| Short Communication | ||
Open Vet. J.. 2022; 12(1): 99-104 Open Veterinary Journal, (2022), Vol. 12(1): 99–104 Short Communication Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central ChileAndrea H. Núñez1, Fabian G. Hidalgo1, Pamela C. Morales1, Victor E. Silva2, Pamela E. Thomson3 and Rodrigo A. Castro1*1Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Talca, Chile 2VSV-Consulting-LATAM, Chile and American Society for Microbiology — ASM, Washington, DC, USA 3Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile *Corresponding Author: Rodrigo A. Castro. Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Talca, Chile. Email: rodrigocastro [at] santotomas.cl Submitted: 12/12/202 Accepted: 22/01/2022 Published: 10/02/2022 © 2022 Open Veterinary Journal
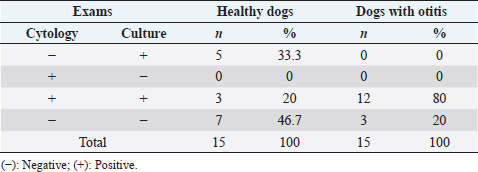
AbstractBackground: External otitis is common in dogs, and one of the main agents involved is Malassezia pachydermatis, a yeast belonging to the otic microbiota. Empirical treatment can fail; therefore, it is essential to know the antifungal susceptibility profile to prescribe appropriate treatment, a fact scarcely reported in Chile. Aim: This study aimed to determine the antifungal sensitivity of M. pachydermatis isolated from the external auditory canal of dogs in central Chile. Methods: Ear swabs from 30 dogs (15 healthy and 15 with external otitis) were used. Samples were subjected to cytology and fungal culture. The antifungal susceptibility was determined according to clinical and laboratory standards institute (CLSI) document M44A-2 using the disk diffusion test from amphotericin B, Caspofungin, fluconazole, nystatin, clotrimazole, and voriconazole were used. Results: The prevalence of M. pachydermatis was 66.7% from 8 healthy dogs and 12 with otitis. While fungal culture was not associated with the patient’s clinical condition (p=0.24), the yeast count by cytology was significantly higher in dogs with otitis (p=0.003). The strains were sensitive to all antifungals except for Caspofungin, where 55% of the strains were resistant. Conclusion: Malassezia pachydermatis is isolated more frequently in dogs with otitis, and the ear cytological examination is useful to differentiate colonized dogs versus dogs with otitis. In addition, most antifungals in vitro are active against this yeast, except Caspofungin, an antifungal used in human medicine. This situation should be further monitored in epidemiological programs to evaluate the possible impact on Chilean public health. Keywords: Dogs, Malassezia pachydermatis, Antifungal susceptibility. IntroductionMalassezia pachydermatis is a part of the cutaneous flora in dogs and cats (Bond et al., 2020) is frequently isolated from the oral, interdigital, and external auditory canal areas in animals (Mayser et al., 2001; Cafarchia et al., 2005). Under humid conditions or disorders of keratinization, this yeast can proliferate (Cafarchia et al., 2006; Torres et al., 2008) and induce an inflammatory response in the host (Bond et al., 2020). The production of lipases, lipoxygenases, proteinases, and phospholipases are described as virulence factors (Chen and Hill, 2005; Ortiz et al., 2013). Malassezia pachydermatis is associated with cases of external otitis in dogs with a prevalence between 10% and 20% (Angus, 2004; Cole, 2004). Frequently, the diagnosis of otitis is made by evaluating the history and clinical signs of the patients, besides the otic cytology (Angus, 2004). To treat the otitis caused by M. pachydermatis, topical azole products such as clotrimazole and/or miconazole are generally prescribed, but their empirical use could fail or generate recurrence of the otitis. Some in vitro studies have reported antifungal resistance in strains with low susceptibility to antifungal agents of the azole family (Rougier et al., 2005; Angileri et al., 2019). For this reason, infections caused by Malassezia require an accurate diagnosis and a careful choice of the antifungal agent to be used, especially in those cases that show recurrent infections or failure to respond to antifungal treatment (Cafarchia et al., 2012). This yeast’s prevalence, isolation, and antifungal resistance are determined by its inhabitants climate, geographical area, and epidemiological variables (Leong et al., 2019), but this information is unknown in our country. This knowledge would allow improving clinical decisions. Therefore, the study of healthy dogs and those with otitis would allow evaluating the sensitivity of the classic diagnostic methods for detecting M. pachydermatis and recognizing the reservoir role that healthy dogs can play in public health under the one health approach. Thus, the present study aims to determine the prevalence and the antifungal susceptibility of M. pachydermatis isolated from the external auditory canal of healthy dogs and dogs with otitis, recognizing its association with epidemiological data in central Chile. Materials and MethodsAnimalsFrom August to December 2018, by means of sterile swabs in stuart medium, 30 routinary samples were obtained from an external auditory canal, from 15 healthy dogs and 15 dogs with otitis externa, males and females of different breeds, whose ages ranged from 1 to 12 years, who attended consultations in veterinary clinics. The clinical diagnosis of otitis was based on the presence of clinical signs such as pain, manifested by shaking and tilting the head to one side, itching of the external auricle, seborrheic odor, otic discharge, redness, and/or inflammation of the external auditory canal. The inclusion criteria corresponded to patients who had not received local or systemic antifungal treatment 30 days before sampling. Before the sampling procedure, all owners of the selected patients signed an informed consent form. All vets who have collaborated in this work have Technical Competence in Animal Health, Husbandry, and Handling. The clinical samples were taken in veterinary clinics of urban areas of the Talca city, located 255 km to the south of Santiago de Chile (35°26′00″S; 71°40′00″W), characterized by continental Mediterranean weather (average temperature 8.2°C to 22.2°C, 792 mm of rain in a year and 67% of relative humidity) (Climate data, 2021). Sample collectionThe samples were transported in Stuart medium under refrigeration conditions (4°–6°) within 24 hours, to be analysed at the Medical Mycology Laboratory at Universidad Mayor, Santiago de Chile. Sample processingThe samples were processed to cytology and fungal culture. Each swab was extended on the sterile slides, stained with Diff Quick Stain, and evaluated under conventional microscopy with an immersion objective (Díaz et al., 2005). To count the number of Malassezia yeast cells, it was recorded as negative (−): absence of yeast cells; (+): 1 to 5 yeasts per field (lpc); (++): 6 to 10 lpc; (+++): more than 10 lpc (Nobre et al., 2001). Subsequently, the same swabs were spread on plates with Sabouraud Agar added with chloramphenicol at concentrations of 100 mg/l, which were incubated at 32°C for 7 days. Strains identificationThe cultures were analyzed macro-morphologically, according to the presence or absence of colonies suggestive of M. pachydermatis, which presented characteristics such as: smooth or slightly rough surface and cream to brown color and subsequent micro-morphological observation of the yeasts for their oval shape and percurrent budding (Carlotti, 2005; Díaz et al., 2005; Ashbee, 2007). Susceptibility testingAntifungal resistance was determined according to CLSI document M44A-2 using the disk diffusion test. Two to three colonies from the culture were suspended in a tube with 0.85% sodium chloride, preparing an inoculum of 0.5 turbidity according to the McFarland scale, equivalent to 0.08–0.11 optical density determined with a spectrophotometer. Subsequently, the inoculum was spread in carpet arrangement on Müller Hinton Agar plates, enriched with 2% glucose and methylene blue at a 0.5 mg/ml concentration. The sensidisks of the antifungals used were: Amphotericin B 10 mcg, Caspofungin 5 mcg, Fluconazole 25 mcg, Nystatin 100 mcg, Clotrimazole 10 mcg, Voriconazole 1 mcg. The Müller Hinton agar