| Original Article | ||
Open Vet. J.. 2021; 11(2): 270-276 Open Veterinary Journal, (2021), Vol. 11(2): 270–276 Original Research Effect of combined intrathecal/intravenous injection of bone marrow derived stromal cells in platelet-rich plasma on spinal cord injury in companion animalsAhmed N. Abdallah1*, Ashraf A. Shamaa2, Omar S. El-Tookhy2 and Mohamed M. Bahr21Pathology department, Animal Health Research Institute, Giza, Egypt 2Surgery, Anesthesiology and Radiology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt *Corresponding Author: Ahmed N. Abdallah. Pathology Department, Animal Health Research Institute, Giza, Egypt. Email: Light_system [at] hotmail.com Submitted: 13/02/2021 Accepted: 04/05/2021 Published: 04/06/2021 © 2021 Open Veterinary Journal
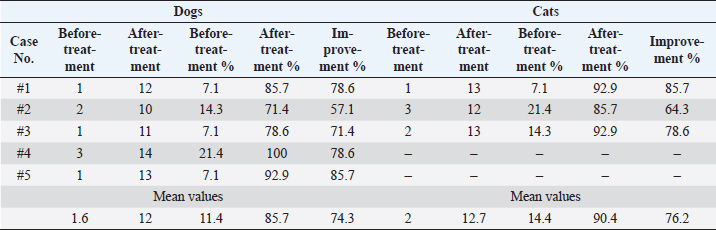
AbstractBackground: Companion animals are prone to spinal cord injuries commonly associated with severe locomotor and sensory complications, which can escalate to a state of irreversible paralysis. Stem cell therapies propose a hope for treating spinal cord injuries via differentiation into neurons and associated glial cells, halting the immune attacks, inhibiting apoptosis and necrosis, and secretion of neurotrophic factors that stimulate the regeneration process. Aim: The study aims to evaluate the use of autologous bone marrow derived stromal cells in platelet-rich plasma carrier for selected clinical cases having chronic spinal cord injuries in dogs and cats via a one-time combined intrathecal/intravenous injection. Methods: Cells were injected in five dogs and three cats suffering from disc protrusion leading to spinal cord injury and in thosewho did not respond to conventional treatment during a clinical trial. Results: Results indicated that the transplanted cells led to the restoration of the weight bearing locomotor function and spinal reflexes in a period less than 90 days with physical rehabilitation. The treatment showed minor changes in the magnetic resonance images of extruded discs. Conclusion: This study concluded that the combined intrathecal/intravenous injection of bone marrow stromal cells is a safe and promising procedure for treating chronic spinal cord injuries in companion animals. Keywords: Spinal cord injury, Stem cell therapy, Bone marrow stromal cells, Platelet-rich plasma. IntroductionSpinal cord damage is caused not only by primary mechanical injuries owed to accidents and fall from heights (Weh and Kraus, 2011) but also as a result of secondary pathologic conditions that include edema, hemorrhage, demyelination, apoptosis, and necrosis (Zhang et al., 2012). Secondary transforms are attributed to biochemical factors, counting the liberation of free radicals, leukotrienes, and prostaglandins that incite further injury to nervous tissue and decreases blood flow to the spinal cord (Bartholdi and Schwab, 1998; Yuan and He, 2013). These injuries lead to severe locomotor deficits that reach irreversible paralysis in some cases, urinary incontinence and uncontrolled defecation with a guarded or very poor prognosis (Shamaa et al., 2018). Even after the corrective operation, there is a possibility that the damage to the spinal cord may not be reversible (Denny and Butterworth, 2000). Stem cells (SCs) pose differentiation capabilities into various type of neurons and associated glial cells such as oligodendrocytes and astrocytes (Neirinckx et al., 2014) and play a role in stimulating the remyelination and axonal mending (Kennea et al., 2009). Moreover, SCs secrete neurotrophic factors having immunomodulatory effects preventing further damage, stopping the apoptosis, stimulating angiogenesis, stimulating resident progenitor cells, and creating a regenerative microenvironment for neuronal repair (Kim et al., 2015). Many animal models assessed the efficiency of the SC therapies for spinal cord injuries and discovered early signals of clinical efficiency and safety (Dasari et al., 2007; Hunt et al., 2008; Kerr et al., 2010; Tetzlaff et al., 2011; Neirinckx et al., 2015). Adult SCs like mesenchymal stem cells (MSCs) collected from several mines, such as bone marrow, adipose tissue, and peripheral blood are considered helpful candidates for neuronal repair (Tondreau et al., 2008; Neirinckx et al., 2013) due to the ease of extraction, trans-differentiation to