| Original Article | ||
Open Vet. J.. 2022; 12(3): 383-390 Open Veterinary Journal, (2022), Vol. 12(3): 383–390 Original Research Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stagesToshie Iseri*, Hiro Horikirizono, Momoko Abe, Harumichi Itoh, Hiroshi Sunahara, Yuki Nemoto, Kazuhito Itamoto, Kenji Tani and Munekazu NakaichiJoint Faculty of Veterinary Science, Yamaguchi University, Yamaguchi, Japan *Corresponding Author: Toshie Iseri. Joint Faculty of Veterinary Science, Yamaguchi University, 1677-1 Yoshida, Yamaguchi-City, Yamaguchi, 753-8515, Japan. Email: iseri [at] yamaguchi-u.ac.jp Submitted: 04/04/2022 Accepted: 16/05/2022 Published: 07/06/2022 © 2022 Open Veterinary Journal
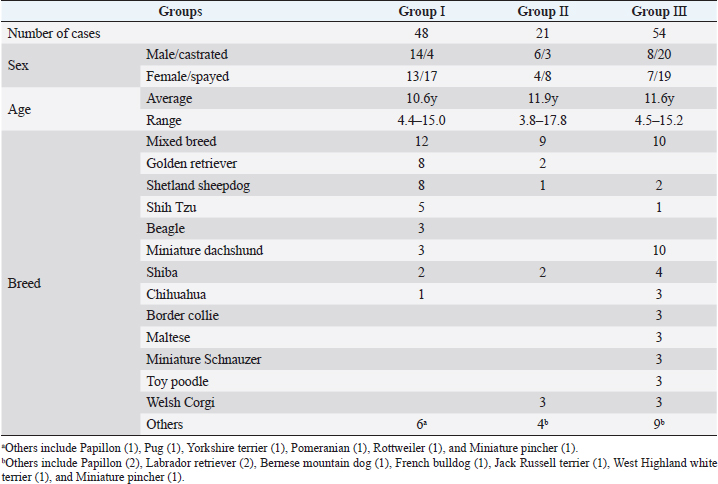
AbstractBackground: Radiation therapy is considered important for the treatment of intranasal tumors in dogs and is believed to be essential for prolonging their survival. Aim: To investigate the contribution of clinical staging to improve outcomes of megavoltage radiotherapy for canine intranasal tumors. Methods: A total of 123 dogs with intranasal tumors were included in the study. Forty-eight dogs received orthovoltage radiotherapy after cytoreductive surgery (Group I), 21 received orthovoltage radiotherapy without surgery (Group II), and 54 received megavoltage radiotherapy without surgery (Group III). All cases in each group were classified into clinical stages 1–4, and the median survival time (MST) was compared for each stage in all groups. Results: The overall MST was not significantly difference among Group I (325 days), Group II (317 days), and Group III (488 days); however, Group III was prolonged than Groups I and II. The MSTs for stages 1, 2, 3, and 4 were 597, 361, 267, and 325 days in Group I; 633, 260, 233, and 329 days in Group II; and 931, 860, 368, and 176 days in Group III, respectively. The MST for stage 2 cases in Group III was significantly prolonged when compared with that in Groups I and II; no significant difference was observed at other stages; however, the MST in Group III was longer in stage 1. These results showed that megavoltage radiotherapy prolonged the MST in dogs with intranasal tumors when compared to orthovoltage radiation with or without cytoreductive surgery, and that improvements in MST at stage 2 contributed significantly to this. Conclusion: The improvement in the MST in dogs with stages 1 and 2 intranasal tumors highlights the importance of starting megavoltage radiotherapy in the early stages. Keywords: Dog, Intranasal tumor, Megavoltage, Orthovoltage, Radiotherapy. IntroductionIntranasal tumors in dogs are reported to account for approximately 1% of all malignant tumors (Lana and Turek, 2020). Intranasal tumors cause facial deformity as they progress and severe neurological symptoms when they invade the cribriform plate and adjacent brain; therefore, early treatment is important. It is widely recognized that radiotherapy plays an important role in the treatment of intranasal tumors in small animal practice (Henry et al. 1998; LaDue et al., 1999; Gieger et al., 2008; Kondo et al., 2008; Adams et al., 2009; Buchholz et al., 2009; Hunley et al. 2010; Maruo et al., 2011; Fujiwara et al., 2013; Mason et al., 2013; Northrup et al., 2001). Radiotherapy for intranasal tumors in dogs may involve either a combination of cytoreductive surgery and postoperative orthovoltage radiation or megavoltage radiotherapy using a linear accelerator. Treatment results for canine intranasal tumors have varied and have been improved by megavoltage radiation (Henry et al., 1998; Gieger et al., 2008; Adams et al., 2009; Buchholz et al., 2009; Hunley et al., 2010; Maruo et al., 2011; Fujiwara et al., 2013; Mason et al., 2013), although the usefulness of orthovoltage radiation has also been reported (LaDue et al., 1999; Northrup et al., 2001; Kondo et al., 2008). However, only a few studies have investigated the factors contributing to prolonged survival in dogs with intranasal tumors after megavoltage radiotherapy (LaDue, et l., 1999; Adams et al., 2009; Mason et al., 2013). In this study, we retrospectively examined clinical outcomes of each treatment methods: orthovoltage radiation in combination with