiveness of commercial insecticidesThe in-vitro effectiveness of the three commercial insecticides was tested as recommended by Balcioğlu et al. (2015) and Kiesewetter et al. (2013) using direct exposure of Rhipicephalus sanguineus ticks to the recommended (by the manufacturer) dose and concentration of the tested insecticides. Rhipicephalus sanguineus ticks were obtained from naturally parasitized dogs and maintained in the laboratory. A total of 216 developmental stage R. sanguineus (72 adults, 72 larvae, and 72 eggs) were divided into replicates of three petri dishes each (3 plates × 8 units × 3 stages/replicate). Each replicate of three plates was exposed to 1 ml of the recommended concentration of each insecticide. The different developmental stages of R. sanguineus ticks were recorded for survival after contact times of 0, 2, 4, 8, 16, and 24 hours. After each contact time, the number of surviving ticks and intact eggs was recorded and the killing efficiencies were calculated and expressed as a percentage (%) using the following formula:
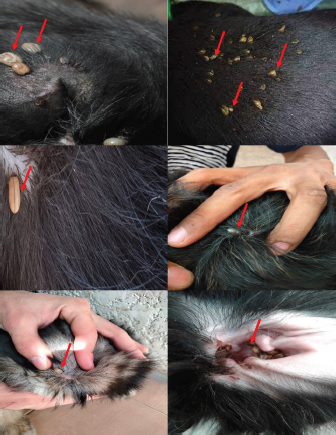
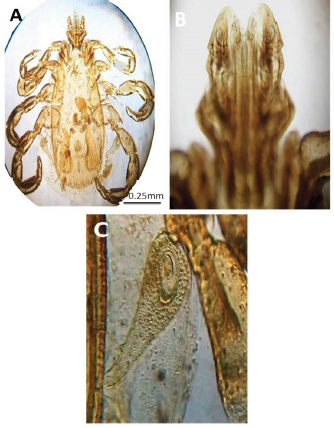
Experimental dogs’ housing microclimateThe laboratory animal units were adjusted prior to housing the dogs to allow biosecurity measures to maintain their health and protection according to Soliman and Abdallah (2020). These biosecurity measures included foot dips supplied with commercial phenol 5% at the entrance of the units, break-back traps to discourage the entrance of rodents, clean feeding, watering bowls, fly proof nets, safe feed storage area, and proper drainage of the units for the removal of the wastes daily. The laboratory animal units were provided with V-shaped side wall windows, ceiling fans, and sidewall suction fans to stimulate the air movement and ventilation act of the rooms that was based on cross-ventilation. Rooms were supplied with white LED lights of 18 watts to be consumed in a continuous lighting regimen following Soliman and Hassan (2019). The floor of the rooms was cleaned sufficiently using hypochlorites and glutaraldehyde, as well the drainage area was lined with slaked lime to achieve maximum cleanliness, dryness, and durability according to Soliman et al. (2018). Experimental animals’ managementSixteen Rottweiler male dogs aged 1 year and 45.5 kg body weight were purchased from a commercial pet shop in Ismailia, Egypt. The animals were handled in human manners, considering all the national and international ethical guidelines in treating the animals. The synchronization in the selected dogs was important to minimize the influences of the physiological variation between the members of each group on the responses to the insecticides used for the treatments (Taktak et al., 2021). Dogs on their arrival were double-checked for their physical conditions and fitness, as well as some random blood samples were collected in a human manner using a local anesthetic to check any signs of internal or external parasitism. Dogs were divided into four groups and placed in four separate previously prepared rooms. The rooms were optimized 24 hours a day at a microclimatic temperature of 37°C using halogen heaters according to Soliman et al. (2021). Dogs were given ad libitum access to clean tap water that was previously stored for de-chlorination, as well as provided with balanced feed twice a day per dog in a routine manner to maintain a healthy lifestyle and prevent the stress that arises from starvation or underfeeding. The first meal was provided in the morning and contained a mix of bread, rice, pasta, boiled well-done chickens, well-cooked liver, and minced meat. The second meal was provided in the evening and contained three-quarter kilogram of commercial dry food with 22%–23% crude protein for each dog. Dogs on their arrival were dewormed as a routine monthly preventive action using praziquantel (5 mg/kg body weight) and fenbendazole (50 mg/kg body weight). Dogs were vaccinated with a booster dose of Vanguard® Plus 5 DHLPPC vaccine (Zeotis® US) against canine parvovirus, canine distemper, parainfluenza, canine adenovirus type I and II, canine hepatitis, leptospirosis, and coronavirus. Dogs were also vaccinated against rabies. The two shots were administered subcutaneously in the back of the neck. Experimental infestation of dogsTicks were collected from naturally parasitized dogs admitted to a veterinary clinic in Ismailia, Egypt, into screw-capped bottles. The bottles were transferred to the laboratory as quickly as possible. In the laboratory, female ticks were identified morphologically and by light microscopy (LM) (10×), then maintained in an incubator at 25°C and 75% relative humidity for 1–2 weeks until they laid eggs. Hatching was encouraged by optimizing microclimatic temperature and relative humidity until the larvae were obtained. The different developmental stages were used for the in-vitro trials. The identified R. sanguineus ticks (five females per dog) were used to infest three out of the four experimental groups (G1, G2, and G3) at the beginning of the second stage of the in-vivo study period (3–4 weeks post-dog arrival) and the fourth group was kept as the control. The dogs of the three infested groups were kept under observation for 2 weeks until the development of ticks up to the adult stage on the animals’ skin. One or two of the infesting ticks were harvested for more confirmation by LM (10×). SamplingA total of 144 samples (3 samples × 16 dogs × 3 types of samples) including 48 ethylenediaminetetraacetic acid (EDTA) blood, 48 whole blood, and 48 sera samples were collected. The samples were transferred to the laboratory in a dry-ice box as quickly as possible. EDTA blood samples on EDTA vacutainers (VACUETTE® TUBE 5 ml K3EDTA 13 × 100 lavender cap-black ring, PREMIUM) were used for hematological analysis. Whole blood samples in erythrocyte sedimentation rates (ESR) tubes (ESR-Vacuum Tubes 1.2 ml for use with the ESR-Auto Plus® and ESR-10 Manual Rack) were examined for the ESR within 1 hour. Sera blood samples on plain serum tubes (BD vacutainer® Serum tubes, 10.0 ml, 16 × 100 mm, Plastic, Additive: Clot Activator, Silicone-Coated, Red Conventional Closure, and Paper Label) were held in a water bath (Thermo® water bath Precision series Standard, 