several neural lineages, the release of paracrine factors, like brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, glial cell line-derived neurotrophic factor, and other nerve growth factor (GF) (Salgado et al., 2010). As neuronal damages and spinal cord injuries are progressive conditions and possibly irreversible, but early therapeutic intervention is lifesaving. As MSCs require prior cultivation for weeks (which can reduce the percentage of improvement), stromal cells or non-expanded SCs offer an alternative therapeutic option due to their huge MSCs content (Gronthos et al., 2001; Le Blanc, 2006). Our previous dog model study assessed the use of non-expanded adipose derived SCs as a single treatment of multiple sclerosis (Abdallah et al., 2019). The study presented very promising results for using stromal cells in the treatment of some degenerative neuronal disease. Conversely, bone marrow mononuclear cells (BM-MNCs) exemplify a significant population of SCs, including hematopoietic SCs, MSCs, and endothelial progenitor cells (Akiyama et al., 2002), which render them good candidates for early therapeutic intervention without further need for tissue cultures, and thus avoiding the continuing damage to neuronal tissues. Degranulation of platelet-rich plasma (PRP) releases a mixture of GFs and cytokines that motivate not only the regeneration of soft tissues and bones but also the nervous tissues (Yu et al., 2011). It can be used as a vehicle to inject the SCs to further augment the regenerative capacity of the treatment. This research aimed to assess the effectiveness of combined intrathecal/intravenous injection of bone marrow stromal cells in a PRP carrier as a sole cure of spinal cord injury in some clinical cases. Materials and MethodsStudy designAll animal’s experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Approval ID#: CU/II/S/23/16. The main inclusion criteria for this study were: chronic spinal cord injury showing no clinical improvement after standard treatment and rehabilitation over 2 months. The exclusion criteria were any record of neoplasia or systemic illness. Study populationThis study included 5 dogs (3 Mongrel dogs and 1 cocker spaniel and 1 Griffon, and 3 cats 1 Persian, 2 Egyptian Maus). Animals were admitted to the surgery hospital suffering from intervertebral disc protrusion at the region of T13-L4 with various degrees during the period from January to April 2020 according to the previously mentioned inclusion and exclusion criteria. Cell preparationAutologous fresh bone marrow was aspirated from the iliac crest of each animal on heparin as an anticoagulant. Bone marrow samples were over-layered on 20 ml Ficoll, then centrifuged for 30 minutes. at 400 × g. BM-MNCs fraction were isolated. The layer of BM-MNCs layer was carefully aspirated and resuspended in PBS and centrifuged for 10 minutes at 200 × g. Then the cell pellet was resuspended in PBS for washing and centrifuged for 10 minutes at 200 × g (Sotiropoulou et al., 2006). PRP preparationA blood sample (20 ml in the case of dogs and 10 ml in the case of cats) was withdrawn on Acid Citrate Dextrose-A anticoagulant, centrifuged at 200 × g for 15 minutes. The plasma layer and the buffy coat were aspirated into a new tube, centrifuged at 200 × g for 30 minutes. The cell pellet and overlaying 3 ml were aspirated, calcium chloride 10% was added to activate the PRP, incubated at 37oC. The resultant was the formation of a gel and kept in a shaking water bath for 2 hours at 37oC till the gel was dissociated. The PRP was added to the BM-MNCs (Amable et al., 2013). InjectionThe SC preparation was divided into two parts, one part (4 × 106 nucleated cells) was injected intrathecally directly in the CSF through the foramen magnum in the proximal spinal cistern under general anesthesia through intravenous injection of a mixture of ketamine 10 mg/kg (Ketalar 5% ® Amoun, Cairo, Egypt) and Xylazine 1mg/kg (Xyla-Ject® 2% ADWIA, Cairo, Egypt) and the other part (6 × 106 nucleated cells) in 50 ml of normal saline were injected systemically through an intravenous cannula and a blood infusion set with a 170 μm filter with a rate of 7–10 ml/minute. EvaluationAll the animals underwent myelography using Omnipaque® 300 mg/ml under general anesthesia as mentioned before, 1 week before the transplantation to