or without cytoreductive surgery and megavoltage radiation alone. Based on these results, we examined the involvement of clinical staging in improving the outcome of megavoltage radiotherapy for canine intranasal tumors. Materials and MethodsThis retrospective study analyzed the medical records of dogs with intranasal tumors that received radiotherapy with a total dose of ≥30 Gy at our veterinary hospital between May 1999 and October 2020. Each owner was informed and given written consent prior to treatment that case data, excluding personal information, would be used for research purposes. Primary intranasal lymphoma cases were excluded, and 123 cases of canine intranasal tumors were included. Group I comprised 48 cases treated by cytoreductive surgery and postoperative orthovoltage radiation (1999–2016); Group II comprised 21 cases treated by orthovoltage radiation alone (1999–2016); and Group III comprised 54 cases treated by megavoltage radiation alone (2016–2020). The background of the cases in each group is summarized in Table 1. Group I comprised 14 males, 4 castrated males, 13 females, and 17 spayed females. Group II comprised 6 males, 3 castrated males, 4 females, and 8 spayed females. Group III comprised 13 males, 17 castrated males, 7 females, and 19 spayed females. The average age was 10.6 years (range, 4.4–15.0 years) in Group I, 11.9 years (range, 3.8–17.8 years) in Group II, and 11.6 years (range, 4.5–15.2 years) in Group III. The major breeds (≥3 cases) included in each group are as follows. Group I: mixed breed (n=12), golden retriever (n=8), Shetland sheepdog (n=8), Shih Tzu (n=5), beagle (n=3), and miniature dachshund (n=3); Group II: mixed breed (n=9) and Welsh corgi (n=3); and Group III: mixed breed (n=10), miniature dachshund (n=10), Shiba (n=4), border collie (n=3), Chihuahua (n=3), Maltese (n=3), miniature schnauzer (n=3), toy poodle (n=3), and Welsh corgi (n=3). Other varieties (n ≤2) are listed in Table 1. In all cases, computed tomography (CT) (Supria™; Hitachi Medico, Tokyo, Japan) confirmed the presence of lesions in the nasal cavity. Bone involvement, extension of the tumor tissue outside the nasal cavity, cribriform plate involvement, and distant metastasis were also examined. Based on the CT findings, the cases included in each group were classified into four clinical stages according to the modified Adams CT staging (Adams et al., 2009.), as follows: stage 1, tumor localized unilaterally to one nasal passage; stage 2, tumor that involved the nasal septum but with no evidence of orbital/subcutaneous/submucosal masses; stage 3, tumor with orbital involvement or subcutaneous/submucosal masses; and stage 4, tumor that invaded the cribriform plate. Following diagnostic imaging, biopsy specimens were obtained from the nasal cavity for histopathological determination. In Group I, a final histopathological diagnosis was made in surgically removed specimens. In Group I, the dogs were first subjected to cytoreductive surgery as described elsewhere (Weeden and Degner, 2016.). The nasal cavity was exposed by excising the nasal bone, and as much tumor tissue as possible was manually removed from the nasal cavity. Hemostasis was performed by electrical coagulation and manual compression of the bleeding site. After removal of the tumor tissues, the inside of the nasal cavity was thoroughly washed, and if possible, the nasal bone was repaired. Postoperative radiotherapy was started 1–2 weeks after surgery. In Groups I and II, a total dose of 36–42 Gy (single dose of 4 or 6 Gy, 6–10 fractions) was delivered to the nasal cavity in all cases using an orthovoltage radiotherapy device (MBR-320; Hitachi Medico). The irradiation conditions are as follows: tube voltage, 300 kVp; tube current, 12 mA; and 1 mm Cu + 1 mm Al as a filter. The irradiation field was selected from squares of 4, 6, or 8 cm on each side, depending on the size of the dog, with an appropriate shielding of the eyeball using a lead plate. After finishing the orthovoltage radiation protocol, the dogs were examined regularly to confirm clinical symptoms, including adverse effects. CT examinations were performed regularly as needed. Table 1. List of case details.
