| Case Report | ||
Open Vet. J.. 2020; 10(1): 31-38 doi: 10.4314/ovj.v10i1.6 Open Veterinary Journal, (2020), Vol. 10(1): 31–38 Case Report DOI: http://dx.doi.org/10.4314/ovj.v10i1.6 Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dogJustin Shmalberg1*, Patrick S. Moyle2, William F. Craft1 and Stuart A. Walton21Department of Comparative, Diagnostic and Population Medicine, College of Veterinary Medicine, University of Florida, Gainesville, FL 32611-0880, USA 2Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL 32611-0880, USA *Corresponding Author: Justin Shmalberg. College of Veterinary Medicine, University of Florida, Gainesville, FL 32611-0880, USA. Email: shmalberg [at] ufl.edu Submitted: 29/05/2019 Accepted: 14/01/2020 Published: 19/02/2020 © 2020 Open Veterinary Journal
AbstractBackground: The oomycete Lagenidium giganteum forma caninum is an uncommon cause of severe dermal and subcutaneous infections in dogs with possible vascular invasion and other fatal sequelae. Infection within the central nervous system of affected dogs has not been previously reported. Case Description: A 6-year-old spayed female mixed-breed dog was evaluated at a referral institution with a 2-month history of suspected fungal infection in the region of the right mandibular lymph node that was refractory to surgical resection and empiric medical therapy. Physical examination identified a 6-cm fluctuant subcutaneous mass caudoventral to the ramus of the right mandible and a second firm mass in the region of the right caudal maxilla. Lesional punch biopsies were submitted for fungal culture and polymerase chain reaction (PCR), which subsequently identified L. giganteum forma caninum infection. Initial treatment consisted of anti-inflammatory doses of prednisone and hyperbaric oxygen therapy. Four weeks following initial evaluation, the patient was presented with progressive neurological signs consistent with a forebrain lesion. Magnetic resonance imaging revealed soft-tissue, contrast-enhancing lesions ventral to the calvarium adjacent to the site of original surgical resection and throughout the brain. Humane euthanasia was elected, and postmortem examination was consistent with the extension of local disease from the right masseter muscle into the right ventral calvarium. Postmortem DNA sequencing confirmed the identity of the organism as L. giganteum forma caninum. Conclusion: This is the first reported case of intracranial lagenidiosis in the dog. PCR distinguished this species from other Lagenidium species and from oomycetes of other genera, such as Pythium insidiosum and Paralagenidium karlingii. Regional extension of cutaneous lagenidiosis should therefore be considered in cases with concurrent or spontaneous neurologic disease. KEYWORDS: Hyperbaric oxygen therapy, Lagenidium giganteum forma caninum, Neurologic disease, Oomycete. IntroductionInfection with the oomycete Lagenidium giganteum, referred to herein as lagenidiosis, is an uncommon disease encountered in veterinary medicine, which was first described in six dogs with chronic, progressive dermal to subcutaneous lesions which subsequently died (Grooters et al., 2003). The infected dogs are generally young to middle age residing in the Southeastern United States with exposure to lakes or ponds (Grooters, 2003; 2014; 2016; Mendoza and Vilela, 2013; Rajeev et al., 2012). The disease typically manifests as firm, cutaneous to subcutaneous, nodular lesions that can be ulcerated, edematous, and necrotic, most frequently involving the extremities, mammary region, perineum, or trunk with enlargement of regional lymph nodes (Dunbar and Wamsley, 2009; Grooters, 2003; 2014; 2016; Mendoza and Vilela, 2013; Rajeev et al., 2012). Sudden death secondary to the erosion of the great vessels and intracavitary hemorrhage has been described in several patients (Grooters, 2016; Grooters et al., 2003). Clinical information is based on few case reports or series (Dunbar and Wamsley, 2009; Grooters et al., 2003; Mendoza and Vilela, 2013; Rajeev et al., 2012). Definitive diagnosis relies on culture with subsequent identification of an isolate based on morphologic features (which can be difficult) or polymerase chain reaction (PCR) and subsequent sequencing (Spies et al., 2016). L. giganteum forma caninum is a common reference for an isolate of L. giganteum derived