20, 30°C–100°C, 392 mm, GP20) at 25°C for 30 minutes and centrifuged at 3,500 rpm for 15 minutes. Clear sera were collected and pipetted using an automatic pipette (Fisherbrand®, variable 200:1,000 micron) into 2.5 ml capacity Eppendorf tubes and stored at −20°C until tested for biochemical and stress marker assay (Soliman et al., 2017). Hematological profileEDTA blood samples were collected (48 from 4 groups of 4 dogs) and examined for the following hematological parameters: red blood cells count (RBCs, ×106/µl), hemoglobin concentrations (Hb, g/dl), hematocrit (HCT, %), mean corpuscular hemoglobin concentrations (MCHC, g/dl), platelet counts (PLT, ×103/µl), white blood cells count (WBCs, ×103/µl), neutrophils (N, %), lymphocytes (L, %), monocytes (M, %), eosinophil (E, %), and basophils (B, %) using Sysmex XP-300 Automated Hematology Analyzer. Whole blood samples were collected (48 from 4 groups of 4 dogs) and examined for ESR (mm/hour) using STATTM PLUS Automated ESR Analyzer. Biochemical profileSera samples were collected (48 from 4 groups of 4 dogs) and examined for the following biochemical parameters: total protein (TP) and expressed as g/dl, alanine aminotransferase (ALT) and expressed as IU/L, urea (UREA) and expressed as mg/dl, creatinine (CREAT) and expressed as mg/dl, glucose (GLUCO) and expressed as mg/dl, total cholesterol (TC) and expressed as mg/dl, and triglycerides (TG) and expressed as mg/dl calorimetrically using ROCHE COBAS Integra 800 chemical analyzer. CORT hormone (mcg/dl) and immunoglobulin G and M concentrations (IgG and IgM, mg/dl) were measured by using ROCHE Elecsys 1010 Immunoassay Analyzer (Wu et al., 2017). Statistical analysisThe statistical analysis was conducted using a statistical Statistical Package for the Social Sciences (SPSS) software version 20 (IBM Corp, 2016—IBM SPSS Statistics 20). Data were analyzed statistically using multifactorial analysis of variance to estimate the effect of insecticides treatments in infested dogs against sampling time. The influence of insecticides treatments on the prevalence of infestations and sampling times and their interactions were displayed in the results tables. The statistical model utilized the following formula: Yijk=µ + αi+ βj + (αβ)ij+ Ɛijk where Yijk is the measurement of the dependent variables; µ is the overall mean; αi is the fixed effect of the different treatments (insecticides), βj is the fixed effect of sampling time, (αβ)ij is the interactions of treatments by sampling time, and Ɛijk is the random error. The killing efficiencies in the in-vitro trials were calculated and expressed as percentage (%). Nonparametric Kruskal–Wallis test was used to determine the significant differences between the reduction percentages. The results were expressed as highly significant at p ≤ 0.01, significant at p ≤ 0.05, and nonsignificant at p > 0.05. Ethical approvalThe study design and animal management system were approved by the Scientific Research Ethics Committee on Animal and poultry researches, Faculty of Veterinary Medicine, Suez Canal University, Egypt, with approval number (2021028). The animals in the current study were handled in a professional way to meet the national and international regulations for experimental animals’ care. Dogs were received and housed in proper housing rooms to meet their requirement with the satisfaction of all the nutritional needs according to the regulation. Infesting animals with R. sanguineus ticks was carried out with a small number of female ticks to minimize the animals’ suffering until treatment with the three appointed insecticides (Doramectin, Fipronil 50 mg/ml, and phenylpyrazole–garlic–camphor mix). The samples’ collection was carried with complete care and the use of local anesthetic to overcome pain during samples collection from animals. ResultsIn-vitro efficacy of chemical and herbal insecticidesThe in-vitro efficacy of the three tested insecticides in Figure 1 show highly significant (p < 0.01) increases in the killing efficiencies as Doramectin (Dectomax®) achieved up to 95.8% after 2 hours and 100% killing efficacy after 8 hours. Fipronil 50 mg/ml (Bars®) achieved up to 91.0% after 2 hours and 100% killing efficacy after 8 hours. Phenylpyrazole–garlic–camphor mix (Safeline®) achieved up to 58.3% after 2 hours and 100% killing efficacy after 24 hours. Experimental infestation of dogs and clinical examinationsRottweiler dogs are shown in Figure 2 during the second stage of the in-vivo experiment (third to fourth week) with high infestation rates with ticks (R. sanguineus). The successful infestation was impacted by the development of a large number of adult ticks on the animal’s skin in the three challenged experimental groups. The LM examination shows in Figure 3 the characterized shape of R. sanguineus (Fig. 3A), the hexagonal shape of the basis capituli (Fig. 3B), and the comma-shaped spiracular plate (Fig. 3C). The clinical examinations of the experimentally infested dogs revealed irritation, lethargy, loss of appetite, fever, swollen lymph nodes, swollen legs, paralysis, shifting leg lameness, and the presence of different developmental stages of ticks on the dogs’ skin.
Fig. 1. In-vitro killing efficiency (%, mean ± SE) of the tested insecticides on ticks.
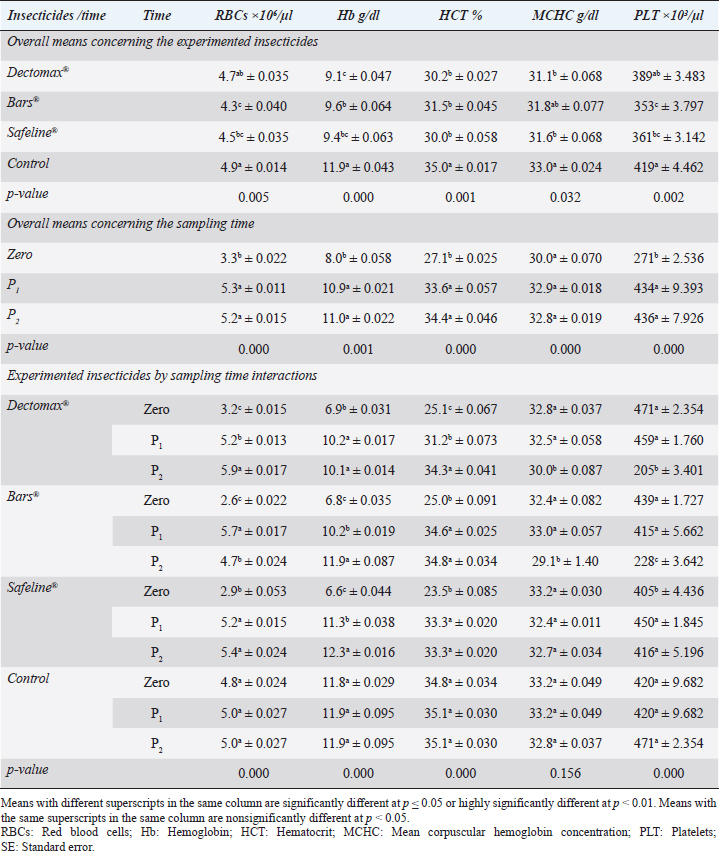
Fig. 2. Photographs of the experimental dogs after experimental infestations. Red arrows point to the ticks. Hematological profile of dogsThe hematological analysis reveals in Table 1 highly significant (p < 0.01) reductions in the overall means of RBCs, HCT, MCHC, and PLT in all groups treated with Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®) compared to the control with more concern toward the phenylpyrazole–garlic–camphor mix (Safeline®). The overall means concerning sampling time reveal in Table 1 highly significant (p < 0.01) reductions in all the measured hematological parameters in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2) with more pronounced improvement in the hematological parameters in the 4-week posttreatment samples.
Fig. 3. LM pictures of (A) R. sanguineus, (B) hexagonal shape of the basis capituli, and (C) comma-shaped spiracular plate. Table 1. Hematological profile (mean ± SE) of infested dogs treated with different experimented insecticides.