plates were incubated at 37°C for 2 days, after which the susceptibility test reading of M. pachydermatis to the antifungals used was performed according to CLSI M44A-2 (CLSI, 2009). Ethical approvalNo ethical approval was needed for this study. Statistical analysisThe results of the otic cytology and otic fungal culture were grouped according to the following patient variables: Clinical condition (healthy; otitis), age (1–3; 4–6; 7–9; 10–12 years old), gender (male; female) and race (purebred; mestizo). Thus, contingency tables were formed, and Fisher’s exact test was used to determine the association between the variables studied. p-value <0.05 was considered to be significant. The specificity, sensitivity, and positive (PPV) and negative predictive values (NPV) for cytology and fungal culture were calculated. The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) statistics program (version 20.0, IBM-SPSS, Armonk, NY). Results and DiscussionThe clinical condition of otitis externa was not associated with the variables age (p=0.42), breed (p=0.12), or sex (p=0.71), in agreement with Nobre et al. (2001). Malassezia pachydermatis belongs to the dermal and otic flora of the dogs; however, the carriage of this yeast varies between different anatomical sites and different breeds of dogs, so this yeast could also be present in the ear canal of healthy dogs (Nardoni et al., 2004; Korbelik et al., 2019). In this study, it was possible to demonstrate the presence of M. pachydermatis in the ears of healthy dogs that live in central Chile. Malassezia can act as a perpetuating factor (Bajwa, 2019), leading to the clinical presentation of external otitis manifested by pruritus, erythema, and cerumen (Bond et al., 2020; Nuttall, 2016). A higher frequency of M. pachydermatis isolation has been reported in dogs with pendulous ears (Girao et al., 2006; Babić et al., 2020); however, in the present study, the pendulous ear trait was not associated with the clinical condition (p=0.7). In our work, the frequency of isolation of M. pachydermatis in dogs with clinical otitis (66.6%) was slightly higher than those described by Magalhães et al. (2017), who reported 60.9% isolation. Otic cytologyOf the 30 dogs studied, 15 (50%) presented M. pachydermatis, of which 12 belonged to the group with clinical otitis and 3 belonged to the healthy dogs, both groups were characterized by 75% females;50% belonged to the age range of 1 to 3 years, and 75% were purebred, the most frequent breed was a poodle. For the otic cytology examination, the sensitivity and specificity were 80%. The PPV and NPV were also 80%. Of the 15 dogs with clinical otitis, 6 had counts of 1 to 5 yeasts per field (lpc), 4 had counts of 6 to 10 lpc, 2 had counts of more than 10 lpc, and 3 had dogs negative to cytology. Of the 15 healthy dogs, 3 had counts of 1 to 5 lpc, and 12 were negative. The yeast count at cytological examination was associated with the presentation of clinical otitis (p=0.003) as obtained by Nobre et al. (2001) and Girao et al. (2006) for the diagnosis of external otitis. This technique is usually used for otitis diagnostic because, in addition to being simple, fast, minimally invasive, and inexpensive, it allows quantification of the yeasts present in the external auditory canal (Cabañes, 2021). In this study, ear cytology examination had high sensitivity to Malassezia (80%), confirming that its use is a very useful tool in the clinical practice of small animals. Otic fungal cultureOf the 30 dogs studied, 20 (66.6%) developed M. pachydermatis, of which 12 belonged to the group with clinical otitis and 8 belonged to the healthy group. Then, 80% of the otitis group and 53.3% of the healthy group were positive for M. pachydermatis culture. This exam had a sensitivity of 60% and specificity of 70%, and its PPV and NPV were 80% and 46%, respectively. The clinical condition of the patients was not associated with the result of the fungal culture (p=0.24). Fifty percent of the samples were positive for both cytology and fungal culture; 33% of the samples were negative for