from the dog and not an isolate from the more common infective target of mosquito larvae, which is referred to as L. giganteum forma giganteum (Spies et al., 2016). However, PCR and phylogenetic analysis cannot distinguish between these two forms (Spies et al., 2016; Vilela et al., 2015; 2019). No efficacious treatment exists, and current treatment recommendations of aggressive surgical resection and antifungal treatment are based on another oomycete, Pythium insidiosum (Grooters, 2014; 2016). Prior to this study, central nervous system (CNS) invasion of any Lagenidium spp. has not been reported in a dog or other mammal. Case DetailsA 6-year-old 31.7-kg spayed female mixed-breed dog presented to an academic referral hospital for the treatment of a suspected fungal infection affecting the skin and subcutaneous tissue adjacent to the right mandible. The patient was evaluated 3 months before at another referral center for a peracute history of hyporexia, lethargy, and a 3-cm firm subcutaneous mass near the right mandibular lymph node. Initial hematology, biochemical analysis, urinalysis, thoracic radiographs, and abdominal ultrasound were unremarkable. Lesion aspirates revealed reactive lymphoid hyperplasia with eosinophilic infiltrates. The patient was empirically treated with tramadol (3.2 mg/kg by mouth (PO) q12hr), ciprofloxacin (16 mg/kg PO q12hr), and clindamycin (9.6 mg/kg PO q12hr). The patient presented 1 week following this initial treatment for worsening mandibular swelling. Computed tomography (CT) revealed a right-sided, soft-tissue attenuating, mildly contrast-enhancing retrobulbar mass (3.5-cm diameter), extending cranial to the caudal maxilla and medially to the nasopharynx with severe enlargement of the retropharyngeal and mandibular lymph nodes. An incisional biopsy showed marked granulomatous/eosinophilic inflammation and fibrosis. Special histochemical stains with Periodic acid–Schiff (PAS) and Grocott’s methenamine silver (GMS) stain failed to identify fungal organisms. Aerobic, anaerobic, and fungal cultures were negative. Prednisone (0.5 mg/kg PO q24hr) was instituted, and all other medications were continued. Six weeks following the initiation of prednisone, the lesion was reduced in size. Follow-up CT revealed a persistent progressive lesion, now extending from the tympanic bulla to the pterygoid bone and ventrally to the mandibular lymph node. Mandibular and retropharyngeal nodes were enlarged. En bloc removal of the right mandibular lymph node and surrounding tissues was performed. Postsurgical medications included cephalexin (25 mg/kg PO q8hr), prednisone (0.5 mg/kg PO q48hr), and tramadol (3.2 mg/kg PO q12hr). Histopathology revealed pyogranulomatous lymphadenitis with irregular, nonseptate, PAS, and GMS-positive bulbous hyphal organisms surrounded by epithelioid macrophages, and vascular encroachment by hyphal organisms was identified. Based on the morphologic features, a presumptive diagnosis of mucormycosis was made by the pathologist who provided consultation to the referral center; the fungal diameter was not reported. Itraconazole (9.7 mg/kg PO q24hr) was initiated and all other medications discontinued. Two weeks following surgery, the patient was referred to the authors’ academic referral institution for hyperbaric oxygen therapy (HBOT) and other adjunctive treatments. On presentation, the patient’s vital parameters were within normal limits. A 6-cm fluctuant subcutaneous mass was present caudoventral to the ramus of the right mandible. A second firm mass was identified over the right caudal maxilla, extending from the maxillary arcade into the ventral orbit. The fluctuant mass was aspirated yielding approximately 125 ml of serous fluid. The lesions, limited histopathological description, and signalment were considered as the most consistent with an infection of oomycete (referred to in this manuscript as oomycosis) or zygomycosis. Zygomycosis is a medical term for infection with coenocytic filamentous fungi in Mucormycota or Entomophthoramycota, groups previously assigned to the phylum Zygomycota that is no longer recognized (Vilela and Mendoza, 2018). Punch biopsies, obtained from the firm swelling and tissue adjacent to the previous surgical incision, were submitted for fungal/oomycete culture, identification, and susceptibility to itraconazole, fluconazole, and