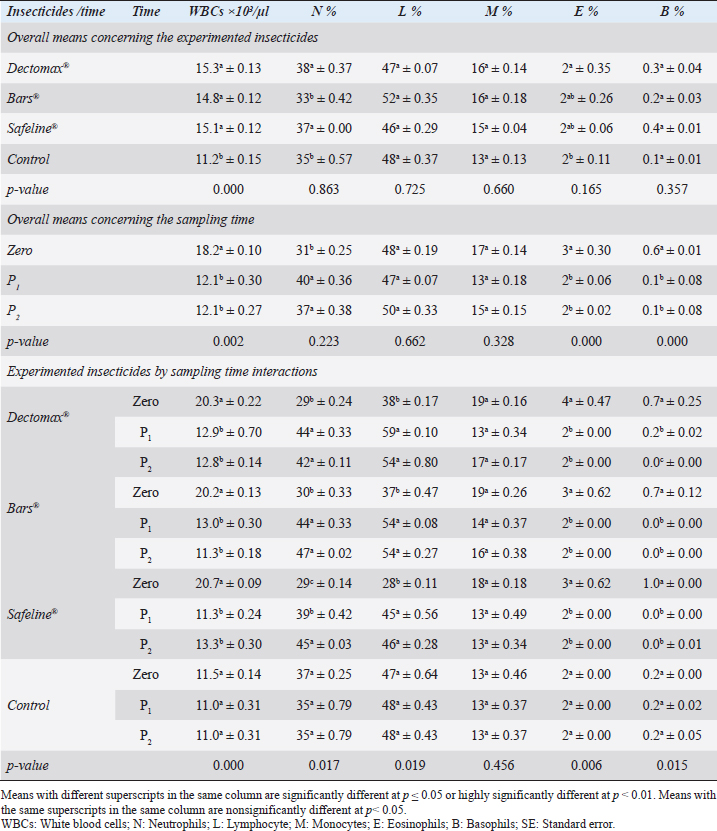
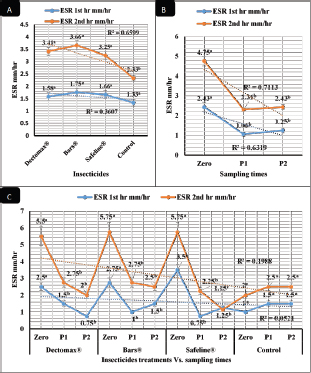
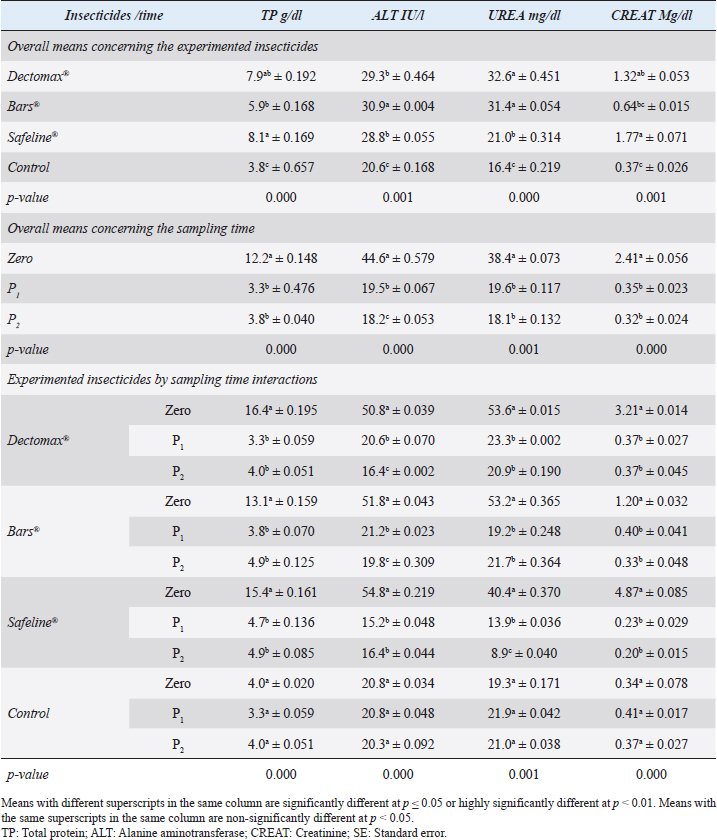
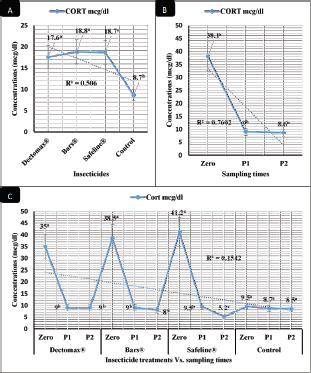
RBCs and HCT interactions in infested dogs treated with Doramectin (Dectomax®) shown in Table 1 reveal highly significant (p < 0.01) increases through all the posttreatment sampling times, while Hb, MCHC, and PLT interactions reveal highly significant (p < 0.01) increases in P1 and P2 with no significant between the two sampling times. Fipronil 50 mg/ml (Bars®) and phenylpyrazole–garlic–camphor mix (Safeline®) reveal highly significant (p < 0.01) increases in the hematological parameters with no significant difference between P1 and P2 samples of HCT, MCHC, and PLT in Bars® and of RBCs, HCT, and MCHC in Safeline®-treated dogs. The total and differential leukocytic counts in Table 2 reveal highly significant (p < 0.01) increases in the overall means of WBCs and N in all groups treated with Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®) compared to the control. Lymphocytes, M, E, and B reveal no significant differences in all dog groups treated with Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®) compared to the control. The overall means concerning sampling time in Table 2 reveal highly significant (p < 0.01) reductions in WBCs, E, and B in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2). Lymphocytes and M reveal no significant differences between the three sampling times. Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®) treatment interactions in Table 2 reveal highly significant (p < 0.01) reductions in WBCs, E, and B, as well as highly significant increases in N and L, and no significant differences in M. Erythrocyte sedimentation rateESR (in the first hour) in Figure 4A show no significant differences between the groups, while highly significant (p < 0.01) increases were recorded in ESR in the second hour with no significant differences between the three dog groups compared to the control. The overall means concerning sampling time in Figure 4B show highly significant (p < 0.01) reductions in ESR in the first hour and ESR in the second hour in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2). The ESR first- and second-hour interactions in Figure 4C show highly significant (p < 0.01) reductions in Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs, especially concerning the strong action of phenylpyrazole–garlic–camphor mix (Safeline®) that reveals highly significant (p < 0.01) reductions between the first- and second-hour ESR. Biochemical profile of dogsThe overall mean shown in Table 3 indicates highly significant (p < 0.01) increases in TP and CREAT in phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs and ALT and UREA in Fipronil 50 mg/ml (Bars®)-treated dogs compared to the other treated groups and control. The overall means concerning sampling time in Table 3 shows highly significant (p < 0.01) reductions in TP, ALT, UREA, and CREAT in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2) in TP, UREA, and CREAT. The results indicate highly significant (p < 0.01) reductions in TP with no significant differences between the two sampling times (P1 and P2); ALT and UREA with no significant differences between the two sampling times (P1 and P2); and CREAT with no significant differences between the two sampling times (P1 and P2). The GLUCO and lipid profile overall means of the treated dogs shown in Table 4 indicate highly significant (p < 0.01) increases in Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs compared to the control. The overall means concerning sampling time shown in Table 4 indicate highly significant (p < 0.01) reductions in GLUCO, TG, and TC in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2). The data shown in Table 4 reveal highly significant (p < 0.01) reductions in GLUCO (Table 4) with no significant differences between the two sampling times (P1 and P2), TG, and TC with no significant differences between the two sampling times (P1 and P2) in Doramectin (Dectomax®) and Fipronil 50 mg/ml (Bars®)-treated dogs. Stress markerThe CORT overall means shown in Figure 5A reveal highly significant (p < 0.01) increases in Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs compared to control with no significant differences between the three groups. The overall means concerning sampling time shown in Figure 5B indicate highly significant (p < 0.01) reductions in CORT levels in the 2-week (P1) and 4-week posttreatment (P2) samples compared to the zero-time samples with no significant differences between the two sampling times (P1 and P2). The data shown in Figure 5C reveal highly significant (p < 0.01) reductions of CORT levels with no significant differences between the two sampling times (P1 and P2) in Doramectin (Dectomax®) and Fipronil 50 mg/ml (Bars®)-treated dogs. DiscussionDog–human interrelationships provide opportunities for enhancing exercise, minimizing stressful events, and developing empathy. The infestation of dogs with external parasites like ticks can be considered a threat to both companions for their abilities to be transmitted easily, as well as for carrying many zoonotic diseases. The control of the external parasites infesting companion animals like dogs and cats is critical for their wellbeing, as control and eradication regimen of infesting external parasites in companion animals like dogs protects not only dogs but also humans from many infectious and zoonotic diseases that can be transmitted by these organisms (Barker and Wolen, 2008; Berg et al., 2021). Table 2. Total and differential leukocytic counts (mean ± SE) of infested dogs treated with different experimented insecticides.