detect any obstruction in the flow of the CSF and the compression over the spinal cord. Changes in animals’ gait were measured according to the standard Olby score for the ataxia, tail movements and proprioception (Olby et al., 2014) before and throughout 90 days period after treatment. Magnetic resonanceUnder general anesthesia magnetic resonance imaging (MRI) was performed before and after treatment using a 1.5 Tesla closed MRI machine. The spinal imaging protocol included sagittal and dorsal T2 weighted and T1-weighted, transverse T2-weighted (TR/TE 3290/99 ms) and T1-weighted and sagittal sequences. Ethical approvalIACUC with approval ID#: CU/II/S/23/16. ResultsBefore treatment, all animals showed similar clinical signs, including paraplegia of the hind limbs, loss of muscle tone, absence of most of the reflexes, especially proprioception reflexes. Lameness score for all cases before and after treatment as listed in Table 1. No adverse reaction was recorded after the injection; no swelling, edema, inflammation, or other complications were recorded. All animals in this study showed major clinical improvement starting from 15 days post injection with physical rehabilitation at home, restored the control over the urination in all animals mostly from the second week post transplantation (Supplementary video showing the clinical improvement of case 1: https://www.openveterinaryjournal.com/case-1), regained the reflexes tone, and regained control of the full weight bearing locomotion without assistance around 60 days post transplantation for dogs and 45 days post transplantation for cats (Figs. 1–3). Magnetic resonance did not show a marked improvement over the 90 days for the intervertebral disc extrusion lesions (Fig. 4). Table 1. Lameness score for all examined cases showing the degree of lameness (Olby’s Score) before and after treatment and the % of improvement after treatment.
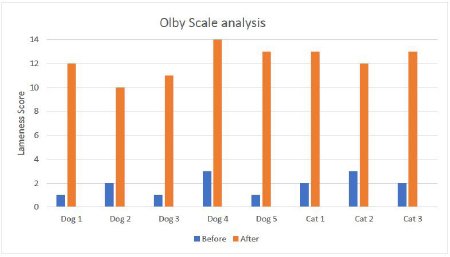
Fig. 1. showing the clinical score analysis according to the Olby scale. Notice all cases reached a near-normal gait after treatment period of 90 days. DiscussionThis study aimed to evaluate the clinical application and the therapeutic effect of BM-MNCs in a PRP vehicle as a one-time combined intrathecal/intravenous injection for chronic spinal cord injuries in selected cases of spinal cord compressions due to incomplete intervertebral disc protrusion that did not respond to conventional treatments. The main inclusion criteria for this study were: chronic spinal cord injury showing no clinical improvement after standard treatment and rehabilitation over 2 months, excluding vertebral fractures. BM-MNCs is a rich source of SCs, enclosing heterogeneous populations of lymphocytes, mesenchymal and hematopoietic SCs, hematopoietic and endothelial progenitor cells. They can be swiftly collected from the bone marrow, easily separated, isolated, and then returned to the recipient animal or human (Suda, 2017). BM-MNCs was chosen as a source for SCs because it is easy to obtain, without any requirement of a donor animal, and bone marrow is rich in mesenchymal, hematopoietic and endothelial SCs (Elawady et al., 2016). The PRP is an autologous concentrated blend of GFs and inflammatory mediators. It has been believed to be potentially valuable for soft and hard tissue restoration and regenerative process (Xie et al., 2014). PRP was chosen as a vehicle for transportation and injection of SCs as it is very rich in GFs and cytokines like NGF and BDNF that will additionally stimulate the spinal cord regeneration processes (Yu et al., 2011). The site of injection of the cells was chosen far from the lesions as SCs homing capacity was evaluated in our previous study model using labelled SCs transplanted intrathecally that was positively homed to lumbar lesions (Abdallah et al., 2019), which did not put a limitation for transplantation at the lesion site and was preferred over lumbar injection because of the ease of application and ensuring that all affected lesions receive an adequate amount of SCs especially when multiple lesions are present. Another delivery method that is not preferred is a direct intraspinal injection, where it is difficult to achieve the precise location of the lesion and requires a more invasive technique to expose the spinal cord (Amemori et al., 2015).