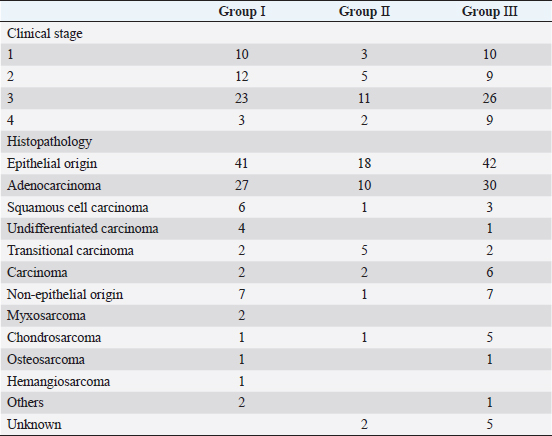
In Group III, diagnosis of the intranasal tumor was made as in Groups I and II. Definitive-intent radiotherapy was performed using the Elekta Synergy Platform™ (Elekta, Stockholm, Sweden) with a multileaf collimator with 5-mm leaves (Agility™; Elekta). The treatment plan was developed using a treatment planning system (Monaco™; Elekta) based on images obtained using planning CT. The gross tumor volume (GTV) was contoured using CT images with a 1.25-mm slice thickness, and the clinical target volume (CTV) was delineated to include 2 mm around the GTV. The planning target volume (PTV) was defined by a margin of 4 mm around the CTV. The organs at risk (OAR), such as eyeballs and brain, were also contoured on the CT images to be excluded from the radiation field. Mandibular and retropharyngeal lymph nodes were irradiated if they were enlarged on CT images. Two irradiation protocols were used [three-dimensional conformal radiotherapy in 11 cases and intensity-modulated radiotherapy (IMRT) in 43 cases], and the goal was for 100% of the PTV to receive ≥ 95% of the prescribed dose. Radiotherapy in Group III was delivered in 10 fractions × 4.2 Gy (total dose of 42 Gy) twice weekly with 4 or 6 megavolts. After finishing the radiation protocol, regular CT examinations were performed as in Groups I and II. Survival of all cases was calculated based on medical records or telephone interviews with referral veterinarians, dog owners, or both. The survival time was defined from the date of the first visit to our veterinary hospital to the date of death. By comparing the median survival time (MST) at each stage in Groups I, II, and III, we examined the effect of the clinical stage on improvements in the outcome of megavoltage radiotherapy. The Kaplan–Meier method and log-rank test were used to analyze survival and test for significant differences. Analyses were conducted using JMP Pro 16.1.0 (SAS Institute Inc. NC,). A P-value of <0.05 was considered significant. ResultsThe dogs included in each group were classified into stages 1–4 according to the modified Adams CT staging (Table 2). In Group I, stages 1–4 cancer were observed in 10, 12, 23, and 3 dogs, respectively. In Group II, stages 1–4 cancer were observed in 3, 5, 11, and 2 dogs, respectively. In Group III, stages 1–4 cancer were observed in 10, 9, 26, and 9 dogs, respectively. For histopathological classification, Group I included 41 epithelial tumors and 7 non-epithelial tumors; Group II included 18 epithelial tumors and 1 non-epithelial tumor; and Group III included 42 epithelial tumors and 7 non-epithelial tumors. No definitive histopathological diagnosis was obtained in the remaining two cases in Group II and five cases in Group III because the tumor tissue was too small or sufficient samples for histological examination could not be obtained. The most common histopathological type was adenocarcinoma in all groups (27 cases in Group I, 10 cases in Group II, and 30 in Group III). The detailed histopathological types in all groups and the number of cases are shown in Table 2. Table 2. Number of cases based on clinical stage and histopathological classification in each group.