Ticks like R. sanguineus widely infest dogs and their kennels (Medlock et al., 2013; Mahai et al., 2021). Ticks are known to be abundant ectoparasite that can infest a wide range of animals including humans. Once infestation takes place, the deterioration actions to the host start via sucking blood, transmitting some pathogens like bacteria, virus, and blood protozoans, and contributing to numerous infectious and zoonotic diseases (Eppleston et al., 2013). Ticks are known to produce a neurotoxin in their salivary secretions following their attachment to the host within 48–72 hours, risking paralysis that might cause deaths (Padula, 2016). Other clinical manifestations include hard respiration, flaccid paralysis, and respiratory failure that contribute eventually to death (Gray et al., 2016).
Fig. 4. Erythrocyte sedimentation (mean ± SE) of infested dogs treated with different experimented insecticides. (A) ESR overall means concerning experimental groups. (B) ESR overall means concerning sampling times. (C) ESR means concerning experimental groups by time interactions. Chemical insecticides have been considered as one of the traditional means used for a long time for insect control, despite the diverse ill effects they might contribute (Singh, 2010). The use of these insecticides has been investigated for the nontarget influence, resistance build-up, and long residual actions (Saeed et al., 2016; Pisa et al., 2021). Herbal eco-friendly insecticides have been researched to overcome all the disadvantages caused by chemical insecticides (Abd-Ella, 2013; Gil et al., 2015; Abd-Ella, 2016; Nettles et al., 2016). The systemic action of insecticides is important to minimize or completely eradicate external parasites like ticks and their vector-borne diseases like Babesia canis, Anaplasma phagocytophilum, and Ehrlichia canis (Honsberger et al., 2016; Jongejan et al., 2016). The current results showed a significant in-vitro efficiency of the three tested insecticides against R. sanguineus with 100% killing efficiency achieved after 8 hours in Doramectin (Dectomax®) and Fipronil 50 mg/ml (Bars®) and 24 hours in phenylpyrazole–garlic–camphor mix (Safeline®). The high insecticidal actions of the three tested insecticides are related to their deep structure and broad-spectrum neurological action on the insect. The same results were recorded by Taenzler et al. (2018), Becskei et al. (2016), Beugnet et al. (2016), Cherni et al. (2016), and Six et al. (2016) who determined the broad-spectrum action of many chemically based insecticides on R. sanguineus ticks, fleas of Ctenocephalides species, mites of Demodex species, sarcoptic mange from Sarcoptes scabiei, and ear mites from Otodectes cynotis. Padula et al. (2020) reported the importance of using effective antisera against ticks for obtaining full protection against their infestations in companion animals like dogs. Poché et al. (2017) recorded that insecticides containing Fipronil 50 mg/kg were able to reduce ectoparasitic infestations up to 95% from their original load after a single time treatment. Dolan et al. (2017) also recorded the ability of Fipronil in reducing ticks up to 76% following 9–17 weeks of treatment. At the chemical structure level, Doramectin (Dectomax®) is a macrocyclic lactone that acts as reported by Page (2018) by modulating chloride ion channel activity in the nervous system of nematodes and arthropods. Macrocyclic lactones bind to receptors that increase membrane permeability to chloride ions. This inhibits the electrical activity of nerve cells in nematodes and muscle cells in arthropods and causes paralysis and the death of the parasites. Fipronil 50 mg/ml (Bars®) is based on its chemical structure of Fipronil, diflubenzuron, and dicarboximide. Fipronil, according to Bonmatin et al. (2015), causes blocking of the GABA-dependent receptors of ectoparasites, impairing the transmission of nerve impulses, which leads to paralysis and death of ectoparasites. Diflubenzuron, as reported by Sankar and Kumar (2021), acts by inhibiting the synthesis of chitin in parasites and disrupting the molting, egg-laying, and hatchery process, which leads to an end to the replenishment of the population. Dicarboximide is a synergist and is used with insecticides to increase their activity. Dicarboximide, as reported by d’Errico et al. (2017), stops microsomal detoxification of the insecticide, increasing its toxicity for the parasite. The extensive use of chemical insecticides lead to resistance against these compounds in many of the arthropods and external parasites, and that is why new lines of insecticides had to be developed. This resistance is multifactorial and usually can be developed by several means through behavioral, biochemical, and metabolic ways (Cossío-Bayúgar et al., 2018; Thangam and Kathiresan, 2021). That is why molecular markers have been developed to target the nucleotide sequence and genes responsible for this resistance as sodium channels, acetylcholinesterase, carboxylesterase, β-adrenergic octopamine receptor, and octopamine–tyramine in the insect as reported by Kumar (2019) and Aguilar et al. (2018). Table 3. Liver and kidney functions (mean ± SE) of infested dogs treated with different experimented insecticides.
Herbal insecticides and acaricides include pyrethroids, neem, and various essential oils, as well as newly based insecticides, such as phenylpyrazole–garlic–camphor mix (Safeline®) that was used in our experiment. Although the herbal insecticide delayed in achieving 100% killing efficacy compared to the other two chemical insecticides, its safety is higher compared to other insecticides. Khare et al. (2019) revealed that the phenylpyrazole–garlic–camphor mix (Safeline®) disrupts the insect central nervous system by blocking GABA-gated chloride channels and GluCl channels. This causes hyperexcitation of contaminated insects’ nerves and muscles. Garlic and camphor oils reported by Krishnananda et al. (2017) and Shaji et al. (2017) inhibit the release of acetylcholinesterase which is essential for insects for their activity and synaptic transmission and act on octopamine (circulating neuromodulator) causing disruption and a complete breakdown of the nervous system in insects, and they are hydrophobic and cause water stress in insects by blocking the spiracles resulting in suffocation and distressing the cuticular waxes. Table 4. GLUCO and lipid profile overall means (mean ± SE) of infested dogs treated with different experimented insecticides.
Doramectin (Dectomax®) is a semisynthetic avermectin, and when administered repetitively, it can diffuse to tissues and contribute to mild toxicity, hepatic toxicity, renal toxicity, and central nervous system depression (Zhang et al., 2016). Usually, it can be deposited in adipose tissue, interfere with hepatocellular activity causing lipid peroxidation and degradation, and suppress the glutathione enzymatic system activity, contributing to the suppression of proliferating cell nuclear antigen (Venkateswarlu et al., 2017). That is why the use of such synthetic insecticides should not be repeated without cause. Care also should be given to the dose and concentration used, whether it is used for prophylaxis or treatment. Doramectin (Dectomax®) is usually recommended in monthly doses for prophylaxis and one dose for treatment as reported by Foy et al. (2019). It is also recommended to use avermectins by injection route to avoid stomach upset and vomiting (Barrett et al., 2016). The in-vivo-tested insecticides were applied as recommended by the manufacturer to the infested dogs using different means of applications: injection in Doramectin (Dectomax®), pour-on in Fipronil 50 mg/ml (Bars®), and spraying in phenylpyrazole–garlic–camphor mix (Safeline®). Despite the differences in applications between the tested insecticides, they were able to act efficiently and maintain the physiological functions of the treated dogs instead of causing physiological alterations and disturbances. The problem with using the insecticides, in general, was their low impacts on the environmental components (Al-Awthan et al., 2012). Abbassy et al. (2014) and Ali et al. (2017) stated that many synthetic insecticides such as Doramectin (Dectomax®) and pour-on in Fipronil 50 mg/ml (Bars®) contributed to extensive influences and accumulation in some environmental components, as well biochemical and hormonal alterations in animals (Nasr et al., 2016). That is why the synthetic insecticides were recommended to be used once as in the current experiment despite infestation. Desai et al. (2016) recommended that prophylactic applications are not recommended by using chemical and organic insecticides. On the other hand, Mossa et al. (2017) reported that natural insecticides such as phenylpyrazole–garlic–camphor mix (Safeline®) in the current study are much recommended for animal treatment for maintaining animal physiological functions and their low impact on the environmental components.