both techniques, and only 16.6% that were negative for cytology were positive for fungal culture, then the results of both exams are statistically associated (p=0.001) (Table 1). Comparatively, a fungal culture is not a routine technique for Malassezia diagnostic, possibly due to its long waiting time for results and its association with cytological results, as evidenced in this study (p=0.001). This result allows us to recommend ear cytology as the method of choice to diagnose clinical otitis for Malassezia. In the treatment of Malassezia otitis, azole antifungal drugs are frequently used topically or systemically, combined with antibiotics and/or glucocorticoids (Negre et al., 2009; Bond, 2010), and in general, patients present a good clinical response, but there are reports of therapeutic failures, presumably due to a poor selection of drugs (Chiavassa et al., 2014). Antifungal therapy requires a responsible and methodical examination, evaluating and selecting a specific therapy for each patient (Velegraki et al., 2015). This may occur because Malassezia otitis occurs secondary to allergic-based diseases such as atopic dermatitis, in which changes in the otic and cutaneous microclimate may occur due to the trauma generated in the dermal barrier because of the different manifestations of canine pruritus (Nardoni et al., 2007; Machado et al., 2010). Regarding the prevention of recurrent otitis due to Malassezia, in vitro studies have demonstrated the efficacy of otic cleansers as cerumenolytics and astringents, so they should be considered as an adjunct to antifungal therapy in the treatment of canine otitis due to M. pachydermatis (Swinney et al., 2008; Mason et al., 2013). In the present study, all strains of M. pachydermatis were sensitive to Amphotericin B, Fluconazole, Nystatin, Clotrimazole, and Voriconazole. However, 55% (11 strains) were resistant to Caspofungin, corresponding to 5 strains from healthy dogs and 6 strains from dogs with external otitis. This is consistent with its low performance on yeasts of clinical interest from the Phylum Basidiomycetes, such as those of the genus Cryptococcus spp. and Trichosporon spp. (Eschenauer et al., 2007). This antifungal belongs to the echinocandin family, a class of agents that act directly on the fungal cell wall by inhibiting the enzyme β-1,3-D-glucan synthase, which catalyzes the biosynthesis of β-1,3-D-glucan, an essential component of the cell wall (Kurtz and Douglas, 1997). This enzyme is not found in mammals, so the risk of toxicity in humans is low (Eschenauer et al., 2007). Caspofungin is used to treat invasive Candida infections and Aspergillosis when patients are refractory or intolerant to other therapies. There are international reports of Caspofungin resistance in M. pachydermatis isolated from dogs with otitis (Prado et al., 2008) and from various anatomic sites in healthy dogs (Brito et al., 2009). Although many studies show the susceptibility of Malassezia strains to commonly used azoles such as itraconazole, ketoconazole, and miconazole (Cafarchia et al., 2012; Weiler et al., 2013), being reported that isolates from canine otitis externa show synergistic interactions between Caspofungin and Itraconazole or Fluconazole (Schlemmer et al., 2019). Regarding the zoonotic potential, some studies are describing human neonatal intensive care patients with low birth weight who received lipid emulsions and were colonized by M. pachydermatis, probably transmitted by the handling of health personnel due to their contact with their own dogs (Chang et al., 1998) and a facial granuloma caused by M. pachydermatis in a dog owner (Fan et al., 2006). Therefore, it is recommended that immunocompromised individuals or people in contact with domestic dogs and cats should practice proper hand hygiene (Morris et al., 2005; Bond et al., 2020; Guillot and Bond, 2020). Table 1. Detection of Malassezia pachydermatis by otic cytology and/or fungal culture according to clinical condition (n=30 dogs).