Fig. 2. Mean values of lameness score for all examined cases before treatment and after 90-days post treatment.
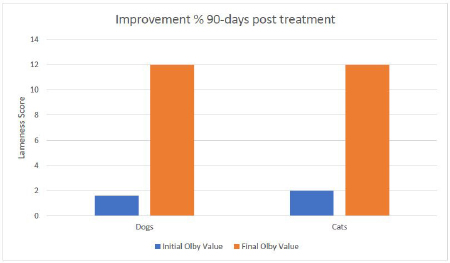
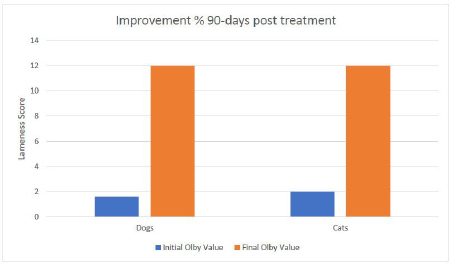
Fig. 3. Mean values of lameness score for all examined cases before treatment and at 15, 30, 45, 60, 75 and 90-days post treatment. The other part of the cells was applied intravenously to obtain full coverage of the lesion to reach the spinal cord or the damaged peripheral nerves too (Akiyama et al., 2002; Kim et al., 2015). The systemic application of cells was safe and did not produce any adverse reactions or complications as stated in the previous studies. The MRI scans did not show a major improvement regarding the protruded discs. This might be attributed to the longer periods required for intervertebral discs to heal (Skovrlj et al., 2015). However, the clinical signs were ameliorated due to the transplanted SCs capable of stopping the inflammation, necrosis, and apoptosis and stimulating the regeneration of the neurons and axons (Kim et al., 2015). This combination proved its safety as no adverse effects were recorded in all animals included in this study from the surgery day till 90 days post-transplantation. The clinical evaluation of the transplanted cells proved its efficacy in the treatment of the spinal cord, which was noticed even before reaching the end of the evaluation period, where animals were able to maintain full weight bearing walking more than 75% of the time without assistance. The final lameness score for all examined cases at the end of the observation period showed great improvements with a mean value percentage of 74.3% and 76.2% for dogs and cats respectively, compared to their initial lameness score values. One dog case showed complete recovery after the 90 days observation period post-treatment. It was worth noticing that the animals starting with a higher degree of lameness (lesser Olby’s values) showed better results at the end of the observation period.
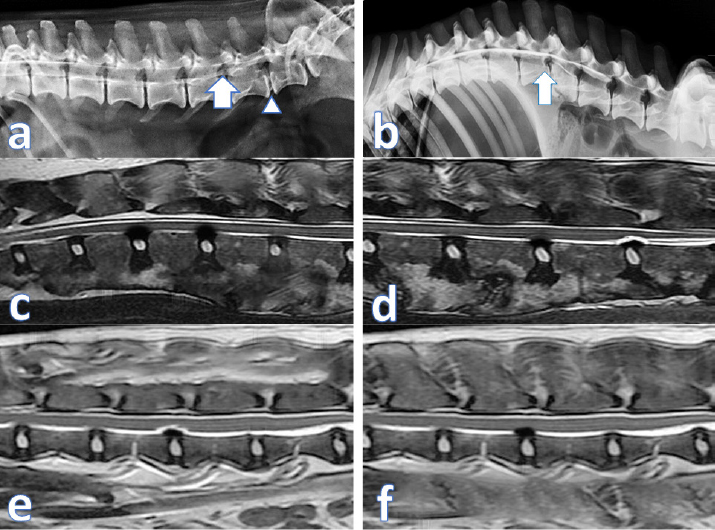
Fig. 4. (a) contrast Myelography where spinal cord of a cat 1 showing mild degree of spinal cord compression (arrow) and severe disc compression and mineralization (arrow head); (b) spinal cord myelography of a dog 1 showing severe compression (arrow); (c) MRI scan of cat 2 showing the protrusion of 2 discs before treatment; (d) MRI scan of Dog 2 showing the same lesion after treatment; (e) MRI scan of cat 2 showing the disc protrusion before treatment; (f) MRI scan of cat 2 showing the same lesion after treatment. Although clinical lameness score analysis for all examined cases showed quick improvements in week two post treatment, where cats showed a slightly better improvement curve than dogs after 15 days from treatment and after that. Noticeable improvements continued until 45 days in cats and 60 days in dogs. The lameness scores became steady for both species until the end of the observation period. These findings coincided with our previous studies that: (1) the injected cells migrated to the site of lesions (homing) within the first week; (2) the effect of inhibiting apoptosis, stimulating regeneration, remyelination and differentiation into neural cell lines occurs from the second week up to a month (Abdallah et al., 2019). By 45 days post treatment, the injected cells cease to exist in the