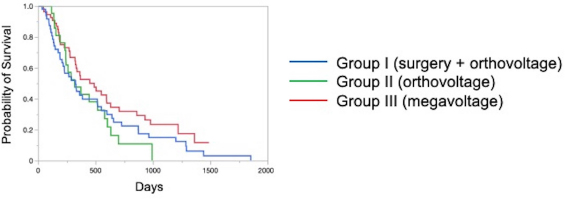
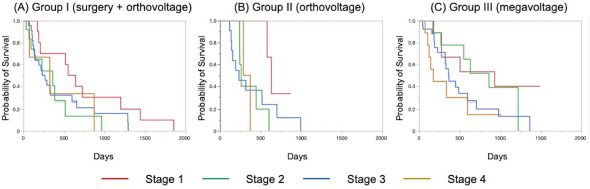
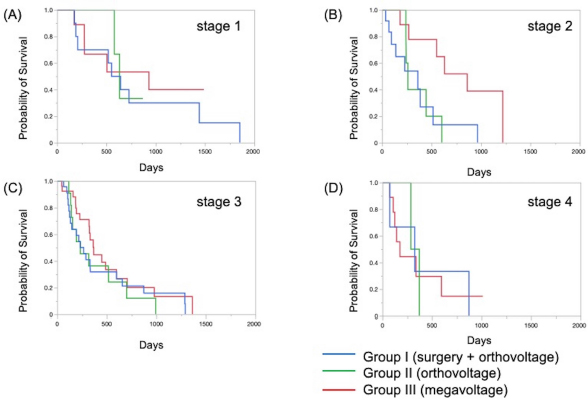
The Kaplan–Meier survival curves of all cases in three groups are shown in Figure 1. The MST was 325 days for Group I, 317 days for Group II, and 488 days for Group III; although there was no significant difference, the MST was prolonged in Group III compared to the other two groups. The Kaplan–Meier survival curves of each clinical stage in three gropes are shown in Figure 2. The MSTs for stages 1, 2, 3, and 4 were 597, 361, 267 and 325 days in Group I; 633, 260, 233, and 329 days in Group II; and 931, 860, 368, and 176 days in Group III, respectively. In Groups I and II, no significant differences in MST were observed among the stages. In Group III, the MST tended to decrease as the stages progressed; however, as in Groups I and II, there were no significant differences among the stages. Figure 3 shows a comparison of the MSTs between the same clinical stages in three groups. There was a significant difference between the MSTs for stage 2 in among the three groups. The MST for stage 2 cases in Group III was significantly longer than that in Groups I and II. However, no significant differences were found for the other stages. DiscussionThere have been many reports on the results of megavoltage radiotherapy for intranasal tumors in dogs. Although there were some differences in detailed conditions and treatment schedules, previous reports showed that megavoltage radiotherapy yielded MSTs of 146–512 days (Mellanby et al., 2002; Gieger et al., 2008; Adams et al., 2009; Buchholz et al., 2009; Kunley et al., 2010; Lawrence et al., 2010; Maruo et al., 2011; Fujiwara et al., 2013). These results appear to have gradually improved over time. Megavoltage radiotherapy is currently considered to be the most effective treatment for intranasal tumors in dogs. Better treatment results with an MST of ≥500 days have been reported for the combination of radiotherapy and intralesional chemotherapy (Lana et al., 1997, 2004). Furthermore, clinical applications of stereotactic radiotherapy and stereotactic body radiotherapy have recently been reported, and the MST for affected dogs has been further extended to 388–586 days (Gieger and Nolan, 2018; Mayer et al., 2019; Fox-Alvarez et al., 2020). Recurrence of intranasal tumors is considered to have a significant effect on survival, and the efficacy and safety of re-irradiation are being investigated for recurrent tumors in order to prolong the survival time of affected dogs (Bommarito et al., 2011; Gieger et al., 2013.). In this study, we compared the MSTs for all cases that received cytoreductive surgery and postoperative orthovoltage radiotherapy (Group I), orthovoltage radiotherapy alone (Group II), and megavoltage radiotherapy alone (Group III). Although not significantly different, the MST for Group III was longer than that for Groups I and II, indicating that megavoltage radiotherapy improved MST in dogs with intranasal tumors. The overall MST of our 54 cases that received megavoltage radiotherapy was 488 days, which seems to be equivalent to that in the report described above. The MSTs of our patients tended to shorten as the clinical stage progressed in Group III, as described previously (Adams et al., 2009). However, few reports have investigated factors related to improvements in treatment results (LaDue et al., 1999; Adams et al., 2009; Mason et al., 2013.). To clarify the factors associated with prolongation of the overall MST in Group III, we compared the MST for each stage among three groups. In stage 1, there was no significant difference among the three groups; however, stage 1 cases in all groups had longer MSTs: 597 days for Group I, 633 days for Group II, and 931 days for Group III. The MST in this study was considered excellent when compared with previous findings (Mellanby et al., 2002; Gieger et al., 2008; Hunley et al., 2010; Lawrence et al., 2010; Maruo et al., 2011). The reason for the good outcome of Group I may be that the tumor was localized unilaterally in the nasal passage, allowing good surgical removal of tumor tissue. Therefore, cytoreductive surgery accompanied by postoperative orthovoltage radiotherapy may be available for the earliest stage 1 intranasal tumors. Unlike