Fig. 5. CORT stress marker (mean ± SE) of infested dogs treated with different experimented insecticides. (A) CORT overall means concerning experimental groups. (B) CORT overall means concerning sampling times. (C) CORT overall means concerning experimental groups by time interactions. The in-vivo results reveal significant improvements of hematological profile (RBCs, HCT, MCHC, PLT, and total and differential leukocytic counts), ESR in the second hour, biochemical profile (TP, CREAT, ALT, UREA, GLUCO, TG, and TC), and CORT levels of the 2-week (P1) and 4-week posttreatment (P2) samples in the infested dogs, considering that the evaluation was carried out for a month after the treatments. These results are in agreement with those of El-Naggar et al. (2017) who reported no adverse effects of the experimented bio-insecticides on the histopathological and physiological functions of liver, kidney, and cerebellum in male Albino mice. Marchiondo et al. (2013, 2019) also reported the necessity to assess the long-term effectiveness of the tested insecticide with intervals of 7–31 days. Segev et al. (2018) also advocate spot-on and injectable doramectin protocols as effective in treating 10 dogs from infestation with some internal and external parasites. Benelli et al. (2017b) recorded that doramectin was efficient and able to enforce good residual action for 63 days during which no external parasitism was noticed on animals. Wanji et al. (2017) reported a significant reduction in microfilaria count and improvement in hematological and biochemical parameters following treatment of external parasitic infestations using ivermectin. Benelli et al. (2016) reported the ability of some chemical insecticides as doramectin to treat animals infested with ticks with significant improvement of the hematological and biochemical profiles of the animals. Benelli et al. (2017a) and Nicoletti et al. (2016) experimented with neem, and Pavela and Benelli (2016) experimented with essential oils as a herbal eco-friendly insecticide related to the phenylpyrazole–garlic–camphor mix (Safeline®) used in our study and they found its actions to be superior to the ideal insecticides in eradication programs of tick infestations. The maintained levels of ALT posttreatment in the current experiment excluded any sign of the development of stress conditions in line with Ahmed et al. (2020) and Khalil and Samrah (2018) who recorded the possible prophylactic effect of insecticides like ivermectin when used in a single dose. They also recommended the use of some herbal eco-friendly alternatives to avoid the alterations in the physiological functions that might occur after treatment. ConclusionThe study revealed the significant in-vitro efficacy of chemicals like Doramectin (Dectomax®) and Fipronil 50 mg/ml (Bars®), and herbal eco-friendly insecticides like the phenylpyrazole–garlic–camphor mix (Safeline®) against R. sanguineus with 100% killing efficacy that was achieved after 8 hours in both Doramectin (Dectomax®) and Fipronil 50 mg/ml (Bars®) and 24 hours in phenylpyrazole–garlic–camphor mix (Safeline®). The insecticidal treatments for infested dogs achieved significant improvements in hematological parameters like RBCs, HCT, MCHC, total and differential leukocytic counts, and ESR, as well biochemical and hormonal profiles, like TP, CREAT, ALT, UREA, GLUCO, TG, TC, and CORT levels in the 2-week (P1) and 4-week posttreatment (P2) samples in Doramectin (Dectomax®), Fipronil 50 mg/ml (Bars®), and phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs with more pronounced recovery in phenylpyrazole–garlic–camphor mix (Safeline®)-treated dogs. Herbal insecticides like phenylpyrazole–garlic–camphor mix (Safeline®) that are based on camphor oil and garlic and according to the current results might be recommended with a higher incidence for prophylaxis and treatment against external parasites’ infestations for their natural contents that provide extended actions, safe use, lower impact on the environment, effective actions, and lower toxicity to the animals compared to the chemical insecticides like Doramectin (Dectomax®, Macrocyclic lactones) and Fipronil 50 mg/ml (Bars®, a mix of Fipronil, diflubenzuron, and dicarboximide). AcknowledgmentThe authors sincerely and honorably thank Prof. MA Sobieh for his guidance and help in summarizing the work design. The current study received no grant from any funding agency in the public and commercial fields and not-for-profit sectors. The authors funded the study. Conflict of interestThe authors declare that they have no financial or personal conflicts which may have inappropriately influenced them in writing this manuscript. Authors’ contributionsESS designed the in-vitro and in-vivo experimental designs, executed the experiment, conducted biochemical analysis, and took a part in writing the manuscript. EMA participated in conducting the in-vivo experiment, conducted hematological analysis, and took a part in writing the manuscript. EMA supervised the tick collection used for both in-vitro and in-vivo experiments, induced the experimental infestation of dogs, and took a part in writing the manuscript. MAS directed the team and group work, supervised the experiments, and took a part in writing the manuscript. ReferencesAbbassy, M.A., Marei, A.E.S.M., Al-Ashkar, M.A.M. and Mossa, A.T.H. 2014. Adverse biochemical effects of various pesticides on sprayers of cotton fields in El-Behira Governorate, Egypt. Biomed. Aging Pathol. 4(3), 251–256. Abd-Ella, A.A. 2013. Toxicity and persistence of selected neonicotinoide insecticides on cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae). Arch. Phytopathol. Plant Prot. 47(3), 366–376. Abd-Ella, A.A. 2016. Evaluation of certain neonicotinoid insecticide seed treatments against cereal aphids on some wheat cultivars. J. Phytopathol. Pest Manag. 3(1), 21–33. Aguilar, G., Olvera, A.M., Carvajal, B.I. and Mosqueda, J. 2018. SNPs and other polymorhisms associated with acaricide resistance in Rhipicephalus microplus. Front. Biosci. Landmark, 23(1), 65–82. Ahmed, A.E., Alshehri, A., Al-Kahtani, M.A., Elbehairi, S.I., Alshehri, M.A., Shati, A.A., Alfaifi, M.Y., Al-Doais, A.A., Taha, R., Morsy, K. and El-Mansi, A.A. 2020. Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar Albino rats. Biomed. Pharmacother. 124, 109841; doi:10.1016/j.biopha.2020.109841 Al-Awthan, Y.S., Al-Douis, M.A., El-Sokkary, G.H. and Aqlan, E.M. 2012. Dimethoate-induced oxidative stress and morphological changes in the liver of guinea pig and the protective effect of vitamin C and E. Asian J. Biol. Sci. 5(1), 9–19. Ali, S., Zhang, C., Wang, Z., Wang, X.M., Wu, J.H., Cuthbertson, A.G.S., Shao, Z. and Qiu, B.L. 2017. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 7(1), Article ID 46558; doi:10.1038/srep46558 Apanaskevich, D.A. and Apanaskevich, M.A. 2016. Description of two new species of Dermacentor Koch, 1844 (Acari: Ixodidae) from Oriental Asia. Syst. Parasitol. 