extracellular space, and their reparative effect is diminished. This would propose a second injection at 45 days that will regain the reparative effect and increase the chances of a full recovery. The proprioception and nociception reflexes were restored, and animals regained control of the urination and defecation, which confirms the studies on animal models like rats and mice (Neirinckx et al., 2015; Elawady et al., 2016) and dogs (Gabr et al., 2015). ConclusionBM-MNCs in PRP vehicle transplantation for spinal cord injury is a safe procedure, time-saving, and provided a passage to functional recovery and restoration of the locomotor and sensory activity in companion animals. The utmost effect of the single injection lasts for 45 days. A second injection at 45 days may increase the chances of full recovery. This treatment shows a promising hope for the treatment of chronic spinal cord injuries. AcknowledgmentsThis paper is based upon the work supported by Science, Technology & Innovation Funding Authority (STDF) under grant (26268). Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionAshraf Shamaa and Omar EL-Tookhy were responsible for case evaluation before and after the treatment. Ahmed Abdallah was responsible for the preparation of the stromal/SCs and PRP. All authors read and approved the final manuscript. Supplementary materialSupplementary video showing the clinical improvement of One link including case 1 as follows: https://www.openveterinaryjournal.com/case-1 ReferencesAbdallah, A.N., Shamaa, A.A. and El-Tookhy, O.S. 2019. Evaluation of treatment of experimentally induced canine model of multiple sclerosis using laser activated non-expanded adipose derived stem cells. Res. Vet. Sci. 125, 71–81; doi:10.1016/j.rvsc.2019.05.016. Akiyama, Y., Radtke, C., Honmou, O. and Kocsis, J.D. 2002. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia 39, 229–236; doi:10.1002/glia.10102. Amable, P.R., Carias, R.B.V., Teixeira, M.V.T., da Cruz Pacheco, I., Corrêa do Amaral, R.J.F., Granjeiro, J.M. and Borojevic, R. 2013. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4, 67; doi:10.1186/scrt218. Amemori, T., Ruzicka, J., Romanyuk, N., Jhanwar-uniyal, M., Sykova, E. and Jendelova, P. 2015. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. Ther. 6, 1–11; doi:10.1186/s13287-015-0255-2. Bartholdi, D. and Schwab, M.E. 1998. Oligodendroglial reaction following spinal cord injury in rat: transient upregulation of MBP mRNA. Glia 23, 278–284. Dasari, V.R., Spomar, D.G., Cady, C., Gujrati, M., Rao, J.S. and Dinh, D.H. 2007. Mesenchymal stem cells from rat bone marrow downregulate caspase-3-mediated apoptotic pathway after spinal cord injury in rats. Neurochem. Res. 32, 2080–2093; doi:10.1007/s11064-007-9368-z. Denny, H.R. and Butterworth, S.J. 2000. Further investigation of spinal diseases. Elawady, M.A., Elmaghrabi, M.M., Ebrahim, N., Elawady, M.A., Sabry, D., Shamaa, A. and Ragaei, A. 2016. Therapeutic potential of bone marrow derived mesenchymal stem cells in modulating astroglyosis of surgical induced experimental spinal cord injury. Adv. Biosci. Biotechnol. 7(6), 251–265. Gabr, H., El-Kheir, W.A., Farghali, H.A.M.A., Ismail, Z.M.K., Zickri, M.B., El Maadawi, Z.M., Kishk, N.A. and Sabaawy, H.E. 2015. Intrathecal transplantation of autologous adherent bone marrow cells induces functional neurological recovery in a canine model of spinal cord injury. Cell Transplant. 