in stage 1, there was a significant difference in the MSTs among the groups in stage 2, with Group III showing a longer MST. An MST of 860 days was obtained in Group III, which was considered acceptable and not significantly different from that of stage 1 cases. In stage 2 cases, tumor tissue extended to both nasal cavities, making cytoreductive surgery more difficult than in stage 1, and it tended to cause earlier recurrence. The predisposition to incomplete surgery in Group I may have been the major causes of the large difference in the MSTs of stage 2 cases. In Groups II and III, factors related to surgical techniques were considered negligible because the dogs in these groups were treated by radiotherapy alone. However, the significantly prolonged MST in Group III compared to Group II indicated that megavoltage radiotherapy had a superior therapeutic effect over orthovoltage radiotherapy alone in more advanced stages of disease. Additionally, these significant differences in the MSTs of stage 2 in among the three groups might have contributed to the difference in the overall MST among the groups. Megavoltage radiotherapy clearly prolonged the MST of canine intranasal tumors, and this improvement in stage 2 cases was considered to contribute to improved therapeutic outcomes after megavoltage radiotherapy. In stage 3, the MST was longer in Group III (368 days) than in Group I (267 days) and in Group II (233 days), but the difference was not significant. In stage 3, the tumor tissue extends outside the nasal cavity, such as to the orbit, which may make treatment difficult. In Group I, it was difficult to remove the tumor tissue that extended outside the nasal cavity by cytoreductive surgery. In Groups II and III, an effort was made to protect radiation-sensitive tissues, such as the eyeball from radiation, which may have resulted in inadequate treatment of tumor tissues in the orbital region. For these reasons, the MSTs may have been short in stage 3 in all groups. However, the reason for the prolonged MST in Group III over Group II was thought that the use of IMRT in Group III allowed the dose to be focused on the target while protecting OAR. In our study, stage 3 cases were most common in all groups; among all 123 cases, stage 3 accounted for 60 cases (49%), and late-stage cases, including stages 3 and 4, accounted for 74 cases (60%). Similarly, previous studies have included many late-stage cases (Rassnick et al., 2006; Adams et al., 2009; Mason et al., 2013). These data indicate that early treatment of canine intranasal tumors remains difficult. Megavoltage radiation to stages 1 and 2 intranasal tumors yielded favorable outcomes; therefore, it is considered important to begin treatment while tumors remain in the early clinical stages.
Fig. 1. Kaplan–Meier curves in all Group I and II cases. The MST of Group III (488 days) was longer than that of Group I (325 days) and Group II (317 days). MST: median survival time.
Fig. 2. Kaplan–Meier curves for each stage in Groups I (A), II (B), and III (C). The MSTs for stages 1, 2, 3, and 4 were 597, 361, 267, and 325 days in Group I; 633, 260, 233, and 329 days in Group II; and 931, 860, 363, and 176 days in Group III, respectively. There was no significant difference in MSTs for each clinical stage in either group. MST: median survival time. Stage 4 cases could not be fully investigated because there were only three in Group I and two in Group II. Surgery or orthovoltage radiation therapy alone for stage 4 cases is rarely performed because a sufficient therapeutic effect cannot be expected. Our study revealed a poor MST (176 days) for stage 4 cases, even with megavoltage radiotherapy. Previous reports have described MSTs of approximately 200 days and 274 days in stage 4 cases (Kondo et al., 2008; Mason et al., 2013). Our MST was inferior to these, but the cause is unknown. Stevens et al. performed intensity-modulated radiotherapy for stage 4 cases and reported an MST of 319 days (Stevens et al., 2020), which is superior to our result. Recently, clinical applications of new therapies, such as stereotactic radiotherapy and stereotactic body radiotherapy, have been reported (Gieger and Nolan, 2018; Mayer et al., 2019; Fox-Alvarez et al., 2020), and their use may extend to stage 4 tumors in the future.