The increasing number of Malassezia spp. infections in humans and animals emphasize the importance of susceptibility testing as a guide for properly antifungal treatment (Bond et al., 2020). Some authors describe that some strains can acquire resistance to fluconazole when this antifungal has been used for a long time, leading to cross-resistance to other azoles (Jesus et al., 2011). Among the risk factors associated with the chronicity or recurrence of Malassezia otitis, the erroneous identification of strains and the failure to control predispose or perpetuating factors. Even though Caspofungin is not an antifungal drug commonly used in veterinary medicine, this study reported a reduced susceptibility for strains of M. pachydermatis, which raises the need to implement surveillance measures against the emergence of strains resistant to other drugs used in veterinary medicine, to provide useful information for a correct therapeutic selection in the management of chronic or recurrent cases (Guillot and Bond, 2020). Our results constitute the first national report of resistance to Caspofungin in M. pachydermatis isolated from dogs. AcknowledgmentsThe authors would like to thank Dr. Gisselle Rojo for her invaluable help in providing the patients for the study and Dr. Benjamin Apeleo for his help in the English translation. Conflict of interestThe authors declare that they have no conflicts of interest. Authors’ contributionAHN and RAC contributed conception and design of the study; FGH collected the samples; FGH and VES performed all the laboratory assays; AHN and RAC statistical analyses, wrote the first draft of the manuscript; PCM, PET and VES critically revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. ReferencesAngileri, M., Pasquetti, M., De Lucia, M. and Peano, A. 2019. Azole resistance of Malassezia pachydermatis causing treatment failure in a dog. Med. Mycol. Case Rep. 23, 58–61. Angus, J. 2004. Otic cytology in health and disease. Vet. Clin. Small Anim. 34(2), 411–424. Ashbee, H. 2007. Update on the genus Malassezia. Med. Mycol. 45, 287–303. Babić, S., Pašić, S. and Zahirović, A. 2020. Prevalence of Malassezia pachydermatis in atopic dogs-50 cases. Veterinaria 69(2), 121–127. Bajwa, J. 2019. Canine otitis externa-treatment and complications. Can. Vet. J. 60(1), 97–99. Bond, R. 2010. Superficial veterinary mycoses. Clin. Dermatol. 28, 226–236. Bond, R., Morris, D.O., Guillot, J., Bensignor, E.J., Robson, D., Mason, K.V., Kano, R. and Hill, P.B. 2020. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats. Clinical consensus guidelines of the world association for veterinary dermatology. Vet. Dermatol. 31, 75. Brito, E., Fontenelle, R., Brilhante, R., Cordeiro, R., Monteiro, A.J.,Sidrim, J.J.C. and Rocha, M.F.G. 2009. The anatomical distribution and antimicrobial susceptibility of yeast species isolated from healthy dogs. Vet. J. 182, 320–326. Cabañes, F.J. 2021. Diagnóstico de las dermatitis y otitis por Malassezia en perros y gatos, ¿es sólo cuestión de contar? Rev. Iberoam. Micol. 38(1), 3–4. Cafarchia, C., Gallo, S., Danesi, P., Capelli, G., Chermette, R., Guillot, J. and Otranto, D. 2005. Frequency, body distribution, and population size of Malassezia species in healthy dogs and in dogs with localized cutaneous lesions. J. Vet. Diagn. Invest. 17, 316–322. Cafarchia, C., Gallo, S., Romito, D., Capelli, G. and Otranto, D. 2006. New insights into the diagnosis and the pathogenicity of Malassezia yeasts. Vet. Res. Commun. 30, 231–234. Cafarchia, C., Figueredo, L.A., Iatta, R., Montagna, M.T. and Otranto, D. 2012. In vitro antifungal susceptibility of Malassezia pachydermatis from dogs with and without skin lesions. Vet. Microbiol. 155, 395–398. Carlotti, D. 2005. Malassezia dermatitis in the dog. In Proceedings of the 30th Congress of the World Small Animal Veterinary Association, May 11–14, México City, México. Chang, H.J., Miller, H.L., Watkins, N., Arduino, M.J., Ashford, D.A., Midgley, G., Aguero, S.M., Pinto-Powell, R., von Reyn, F., Edwards, W., McNeil, M.M. and Jarvis, W.R. 1998. An Epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers´pet dogs. N. Engl. J. Med. 338(11), 706–711. Chen, T.A. and Hill, P.B. 2005. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet. Dermatol. 16, 4–26. Chiavassa, E., Tizzani, P. and Peano, A. 2014. In vitro antifungal susceptibility of Malassezia pachydermatis strains isolated from dogs with chronic and acute otitis externa. Mycopathologia 178, 315–319. Climate data. 2021. Chile, VII region del Maula, Talca. Available via https://es.climate-data.org/america-del-sur/chile/vii-region-del-maule/talca-942/ (Accessed 18 June 2021). CLSI. 2009. Method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline, 2nd edition, M44A2, Agosto, Wayne, Pennsylvania, USA. Cole, L.K. 2004. Otoscopic evaluation of the ear canal. Vet. Clin. North Am. Small Anim. Pract. 34, 397–410. Díaz, M., Silva, V. and Hermosilla, G. 2005. Manual práctico. Santiago, Chile: Curso Internacional de Micología Médica. Eschenauer, G., DePestel, D. and Carver, P. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 3(1), 71–97. Jesus, F.P.K., Lautert, C., Zanette, R.A., Mahl, D.L., Azevedo, M.I., Machado, M.L.S., Dutra, V., Botton, S.A., Alves, S.H. and Santurio, J.M. 2011. In vitro susceptibility of fluconazole-susceptible and -resistant isolates of Malassezia pachydermatis against azoles. Vet. Microbiol. 152, 161–164. Fan, Y., Huang, W., Li, S., Wu, G., Lai, K. and Chen, R. 2006. Granulomatous skin infection caused by Malassezia pachydermatis in a dog owner. Arch. Dermatol. 142(9), 1181–1184. Girao, M.D., Prado, M.R., Brilhante, R.S.N., Cordeiro, R.A., Monteiro, A., Sidrim, J.J.C. and Rocha, M.F.G. 2006. Malassezia pachydermatis isolated from normal and diseased external ear canals in dogs: a comparative analysis. Vet. J. 172, 544–548. Guillot, J. and Bond, R. 2020. Malassezia yeasts in veterinary dermatology: an updated overview. Front. Cell Infect. Microbiol. 10, 1–12. Korbelik, J., Singh, A., Rousseau, J. and Weese, J.S. 2019. Characterization of the otic bacterial microbiota in dogs with otitis externa compared to healthy individuals. Vet. Dermatol. 30, 228–e70. Kurtz, M.B. and Douglas, C.M. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35, 79–86. Leong, C., Schmid, B., Toi, M., Wang, J., Irudayaswamy, A., Goh, J., Bosshard, P., Glatz, M. and Dawson, T. 2019. Geographical and ethnic differences influence culturable commensal yeast diversity on healthy skin. Front. Microbiol. 10, 1–11. Machado, M.L., Ferreiro, L., Ferreira, R.R., Corbellini, L.G., Deville, M., Berthelemy, M. and Guillot, J. 2010. Malassezia dermatitis in dogs in Brazil: diagnosis, evaluation of clinical signs and molecular identification. Vet. Dermatol. 22, 46–52. Magalhães, N.R., Moraes, S.F.S., Dresch, D. and Kataoka, A. 2017. Frequência de Malassezia spp. em cães apresentando otite externa. Sci. Elec. Arch. 10(6), 50–55. Mason, C., Steen, S., Paterson, S. and Cripps, P. 2013. Study to assess in vitro antimicrobial activity of nine ear cleaners against 50 Malassezia pachydermatis isolates. Vet. Dermatol. 24, 362–366, e80–e81. Mayser, P., Schutz, M., Schuppe, H.C., Jung, A. and Schill, W.B. 2001. Frequency and spectrum of Malassezia yeasts in the area of the prepuce and glans penis. BJU Int. 88, 554–558. Morris, D., O’Shea, K., Shofer, F. and Rankin, S. 2005. Malassezia pachydermatis carriage in dog owners. Emerg. Infect. Dis. 11(1), 83–88. Nardoni, S., Mancianti, F., Corazza, M. and Rum, A. 2004. Occurrence of Malassezia species in healthy and dermatologically diseased dogs. Mycopathologia 157, 383–388. Nardoni, S., Dini, M., Taccini, F. and Mancianti, F. 2007. Occurrence, distribution and population size of Malassezia pachydermatis on skin and mucosae of atopic dogs. Vet. Microbiol. 122, 172–177. Negre, A., Bensignor, E. and Guillot, J. 2009. Evidence-based veterinary dermatology: a systematic review of interventions for Malassezia dermatitis in dogs. Vet. Dermatol. 20, 1–12. Nobre, M.O., De Castro, A.P., Nascente, P., Ferreiro, L. and Meireles, M.C. 2001. Occurrency of Malassezia pachydermatis and other infectious agents as cause of external otitis in dogs from Río Grande do Sul State, Brazil (1996/1997.) Braz. J. Microbiol. 