24, 1813–1827; doi:10.3727/096368914X683025. Gronthos, S., Franklin, D.M., Leddy, H.A, Robey, P.G., Storms, R.W. and Gimble, J.M. 2001. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189, 54–63; doi:10.1002/jcp.1138. Hunt, D.P.J., Irvine, K.A., Webber, D.J., Compston, D.A.S., Blakemore, W.F. and Chandran, S. 2008. Effects of direct transplantation of multipotent mesenchymal stromal/stem cells into the demyelinated spinal cord. Cell Transplant. 17, 865–873; doi:10.3727/096368908786516738. Kennea, N.L., Waddington, S.N., Chan, J., O’Donoghue, K., Yeung, D., Taylor, D.L., Al-Allaf, F.A, Pirianov, G., Themis, M., Edwards, A.D., Fisk, N.M. and Mehmet, H. 2009. Differentiation of human fetal mesenchymal stem cells into cells with an oligodendrocyte phenotype. Cell Cycle 8, 1069–1079; doi:10.4161/cc.8.7.8121. Kerr, C.L., Letzen, B.S., Hill, C.M., Agrawal, G., Thakor, N.V, Sterneckert, J.L., Gearhart, J.D. and All, A.H. 2010. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int. J. Neurosci. 120, 305–313; doi:10.3109/00207450903585290. Kim, Y., Jo, S., Kim, W.H. and Kweon, O.-K. 2015. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 6, 229; doi:10.1186/s13287-015-0236-5. Le Blanc, K. 2006. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy 8, 559–561; doi:10.1080/14653240601045399. Neirinckx, V., Agirman, G., Coste, C., Marquet, A., Dion, V., Rogister, B., Franzen, R. and Wislet, S. 2015. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res. Ther. 6, 211; doi:10.1186/s13287-015-0202-2. Neirinckx, V., Cantinieaux, D., Coste, C., Rogister, B., Franzen, R. and Wislet-Gendebien, S. 2014. Concise review: spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge. Stem Cells 32, 829–843; doi:10.1002/stem.1579. Neirinckx, V., Marquet, A., Coste, C., Rogister, B. and Wislet-Gendebien, S. 2013. Adult bone marrow neural crest stem cells and mesenchymal stem cells are not able to replace lost neurons in acute MPTP-lesioned mice. PLoS One 8, 1–10; doi:10.1371/journal.pone.0064723. Olby, N.J., Lim, J.-H., Babb, K., Bach, K., Domaracki, C., Williams, K., Griffith, E., Harris, T. and Muguet-Chanoit, A. 2014. Gait scoring in dogs with thoracolumbar spinal cord injuries when walking on a treadmill. BMC Vet. Res. 10, 58; doi:10.1186/1746-6148-10-58. Salgado, A.J., Reis, R.L., Sousa, N. and Gimble, J.M. 2010. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 5, 103–110. Shamaa, A.A., El-Tookhy, O.S. and Abdallah, A.N. 2018. Progressive model of multiple sclerosis following ethidium bromide injection in dogs’ spinal cord: failure of endogenous remyelination. Biosci. Res. 15, 2327–2337. Skovrlj, B., Qureshi, S. and Singh, K. 2015. Mesenchymal stem cells for intervertebral disc repair and regeneration. Semin. Spine Surg. 27, 76–81; doi:10.1053/j.semss.2015.03.002 Sotiropoulou, P.A., Perez, S.A., Salagianni, M., Baxevanis, C.N. and Papamichail, M. 2006. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24, 462–471; doi:10.1634/stemcells.2004-0331. Suda, S. 2017. Bone marrow-derived mononuclear cells. In: Cell therapy against cerebral stroke: comprehensive reviews for translational researches and clinical trials. Eds., Houkin, K., Abe, K., Kuroda, S. Springer, Berlin, Germany, pp: 3–14; doi:10.1007/978-4-431-56059-3_1 Tetzlaff, W., Okon, E.B., Karimi-Abdolrezaee, S., Hill, C.E., Sparling, J.S., Plemel, J.R., Plunet, W.T., Tsai, E.C., Baptiste, D., Smithson, L.J., Kawaja, M.D., Fehlings, M.G. and Kwon, B.K. 2011. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682; doi:10.1089/neu.2009.1177. Tondreau, T., Dejeneffe, M., Meuleman, N., Stamatopoulos, B., Delforge, A., Martiat, P., Bron, D. and Lagneaux, L. 2008. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics 9, 166; doi:10.1186/1471-2164-9-166. Weh, M. and Kraus, K.H. 2011. Spinal fractures and luxations. In Veterinary surgery: small animal. Eds., Tobias, K.M., Johnston, S.A. St. Louis, MO: Saunders, pp: 487–503. Xie, X., Zhang, C. and Tuan, R.S. 2014. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 16, 204; doi:10.1186/ar4493 Yu, W., Wang, J. and Yin, J. 2011. Platelet-rich plasma: a promising product for treatment of peripheral nerve regeneration after nerve injury. Int. J. Neurosci. 121, 176–180; doi:10.3109/00207454.2010.544432 Yuan, Y.-M. and He, C. 2013. The glial scar in spinal cord injury and repair. Neurosci. Bull. 29, 421–435; doi:10.1007/s12264-013-1358-3. Zhang, N., Yin, Y., Xu, S., Wu, Y. and Chen, W. 2012. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 135, 287–296. | ||
| How to Cite this Article |
| Pubmed Style Abdallah ANE, Shamaa AA, El-tookhy OS, Bahr MM. Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Vet. J.. 2021; 11(2): 270-276. doi:10.5455/OVJ.2021.v11.i2.10 Web Style Abdallah ANE, Shamaa AA, El-tookhy OS, Bahr MM. Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. https://www.openveterinaryjournal.com/?mno=49836 [Access: January 24, 2026]. doi:10.5455/OVJ.2021.v11.i2.10 AMA (American Medical Association) Style Abdallah ANE, Shamaa AA, El-tookhy OS, Bahr MM. Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Vet. J.. 2021; 11(2): 270-276. doi:10.5455/OVJ.2021.v11.i2.10 Vancouver/ICMJE Style Abdallah ANE, Shamaa AA, El-tookhy OS, Bahr MM. Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Vet. J.. (2021), [cited January 24, 2026]; 11(2): 270-276. doi:10.5455/OVJ.2021.v11.i2.10 Harvard Style Abdallah, A. N. E., Shamaa, . A. A., El-tookhy, . O. S. & Bahr, . M. M. (2021) Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Vet. J., 11 (2), 270-276. doi:10.5455/OVJ.2021.v11.i2.10 Turabian Style Abdallah, Ahmed Nour Eldine, Ashraf Aly Shamaa, Omar Salah El-tookhy, and Mohamed Moustafa Bahr. 2021. Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Veterinary Journal, 11 (2), 270-276. doi:10.5455/OVJ.2021.v11.i2.10 Chicago Style Abdallah, Ahmed Nour Eldine, Ashraf Aly Shamaa, Omar Salah El-tookhy, and Mohamed Moustafa Bahr. "Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals." Open Veterinary Journal 11 (2021), 270-276. doi:10.5455/OVJ.2021.v11.i2.10 MLA (The Modern Language Association) Style Abdallah, Ahmed Nour Eldine, Ashraf Aly Shamaa, Omar Salah El-tookhy, and Mohamed Moustafa Bahr. "Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals." Open Veterinary Journal 11.2 (2021), 270-276. Print. doi:10.5455/OVJ.2021.v11.i2.10 APA (American Psychological Association) Style Abdallah, A. N. E., Shamaa, . A. A., El-tookhy, . O. S. & Bahr, . M. M. (2021) Effect of combined intrathecal/intravenous injection of Bone Marrow Derived Stromal Cells in Platelet Rich Plasma on Spinal Cord Injury in Companion Animals. Open Veterinary Journal, 11 (2), 270-276. doi:10.5455/OVJ.2021.v11.i2.10 |