Fig. 3. Kaplan–Meier curves for each clinical stage in Groups I, II, and III. (A) Stage 1, (B) stage 2, (C) stage 3, and (D) stage 4. In stage 2 cases, the survival time of Group III was significantly prolonged when compared to that in Groups I and II (p=0.034). However, there were no significant differences at other stages. In addition to the clinical stage, many factors are considered to affect MSTs; one of these is the histopathological type of tumor (Henry et al., 1998; Gieger et al., 2008; Adams et al., 2009; Morgan et al., 2018; Sones et al., 2013). There are reports of shorter MSTs for epithelial tumors and adenocarcinomas (Gieger et al., 2008), and non-epithelial tumors are reportedly associated with long survival times (Rassnick et al., 2006). However, one study reported that the volume reduction effect on CT images was smaller for non-epithelial tumors than for epithelial tumors, suggesting that non-epithelial tumors are radio-resistant (Morgan et al., 2018). At present, no definitive conclusion has been reached regarding the effect of histopathological type on the survival of dogs with intranasal tumors. Although the number of cases was limited in our study, the outcome of megavoltage radiotherapy for non-epithelial tumors did not differ significantly from that of epithelial tumors (data not shown). It will be necessary to continue to study the effects of histopathological types on patient survival in the future. In conclusion, megavoltage radiotherapy for canine intranasal tumors improved outcomes primarily in stage 2 cases when compared with surgery and postoperative orthovoltage radiation, and orthovoltage radiation without surgery. This effect was considered to contribute to the improvement of overall MSTs in dogs with intranasal tumors; however, no definitive conclusion was reached regarding the effect of histopathological type on the efficacy of megavoltage radiotherapy. Furthermore, for megavoltage radiotherapy, the treatment outcome of stage 1 and 2 canine intranasal tumors was excellent. This emphasizes the importance of megavoltage radiotherapy for early treatment of canine intranasal tumors. In the future, it will be necessary to examine in detail the factors that affect treatment outcomes while improving outcomes by introducing new radiotherapy techniques. AcknowledgmentThe authors thank Cathel Kerr, BSc, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsRadiation therapy was performed by Toshie Iseri, Hiro Horikirizono, Harumichi Itoh, Hiroshi Sunahara, Yuki Nemoto, Kazuhito Itamoto, Kenji Tani, and Munekazu Nakaichi. Toshie Iseri, Abe Momoko, and Munekazu Nakaichi were involved in the writing of the first draft, and all authors read and approved the final draft. ReferencesAdams, W.M., Kleiter, M.M., Thrall, D.E., Klauer, J.M., Forrest, L.J., La Due, T.A. and Havighurst T.C. 2009. Prognostic significance of tumor histology and computed tomographic staging for radiation treatment response of canine nasal tumors. Vet. Radiol. Ultrasound. 50, 330–335. Bommarito, D.A., Kent, M.S., Selting, K.A., Henry, C.J. and Lattimer. J.C. 2011. Reirradiation of recurrent canine nasal tumors. Vet. Radiol. Ultrasound. 52, 207–212. Buchholz, J., Hagen, R., Leo, C., Ebling, A., Roos, M., Kaser-Hotz, B. and Bley, C.R. 2009. 3D conformal radiation therapy for palliative treatment of canine nasal tumors. Vet. Radiol. Ultrasound. 50, 679–683. Fox-Alvarez, S., Shiomitsu, K., Lejeune, A.T., Szivek, A. and Kubicek, L. 2020. Outcome of intensity-modulated radiation therapy-based stereotactic radiation therapy for treatment of canine nasal carcinomas. Vet. Radiol. Ultrasound. 61, 370–378. Fujiwara, A., Kobayashi, T., Kazato, Y., Yayoshi, N. and Fujita, M. 2013. Efficacy of hypofractionated radiotherapy for nasal tumours in 38 dogs (2005-2008). J. Small Anim. Pract. 