93(2), 159–171. Apanaskevich, D.A., Chaloemthanetphong, A., Vongphayloth, K., Ahantarig, A., Apanaskevich, M.A., Brey, P.T., Hertz, J.C., Lakeomany, K., Sutherland, I.W. and Trinachartvanit, W. 2019. Description of a new species of Dermacentor Koch, 1844 (Acari: Ixodidae) from Laos and Thailand. Syst. Parasitol. 96(2), 1–10. Balcioğlu, I.C., Mehmet, K., Arserim, S.K., Limoncu, M.E., Tőz, S., Baştemur, S., Őncel, K. and Őzbel, Y. 2015. Comparing the efficacy of commercially available insecticide and dimeticone based solutions on head lice, pediculus capitis: in vitro trials. Turk. Parazitol. Derg. 39(4), 305–309. Barker, S.B. and Wolen, A.R. 2008. The benefits of human-companion animal interaction: a review. J. Vet. Med. Educ. 35(4), 487–495. Barrett, J., Broderick, C., Soulsby, H., Wade, P. and Newsholme. W. 2016. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: two case reports and a discussion of the literature. J. Antimicrob. Chemother. 71(1), 220–225. Becskei, C., De Bock, F., Illambas, J., Cherni, J.A., Fourie, J.J., Lane, M., Mahabir, S.P. and Six, R.H. 2016. Efficacy and safety of a novel oral isoxazoline, sarolaner (SimparicaTM), for the treatment of sarcoptic mange in dogs. Vet. Parasitol. 222, 56–61. Benelli, G., Canale, A., Toniolo, C., Higuchi, A., Murugan, K., Pavela, R. and Nicoletti, M. 2017a. Neem (Azadirachta indica): towards the ideal insecticide? Nat. Prod. Res. 31(4), 369–386. Benelli, G., Caselli, A., Giuseppe, G.D. and Canale, A. 2017b. Control of biting lice, Mallophaga- a review. Acta Trop. 177, 211–219. Benelli, G., Pavela, R., Canale, A. and Mehlhorn, H. 2016. Tick repellents and acaricides of botanical origin: a green roadmap to control tick-borne diseases? Parasitol. Res. 115(7), 2545–2560. Berg, H.V., Velayudhan, R. and Yadav, R.S. 2021. Management of insecticides for use in disease vector control: lessons from six countries in Asia and the Middle East. PLoS Negl. Trop. Dis. 15(4), e0009358; doi:10.1371/journal.pntd.0009358 Beugnet, F., Halos, L., Larsen, D. and de Vos, C. 2016. Efficacy of oral afoxolaner for the treatment of canine generalized demodicosis. Parasite 23, 14; doi:10.1051/parasite/2016014 Bonmatin, J.M., Giorio, C., Girolami, V., Goulson, D., Kreutzweiser, D., Krupke, C., Liess, M., Long, E., Marzaro, M. and Mitchell, E.A. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22(1), 35–67. Chen, T., Yu, X., Tian, X., Hu, J., Chen, Y., Long, G., Xu, H. and Yang, G.F. 2022. Study on the environmental fate of three insecticides in garlic by in vivo sampling rate calibrated-solid phase microextraction-gas chromatography-mass spectrometry. Food Chem. 367, 130740; doi:10.1016/j.foodchem.2021.130740 Cherni, J.A., Mahabir, S.P. and Six, R.H. 2016. Efficacy and safety of sarolaner (SimparicaTM) against fleas on dogs presented as veterinary patients in the United States. Vet. Parasitol. 222, 43–48. Cochi, S., de Quadros, C., Dowdle, W., Goodman, R., Ndumbe, P. and Salisbury, D. 1998. Post-Conference small group report. In global disease elimination and eradication as public health strategies. Eds., Goodman, R.A., Foster, K.L., Trowbridge, F.L. and Figueroa J.P. Bulletin of the World Health Organization, vol. 76, no. Suppl. 2, p 113. Cossío-Bayúgar, R., Martínez-Ibañez, F., Aguilar-Díaz, H. and Miranda-Miranda, E. 2018. Pyrethroid acaricide resistance is proportional to P-450 cytochrome oxidase expression in the cattle tick Rhipicephalus microplus. Biomed. Res. Int. Article ID: 8292465; doi:10.1155/2018/8292465 De Leeuw, W. The council of Europe. In Proceedings of an ILAR international workshop, 2003 Nov 15–17, Washington, DC, 2003. d’Errico, G., Giacometti, R., Roversi, P.F., d’Errico, S. and Woo, S.L. 2017. Mode of action and efficacy of iprodione against the root-knot nematode Meloidogyne incognita. Ann. Appl. Biol. 171(3), 506–510. Desai, K.R., Moid, N., Patel, P.B. and Highland, H.N. 2016. Evaluation of Deltamethrin induced reproductive toxicity in male Swiss Albino mice. Asian Pac. J. Reprod. 5(1), 24–30. Dolan, M.C., Schulze, T.L., Jordan, R.A., Schulze, C.J., Ullmann, A.J., Hojgaard, A., Williams, M.A. and Piesman, J. 2017. Evaluation of doxycycline-laden oral bait and topical fipronil delivered in a single bait box to control Ixodes scapularis (Acari: Ixodidae) and reduce Borrelia burgdorferi and Anaplasma phagocytophilum infection in small mammal reservoirs and host-seeking ticks. J. Med. Entomol. 54(2), 403–410. Elbrense, H., El Husseiny, I., Abo El makarem, H., Abo Arab, R. and El Kholy, S. 2022. Insecticidal, Antifeedant and repellent efficacy of certain essential oils against adult rust-red flour beetle, Tribolium castaneum. Egyptian J. Chem. 65(1), 167–178. El-Naggar, S.A., Eltantawi, H., Ibrahim, M.A. and Alm-Eldeen, A. 2017. Assessment of the toxicity of sub-chronic low and high doses of the bio-insecticide spinosad on the liver, kidney and the cerebellum in male albino mice. Braz. Arch. Biol. Technol. 60, e17160179; doi:10.1590/1678-4324-2017160179 Eppleston, K.R., Kelman, M. and Ward, M.P. 2013. Distribution, seasonality and risk factors for tick paralysis in Australian dogs and cats. Vet. Parasitol. 196, 460–468. Foy, B.D., Alout, H., Seaman, J.A., Rao, S., Magalhaes, T., Wade, M., Parikh, S., Soma, D.D., Sagna, A.B., Fournet, F., Slater, H.C., Bougma, R., Drabo, F., Diabaté, A., Coulidiaty, A.G.V., Rouamba, N. and Dabiré, R.K. 2019. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet 393, 1517–1526. Gil, F.N., Moreira-Santos, M., Chelinho, S., Pereira, C., Feliciano, J.R., Leitão, J.H., Sousa, J.P., Ribeiro, R. and Viegas, C.A. 2015. Suitability of a Saccharomyces cerevisiae-based assay to assess the toxicity of pyrimethanil sprayed soils via surface runoff: comparison with standard aquatic and soil toxicity assays. Sci. Total Environ. 505, 161–171. González-Macedo, M., Cabirol, N. and Rojas-Oropeza, M. 2021. Assessment of the ancestral use of garlic (Allium sativum) and nettle (Urtica dioica) as botanical insecticides in the protection of mesquite (Prosopis laevigata) seeds against bruchins. Plant Prot. Res. 61(2), 170–175. Gray, J.S., Kahl, O., Lane, R.S., Levin, M.L. and Tsao, J.L. 2016. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick-Borne Dis. 7(5), 992–1003. Hafez, A.M. and Abbas, N. 2021. Insecticide resistance to insect growth regulators, avermectins, spinosyns and diamides in Culex quinquefasciatus in Saudi Arabia. Parasites Vectors 14, Article number: 225; doi: 10.1186/s13071-021-05068-8 Honsberger, N.A., Six, R.H., Heinz, T.J., Weber, A., Mahabir, S.P. and Berg, T.C. 2016. Efficacy of sarolaner in the prevention of Borrelia burgdorferi and Anaplasma phagocytophilum transmission from infected Ixodes scapularis to dogs. Vet. Parasitol. 