32, 245–249. Nuttall, T. 2016. Successful management of otitis externa. In Pract. 38(2), 17–21. Ortiz, G., Martín, M.C., Carrillo-Muñoz, A.J. and Payá, M.J. 2013. Producción de fosfolipasa y proteinasa en cepas de Malassezia pachydermatis aisladas de perros con otitis y sin otitis. Rev. Iberoam. Micol. 30(4), 235–238. Prado, M.R., Brito, E., Brilhante, R., Cordeiro, R., Leite, J.J.G., Sidrim, J.J.C. and Rocha, M.F.G. 2008. Subculture on potato dextrose agar as a complement to the broth microdilution assay for Malassezia pachydermatis. J. Microbiol. Methods 75, 341–343. Rougier, S., Borell, D., Pheulpin, S., Woehrle, F. and Boisrame, B. 2005. A comparative study of two antimicrobial/anti-inflammatory formulations in the treatment of canine otitis externa. Vet. Dermatol. 16, 299–307. Schlemmer, K.B., de Jesus, F.P.K., Loreto, E.S., Farias, J.B, Alves, S.H., Ferreiro, L. and Santurio, J.M. 2019. In vitro combination of antifungal agents against Malassezia pachydermatis. Med. Mycol. 57, 324–327. Swinney, A., Fazakerley, J., McEwan, N. and Nuttall, T. 2008. Comparative in vitro antimicrobial efficacy of commercial ear cleaners. Vet. Dermatol. 19, 373–379. Torres, E., Arenas, R. and Atoche-Diéguez, C. 2008. Infecciones causadas por el género Malassezia. Med. Cutan. Iber. Lat. Am. 36(6), 265–284. Velegraki, A., Cafarchia, C., Gaitanis, G., Iatta, R. and Boekhout, T. 2015. Malassezia infections in humans and animals: pathophysiology, detection, and treatment. PLoS Pathog. 11(1), e1004523. Weiler, C.B., de Jesus, F.P.K., Nardi, G.H., Loreto, E.S., Santurio, J.M., Coutinho, S.D.A. and Alves, S.H. 2013. Susceptibility variation of Malassezia pachydermatis to antifungal agents according to isolate source. Braz. J. Microbiol. 44(1), 174–178. | ||
| How to Cite this Article |
| Pubmed Style Nuñez AH, Hidalgo FG, Morales PC, Silva VE, Thomson PE, Castro RA. Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Vet. J.. 2022; 12(1): 99-104. doi:10.5455/OVJ.2022.v12.i1.12 Web Style Nuñez AH, Hidalgo FG, Morales PC, Silva VE, Thomson PE, Castro RA. Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. https://www.openveterinaryjournal.com/?mno=46722 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.12 AMA (American Medical Association) Style Nuñez AH, Hidalgo FG, Morales PC, Silva VE, Thomson PE, Castro RA. Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Vet. J.. 2022; 12(1): 99-104. doi:10.5455/OVJ.2022.v12.i1.12 Vancouver/ICMJE Style Nuñez AH, Hidalgo FG, Morales PC, Silva VE, Thomson PE, Castro RA. Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 99-104. doi:10.5455/OVJ.2022.v12.i1.12 Harvard Style Nuñez, A. H., Hidalgo, . F. G., Morales, . P. C., Silva, . V. E., Thomson, . P. E. & Castro, . R. A. (2022) Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Vet. J., 12 (1), 99-104. doi:10.5455/OVJ.2022.v12.i1.12 Turabian Style Nuñez, Andrea Haydee, Fabian Guillermo Hidalgo, Pamela Carolina Morales, Victor Eugenio Silva, Pamela Evelyn Thomson, and Rodrigo Andres Castro. 2022. Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Veterinary Journal, 12 (1), 99-104. doi:10.5455/OVJ.2022.v12.i1.12 Chicago Style Nuñez, Andrea Haydee, Fabian Guillermo Hidalgo, Pamela Carolina Morales, Victor Eugenio Silva, Pamela Evelyn Thomson, and Rodrigo Andres Castro. "Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.." Open Veterinary Journal 12 (2022), 99-104. doi:10.5455/OVJ.2022.v12.i1.12 MLA (The Modern Language Association) Style Nuñez, Andrea Haydee, Fabian Guillermo Hidalgo, Pamela Carolina Morales, Victor Eugenio Silva, Pamela Evelyn Thomson, and Rodrigo Andres Castro. "Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.." Open Veterinary Journal 12.1 (2022), 99-104. Print. doi:10.5455/OVJ.2022.v12.i1.12 APA (American Psychological Association) Style Nuñez, A. H., Hidalgo, . F. G., Morales, . P. C., Silva, . V. E., Thomson, . P. E. & Castro, . R. A. (2022) Antifungal susceptibility of Malassezia pachydermatis isolated from the external auditive conduct from dogs, in central Chile.. Open Veterinary Journal, 12 (1), 99-104. doi:10.5455/OVJ.2022.v12.i1.12 |