54, 80–86. Gieger, T., Rassnick, K., Siegel, S., Proulx, D., Bergman, P., Anderson, C., LaDue, T., Smith, A., Northrup, N. and Roberts, R. 2008. Palliation of clinical signs in 48 dogs with nasal carcinomas treated with coarse-fraction radiation therapy. J. Am. Anim. Hosp. Assoc. 44, 116–123. Gieger, T., Siegel, S., Rosen, K., Jackson, D., Ware, K., Kiselow, M. and Shiomitsu. K. 2013. Reirradiation of canine nasal carcinomas treated with coarsely fractionated radiation protocols: 37 cases. J. Am. Anim. Hosp. Assoc. 49, 318–324. Gieger, T.L. and Nolan, M.W. 2018. Linac-based stereotactic radiation therapy for canine non-lymphomatous nasal tumours: 29 cases (2013-2016). Vet. Comp. Oncol. 16, E68–E75. Henry, C.J., Brewer Jr., W.G., Tyler, J.W., Brawner, W.R., Henderson, R.A. Hankes, G.H. and Royer, N. 1998. Survival in dogs with nasal adenocarcinoma: 64 cases (1981-1995). J. Vet. Intern. Med. 12, 436–439. Hunley, D.W., Mauldin, G.N., Shiomitsu, K. and Mauldin, G.E. 2010. Clinical outcome in dogs with nasal tumors treated with intensity-modulated radiation therapy. Can. Vet. J. 51, 293–300. Kondo, Y., Matsunaga, S., Mochizuki, M., Kadosawa, T., Nakagawa, T., Nishimura, R. and Sasaki, N. 2008 Prognosis of canine patients with nasal tumors according to modified clinical stages based on computed tomography: a retrospective study. J. Vet. Med. Sci. 70, 207–212. LaDue, T.A., Dodge, R., Page, R.L., Price, G.S., Hauck, M.L. and Thrall, D.E. 1999. Factors influencing survival after radiotherapy of nasal tumors in 130 dogs. Vet. Radiol. Ultrasound. 40, 312–317. Lana, S.E. and Turek, M.M. 2020. Canine Nasosinal Tumors. In Withrow and MacEwen’s small animal clinical oncology, 6th ed., Eds., Vail, D.M., Thamm, D.H. and Liptak, J.M. St.Louis, MO: Saunders Elsevier, pp: 494–503. Lana, S.E., Dernell, W.S., Lafferty, M.H., Withrow, S.J. and LaRue. S.M. 2004. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet. Radiol. Ultrasound. 45, 577–581. Lana, S.E., Dernell, W.S., LaRue, S.M., Lafferty, M.J., Douple, E.B., Brekke, J.H. and Withrow, S. 1997. Slow release cisplatin combined with radiation for the treatment of canine nasal tumors. Vet. Radiol. Ultrasound. 38, 474–478. Lawrence, J.A., Forrest, L.J., Turek, M.M., Miller, P.E., Mackie, T.R., Jaradat, H.A., Vail, D.M., Dubielzig, R.R., Chappell, R. and Mehta, M.P. 2010. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity-modulated radiation therapy. Vet. Radiol. Ultrasound. 51, 561–570. Maruo, T., Shida, T., Fukuyama, Y., Hosaka, S., Noda, M., Ito, T., Sugiyama, H., Ishikawa, T. and Madarame, H. 2011. Retrospective study of canine nasal tumor treated with hypofractionated radiotherapy. J. Vet. Med. Sci. 73, 193–197. Mason, S.L., Maddox, T.W., Lillis, S.M., Blackwood, L. 2013. Late presentation of canine nasal tumours in a UK referral hospital and treatment outcomes. J. Small Anim. Pract. 54, 347–353. Mayer, M.N., DeWalt, J.O., Sidhu, N., Mauldin, G.N., Waldner. C.L. 2019. Outcomes and adverse effects associated with stereotactic body radiation therapy in dogs with nasal tumors: 28 cases (2011-2016). J. Am. Vet. Med. Assoc. 254, 602–612. Mellanby, R.J., Stevenson, R.K., Herrtage, M.E., White, R.A. and Dobson. J.M. 2002. Long-term outcome of 56 dogs with nasal tumours treated with four doses of radiation at intervals of seven days. Vet. Rec. 151, 253–257. Morgan, M.J., Lurie, D.M. and Villamil, A.J. 2018. Evaluation of tumor volume reduction of nasal carcinomas versus sarcomas in dogs treated with definitive fractionated megavoltage radiation: 15 cases (2010-2016). BMC Res. Notes. 