222, 67–72. Jongejan, F., Crafford, D., Erasmus, H., Fourie, J.J. and Schunack, B. 2016. Comparative efficacy of oral administrated afoxolaner (NexGard™) and fluralaner (Bravecto™) with topically applied permethrin/imidacloprid (Advantix®) against transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs. Parasites Vectors 9, Article number: 348; doi:10.1186/s13071-016-1636-9 Khalil, A.M. and Abu Samrah, H.M. 2018. In vivo combined treatment of rats with ivermectin and aged garlic extract attenuates ivermectin-induced cytogenotoxicity in bone marrow cells. Res. Vet. Sci. 120, 94–100. Khare, R.K., Das, G., Kumar, S., Bendigeri, S., Sachan, S., Saiyam, R., Banerjee, D.K. and Khare, D.S. 2019. Herbal insecticides and Acaricides: challenges and constraints. Int. J. Chem. Stud. 7(4), 118–125. Khorrami, F. and Soleyman, A. 2021. Efficacy of some chemical insecticides and plant extracts combined with Bacillus thuringiensis against Phthorimaea operculella. Acta Phytopathol. Entomol. Hung. 56(2), 169–179. Kiesewetter, T., Ariza, L., Martins, M.M., Limongi, J.E., da Silva, J.J., Mendes, J., Calheiros, C.M.L., Becher, H. and Heukelbach, J. 2013. In vitro efficacy of four insecticides against eggs of tunga penetrans (Siphonaptera). Open Dermatol. J. 7(1), 15–18. Krishnananda, P.I., Deshmukh, A.G., Padole, D.A., Dudhare, M.S., Moharil, M.P. and Khelurkar, V.C. 2017. Phytochemicals: extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 6(1), 32–36. Kumar, R. 2019. Molecular markers and their application in the monitoring of acaricide resistance in Rhipicephalus microplus. Exp. Appl. Acarol. 78, 149–172. Kwak, M.L., Chavatte, J.M., Chew, K.L. and Lee, B.P.Y.H. 2021. Emergence of the zoonotic tick Dermacentor (Indocentor) auratus Supino, 1897. (Acari: Ixodidae) in Singapore. Ticks Tick-borne Dis. 12(1), Article number: 101574. Lucia, A. and Guzmán, E. 2021. Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Adv. Colloid Interface Sci. 287, 102330; doi:j.cis.2020.102330 Mahai, G., Wan, Y., Xia, W., Wang, A., Shi, L., Qian, X., He, Z. and Xu, S. 2021. A nationwide study of occurrence and exposure assessment of neonicotinoid insecticides and their metabolites in drinking water of China. Water Res. 189, 116630; doi:10.1016/j.watres.2020.116630 Mansour, R., Grissa-Lebdi, K., Suma, P., Mazzeo, G. and Russo, A. 2017. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: host plants, bio-ecological characteristics, natural enemies and pest management strategies – a review. Plant Prot. Sci. Czech Acad. Agric. Sci. 53(1), 1–14. Marchiondo, A.A., Cruthers, L.R. and Fourie, J.J. 2019. Parasiticide screening. Volume 1: in vitro and in vivo tests with relevant parasite rearing and host infection/infestation methods. London, UK: Academic Press, pp: 321–331. Marchiondo, A.A., Holdsworth, P.A., Fourie, L.J., Rugg, D., Hellmann, K., Snyder, D.E. and Dryden, M.W. 2013. World Association for the Advancement of Veterinary Parasitology (WAAVP) second edition: guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestations on dogs and cats. Vet. Parasitol. 194(1), 84–97. Medlock, J.M., Hansford, K.M., Bormane, A., Derdakova, M., Estrada-Peña, A., George, J.C., Golovljova, I., Jaenson, T.G., Jensen, J.K., Jensen, P.M., Kazimirova, M., Oteo, J.A., Papa, A., Pfister, K., Plantard, O., Randolph, S.E., Rizzoli, A., Santos-Silva, M.M., Sprong, H., Vial, L., Hendrickx, G., Zeller, H. and Bortek, W.V. 2013. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 6, 1; doi:10.1186/1756-3305-6-1 Metcalf, R.L. 2002. Insect control. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH; doi:10.1002/14356007.a14_263 Minetti, C., Ingham, V.A. and Ranson, H. 2020. Effects of insecticide resistance and exposure on Plasmodium development in Anopheles mosquitoes. Curr. Opin. Insect Sci. 39, 42–49. Moore, G. Assessment of animal housing needs in the research setting using a peer-reviewed literature approach: dogs and cats. In The Development of Science-Based Guidelines for Laboratory Animal Care: Proceedings of the November 2003 International Workshop. 2003. Mossa, A.T.H., Abdel Rasoul, M.A. and Mohafrash, S.M.M. 2017. Lactational exposure to abamectin induced mortality and adverse biochemical and histopathological effects in suckling pups. Environ. Sci. Poll. Res. 24(11), 10150–10165. Nasr, H.M., El-Demerdash, F.M. and El-Nagar, W.A. 2016. Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats: toxicity of insecticide mixture. Environ. Sci. Poll. Res. 23(2), 1852–1859. National Research Council (NRC). 1996. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press. Nettles, R., Watkins, J., Ricks, K., Boyer, M., Licht, M., Atwood, L.W., Peoples, M., Smith, R.G., Mortensen, D.A. and Koide, R.T. 2016. Influence of pesticide seed treatments on rhizosphere fungal and bacterial communities and leaf fungal endophyte communities in maize and soybean. Appl. Soil Ecol. 102, 61–69. Nicoletti, M., Murugan, K., Canale, A. and Benelli, G. 2016. Neem-borne molecules as eco-friendly control tools against mosquito vectors of economic importance. Curr. Org. Chem. 20(25), 2681–2689. Oliveira, L.H., Trigueiro, P., Souza, J.S.N., Carvalho, M.S., Osajima, J.A., Silva-Filho, E.C. and Fonseca, M.G. 2022. Montmorillonite with essential oils as antimicrobial agents, packaging, repellents, and insecticides: an overview. Colloids Surf. B Biointerfaces 209(2), 112186; doi:10.1016/j.colsurfb.2021.112186 Padula, A.M. 2016. Tick paralysis of animals in Australia. In Clinical toxinology in Asia Pacific and Africa. Dordrecht, Netherlands: Springer Science+Business Media; doi:10.1007/978-94-017-7438-3 Padula, A.M., Leister, E.M. and Webster, R.A. 2020. Tick paralysis in dogs and cats in Australia: treatment and prevention deliverables from 100 years of research. Aust. Vet. J. 98(1–2), 53–59. Page, A.P. 2018. The sensory amphidial structures of Caenorhabditis elegans are involved in macrocyclic lactone uptake and anthelmintic resistance. Int. J. Parasitol. 48(13), 1035–1042. Pavela, R. and Benelli, G. 2016. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 21(12), 1000–1007. Pisa, L., Goulson, D., Yang, E.C., Gibbons, D., Sánchez-Bayo, F., Mitchell, E., Aebi, A., Sluijs, J.V., MacQuarrie, C.J.K., Giorio, C., Long, E.Y., McField, M., Lexmond, M.B. and Bonmatin, J.M. 2021. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ. Sci. Poll. Res. 28, 11749–11797. Poché, D.M., Hartman, D., Polyakova, L. and Poché, R.M. 2017. Efficacy of a fipronil bait in reducing the number of fleas (Oropsylla spp.) infesting wild black-tailed prairie dogs. J. Vector Ecol. 42(1), 171–177. Rasouli, S., Tehrani, A., Hifian, H., Athayi, M., Ghafarzadeh, S., Pirbudaghi, H., Hoseini, E. and Ghasemzade, E. 2011. A report over the infection with the louse Polyplax spinulosa in typical rats belonging to the Wistar strain kept in the laboratory animal breeding and keeping Center of Urmia University. Glob. Vet. 6(6), 547–550. Saad, A.M., El-Saadony, M.T., El-Tahan, A.M., Sayed, S., Moustafa, M.A.M., Taha, T.F. and Ramadan, M.M. 2021. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudia J. Biol. Sci. 28(10), 5674–5683. Saeed, R., Razaq, M. and Hardy, I.C.W. 2016. Impact of neonicotinoid seed treatment of cotton on the cotton leafhopper, Amrasca devastans (Hemiptera: Cicadellidae), and its natural enemies. Pest Manag. Sci. 72(6), 1260–1267. Sankar, M. and Kumar, S. 2021. A systematic review on the eco-safe management of mosquitoes with diflubenzuron: an effective growth regulatory agent. Acta Ecol. Sinica (in press); doi:10.1016/j.chnaes.2021.09.019 Segev, G., Rojas, A., Lavy, E., Marganit, Y., Aroch, I. and Baneth, G. 2018. Evaluation of a spot-on imidacloprid-moxidectin formulation (Advocate®) for the treatment of naturally occurring esophageal spirocercosis in dogs: a double-blinded, placebo-controlled study. Parasites Vectors 11, Article number: 127; doi:10.1186/s13071-018-2731-x Shaji, S.M., Shahana, J., Thomas, A., Jiju, V. and Elessy. A. 2017. Herbal insecticide and pesticide—save the life and future. J. Pharm. Sci. Pharmacol. 4(3), 34–40. Shirazi, Sh., Bahadori, F., Mostafaei, T. and Ronaghi, H. 2013. First report of Polyplax sp. in a Persian Squirrel (Scuirus anomalus) in Tabriz, Northwest of Iran. Turk. Parazitol. Derg. 37(4), 299–301. Singh, S.S. 2010. Comparative efficacy of certain bio-rational insecticide and Bacillus thuringinesis based bio-insecticides against Leucinodes orbonalis Guenee. In Brinjal. Indian J. Hortic. 67(3), 353–356. Six, R.H., Geurden, T., Carter, L., Everett, W.R., McLoughlin, A., Mahabir, S.P., Myers, M.R. and Slootmans, N. 2016. Evaluation of the speed of kill of sarolaner (SimparicaTM) against induced infestations of three species of ticks (Amblyomma maculatum, Ixodes scapularis, Ixodes ricinus) on dogs. Vet. Parasitol. 222, 37–42. Soliman, E.S. and Abdallah, M.S. 2020. Assessment of biosecurity measures in broiler’s farms in the Suez Canal area—Egypt using a seasonal prevalence of Salmonellosis. Vet. World 13(4), 622–632. Soliman, E.S. and Hassan, R.A. 2019. Impact of lighting color and duration on productive performance and Newcastle disease vaccination efficiency in broiler chickens. Vet. World 12(7), 1052–1059. Soliman, E.S., Ali, A.A. and Gafaar, R.E.M. 2021. Impact of heating systems on air and litter quality in broiler houses, performance, behavior, and immunity in broiler chickens. Adv. Anim. Vet. Sci. 9(2), 301–314. Soliman, E.S., Hamad, R.T. and Ahmed, A. 2017. Prophylactic and immune modulatory influences of Nigella sativa Linn. in broilers exposed to biological challenge. Vet. World 10(12), 1447–1455. Soliman, E.S., Sallam, N.H. and Abouelhassan, E.M. 2018. Effectiveness of poultry litter amendments on bacterial survival and Eimeria oocyst sporulation. Vet. World 11(8), 1064–1073. SPSS. 2016. Statistical Packages of Social Sciences. Version 21 for windows. Chicago, IL: SPSS. Inc. Taenzler, J., de Vos, C., Roepke, R.K.A. and Heckeroth, A.R. 2018. Efficacy of fluralaner plus moxidectin (Bravecto® Plus spot-on solution for cats) against Otodectes cynotis infestations in cats. Parasite Vectors 11(1), 595; doi:10.1186/s13071-018-3167-z Taktak, N.E.M., Badawy, M.E.I., Awad, O.M., Abou El-Ela, N.E. and Abdallah, S.M. 2021. Enhanced mosquitocidal efficacy of pyrethroid insecticides by nanometric emulsion preparation towards Culex pipiens larvae with biochemical and molecular docking studies. J. Egypt. Public Health Assoc. 96, Article number: 21; doi:10.1186/s42506-021-00082-1 Thangam, T.S. and Kathiresan, K. 2021. Mosquito larvicidal activity of marine plant extracts with synthetic insecticides. Eds., Borowitzka, M.A. and G.T. Boalch. Berlin, Boston; Germany, MA: De Gruyter, pp: 537–540. Tong, L., Nieh, J.C. and Tosi, S. 2019. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 237, Article number: 124408; doi:10.1016/j.chemosphere.2019.124408 Venkateswarlu, K., Heerasingh, T., Babu, C.N., Triveni, S., Manasa, S. and Babu, T.N.B. 2017. Preclinical evaluation of nephroprotective potential of a probiotic formulation LOBUN on Cyclosporine-A induced renal dysfunction in Wistar rats. Braz. J. Pharm. Sci. 53(2), e16041; doi:10.1590/s2175-97902017000216041 Wanji, S., Eyong, E.E.J., Tendongfor, N., Ngwa, C.J., Esuka, E.N., Kengne-Ouafo, A.J., Datchoua-Poutcheu, F.R., Enyong, P., Agnew, D., Eversole, P.R., Hopkins, A. and Mackenzi, C.D. 2017. Ivermectin treatment of Loa loa hypermicrofilaraemic baboons (Papio anubis): assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl. Trop. Dis. 11(7), Article e0005576; doi:10.1371/journal.pntd.0005576 Wu, Y.N., Yan, F.F., Hu, J.Y., Chen, H., Tucker, C.M., Green, A.R. and Cheng, H.W. 2017. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult. Sci. 96(6), 1524–1530. Zendehfili, H., Zahirnia, A.H., Maghsood, A.H., Khanjani, M. and Fattah, M. 2015. Ectoparasites of rodents captured in Hamedan, Western Iran. Short Commun. J. Arthropod Borne Dis. 9(2), 267–273. Zhang, Y., Luo, M., Xu, W., Yang, M., Wang, B., Gao, J., Li, Y. and Tao, L. 2016. Avermectin confers its cytotoxic effects by inducing DNA damage and mitochondria-associated apoptosis. J. Agric. Food Chem. 64, 6895–6902. | ||
| How to Cite this Article |
| Pubmed Style Aboelela EM, Sobieh MA, Abouelhassan EM, Farid DS, Soliman ES. The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Vet. J.. 2022; 12(1): 44-60. doi:10.5455/OVJ.2022.v12.i1.6 Web Style Aboelela EM, Sobieh MA, Abouelhassan EM, Farid DS, Soliman ES. The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. https://www.openveterinaryjournal.com/?mno=4033 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.6 AMA (American Medical Association) Style Aboelela EM, Sobieh MA, Abouelhassan EM, Farid DS, Soliman ES. The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Vet. J.. 2022; 12(1): 44-60. doi:10.5455/OVJ.2022.v12.i1.6 Vancouver/ICMJE Style Aboelela EM, Sobieh MA, Abouelhassan EM, Farid DS, Soliman ES. The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 44-60. doi:10.5455/OVJ.2022.v12.i1.6 Harvard Style Aboelela, E. M., Sobieh, . M. A., Abouelhassan, . E. M., Farid, . D. S. & Soliman, . E. S. (2022) The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Vet. J., 12 (1), 44-60. doi:10.5455/OVJ.2022.v12.i1.6 Turabian Style Aboelela, Eman M., Mohamed A. Sobieh, Eman M. Abouelhassan, Doaa S. Farid, and Essam S. Soliman. 2022. The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Veterinary Journal, 12 (1), 44-60. doi:10.5455/OVJ.2022.v12.i1.6 Chicago Style Aboelela, Eman M., Mohamed A. Sobieh, Eman M. Abouelhassan, Doaa S. Farid, and Essam S. Soliman. "The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs." Open Veterinary Journal 12 (2022), 44-60. doi:10.5455/OVJ.2022.v12.i1.6 MLA (The Modern Language Association) Style Aboelela, Eman M., Mohamed A. Sobieh, Eman M. Abouelhassan, Doaa S. Farid, and Essam S. Soliman. "The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs." Open Veterinary Journal 12.1 (2022), 44-60. Print. doi:10.5455/OVJ.2022.v12.i1.6 APA (American Psychological Association) Style Aboelela, E. M., Sobieh, . M. A., Abouelhassan, . E. M., Farid, . D. S. & Soliman, . E. S. (2022) The in-vivo and in-vitro Effectiveness of Three Insecticides Types for Eradication of The Tick Rhipicephalus sanguineus in Dogs. Open Veterinary Journal, 12 (1), 44-60. doi:10.5455/OVJ.2022.v12.i1.6 |