11, 70. Northrup, N.C., Etue, S.M., Ruslander, D.M., Rassnick, K.M., Hutto, D.L., Bengtson, A., Rand, W. and Moore, A.S. 2001. Retrospective study of orthovoltage radiation therapy for nasal tumors in 42 dogs. J. Vet. Intern. Med. 15, 183–189. Rassnick, K.M., Goldkamp, C.E., Erb, H.N., Scrivani, P.V., Njaa, B.L., Gieger, T.L., Turek, M.M., McNiel, E.A., Proulx, D.R., Chun, R., Mauldin, G.E., Phillips, B.S. and Kristal, O. 2006. Evaluation of factors associated with survival in dogs with untreated nasal carcinomas: 139 cases (1993-2003). J. Am. Vet. Med. Assoc. 229, 401–406. Stevens, A., Turek, M., Vail, D., Christensen, N. and Forrest, L. 2020. Definitive-intent intensity modulated radiotherapy for modified-Adams’ stage 4 canine sinonasal cancer: A retrospective study of 29 cases (2011-2017). Vet. Radiol. Ultrasound. 61, 718–725. Sones, E., Smith, A., Schleis, S., Brawner, W., Almond, G., Taylor, K., Haney, S., Wypij, J., Keyerleber, M., Arthur, J., Hamilton, T., Lawrence, J., Gieger, T., Sellon, R. and Wright Z. 2013. Survival times for canine intranasal sarcomas treated with radiation therapy: 86 cases (1996-2011). Vet. Radiol. Ultrasound. 54, 194–201. Weeden, A.M. and Degner, D.A. 2016. Surgical approaches to the nasal cavity and sinuses. Vet. Clin. North Am. Small Anim. Pract. 46, 719–733. | ||
| How to Cite this Article |
| Pubmed Style Iseri T, Horikirizono H, Abe M, Itoh H, Sunahara H, Nemoto Y, Itamoto K, Tani K, Nakaichi M. Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Vet. J.. 2022; 12(3): 383-390. doi:10.5455/OVJ.2022.v12.i3.12 Web Style Iseri T, Horikirizono H, Abe M, Itoh H, Sunahara H, Nemoto Y, Itamoto K, Tani K, Nakaichi M. Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. https://www.openveterinaryjournal.com/?mno=5020 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i3.12 AMA (American Medical Association) Style Iseri T, Horikirizono H, Abe M, Itoh H, Sunahara H, Nemoto Y, Itamoto K, Tani K, Nakaichi M. Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Vet. J.. 2022; 12(3): 383-390. doi:10.5455/OVJ.2022.v12.i3.12 Vancouver/ICMJE Style Iseri T, Horikirizono H, Abe M, Itoh H, Sunahara H, Nemoto Y, Itamoto K, Tani K, Nakaichi M. Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Vet. J.. (2022), [cited January 25, 2026]; 12(3): 383-390. doi:10.5455/OVJ.2022.v12.i3.12 Harvard Style Iseri, T., Horikirizono, . H., Abe, . M., Itoh, . H., Sunahara, . H., Nemoto, . Y., Itamoto, . K., Tani, . K. & Nakaichi, . M. (2022) Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Vet. J., 12 (3), 383-390. doi:10.5455/OVJ.2022.v12.i3.12 Turabian Style Iseri, Toshie, Hiro Horikirizono, Momoko Abe, Harumichi Itoh, Hiroshi Sunahara, Yuki Nemoto, Kazuhito Itamoto, Kenji Tani, and Munekazu Nakaichi. 2022. Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Veterinary Journal, 12 (3), 383-390. doi:10.5455/OVJ.2022.v12.i3.12 Chicago Style Iseri, Toshie, Hiro Horikirizono, Momoko Abe, Harumichi Itoh, Hiroshi Sunahara, Yuki Nemoto, Kazuhito Itamoto, Kenji Tani, and Munekazu Nakaichi. "Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages." Open Veterinary Journal 12 (2022), 383-390. doi:10.5455/OVJ.2022.v12.i3.12 MLA (The Modern Language Association) Style Iseri, Toshie, Hiro Horikirizono, Momoko Abe, Harumichi Itoh, Hiroshi Sunahara, Yuki Nemoto, Kazuhito Itamoto, Kenji Tani, and Munekazu Nakaichi. "Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages." Open Veterinary Journal 12.3 (2022), 383-390. Print. doi:10.5455/OVJ.2022.v12.i3.12 APA (American Psychological Association) Style Iseri, T., Horikirizono, . H., Abe, . M., Itoh, . H., Sunahara, . H., Nemoto, . Y., Itamoto, . K., Tani, . K. & Nakaichi, . M. (2022) Outcomes of megavoltage radiotherapy for canine intranasal tumors and its relationship to clinical stages. Open Veterinary Journal, 12 (3), 383-390. doi:10.5455/OVJ.2022.v12.i3.12 |