amphotericin B (Fungus Testing Laboratory, University of Texas Health Science Center at San Antonio, San Antonio, TX). Prednisone (1 mg/kg PO q24hr for 3 days and then 0.67 mg/kg PO q24hr) and HBOT (99% O2 at 15 pounds per square inch (PSI) for 45 minutes) were initiated. HBOT was administered 2–3 times weekly at an outpatient facility with subjective reduction of the masses reported by the owner. Three weeks after an initial evaluation, the patient presented to the academic referral hospital with acute blindness and was confirmed nonvisual in both eyes (OU). The patient displayed absent menace response bilaterally and decreased conscious proprioception in the left pelvic limb. Direct and consensual pupillary light reflexes (PLRs) were present in the right eye (OD). Direct PLR was present, but consensual PLR was inconsistent, in the left eye (OS). The rest of the neurologic and ophthalmologic assessment was unremarkable. The presumptive lesion localization was multifocal; the proprioceptive deficits were best explained by an incidental left-sided T3-L3 myelopathy and the acute blindness by a cortical lesion. The localization for the inconsistent indirect PLR with concurrent blindness was unclear, but the possibility of concurrent pre- and postchiasmal lesions was considered. Abnormalities on hematology and serum chemistry included mild hypoalbuminemia (2.5 g/dl [reference range (rr): 2.9–3.8 g/dl]), a mild elevation of blood urea nitrogen (BUN) (36 mg/dl [reference range (rr): 8–25 mg/dl]), and a moderate hypoglycemia (55 mg/dl, rr: 79–120 mg/dl]). A mean systolic blood pressure of 110 mm Hg was obtained by using Doppler sphygmomanometry (Doppler flow detector, Parks Medical Electronics Inc., Aloha, OR and sphygmomanometer, Welch Allyn, Skaneateles Falls, NY). Magnetic resonance imaging (MRI) was declined by the owner in favor of supportive care. The previous culture yielded the colonies displaying coenocytic hyphae similar to that in nonseptate fungi or oomycota after growth at 37°C on blood agar for 48 hours. L. giganteum was identified using standard PCR primers on extracted DNA for amplification of the following regions: D1/D2 28S rDNA, internal transcribed spacer (ITS) 1, ITS2, β-tubulin (TUBB), and heat shock protein 90 (hsp90) (oomycete PCR testing, Zoological Medicine Infectious Disease Laboratory, College of Veterinary Medicine, University of Florida, Gainesville, FL). Nucleotide sequences were deposited in GenBank (accession KY965924-7). Phylogenetic analysis was inferred using the maximum likelihood method based on the general time-reversible model following alignment and concatenation of ITS1, ITS2, and β-tubulin using commercially available software (Mega7 for OS X) (Fig. 1). Antifungal susceptibility showed resistance to itraconazole, fluconazole, and amphotericin B as no growth inhibition was noted for any agent. Subsequently, the patient was discharged on minocycline (10 mg/kg PO q24hr), mefenoxam (Mefenoxam 2 AQ, Control Solutions Inc., Pasadena, TX 77507) (8.3 mg/kg PO q24hr), and continued on prednisone (0.67 mg/kg PO q24hr). Three days following discharge, the patient presented with acute onset vestibular ataxia, altered mentation, and left-sided head tilt with circling to the right. Conscious proprioception was absent in the left forelimb and hindlimb. Spinal reflexes were intact. Presumptive lesion localization was again multifocal and inconsistent, with altered mentation and blindness being presumptive cortical changes, the proprioceptive deficits, and head tilt ascribed to central vestibular disease affecting the left side of the brainstem, although a concurrent right sensory cortical lesion was considered, and the circling was consistent with a right forebrain lesion or right central vestibular disease. There was no evidence of an acute cause for any possible peripheral vestibular disease. In the case of a cerebellar lesion causing a paradoxical head tilt, circling to the left would have been expected. Unappreciated and asymmetric partial visual function was considered as a cause for the circling to the right or head tilt.
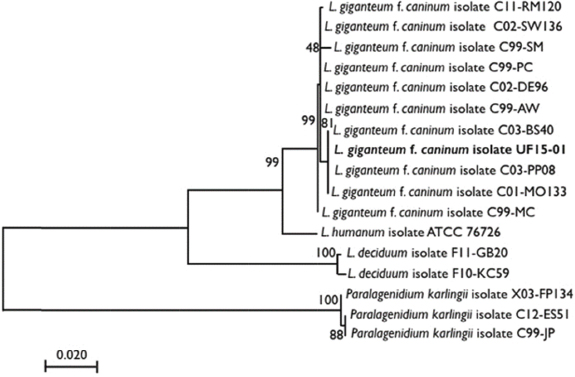
Fig. 1. The molecular phylogenetic analysis of highest log likelihood for the subject isolate and publically available sequences of L. giganteum obtained from other dogs, Lagenidium deciduum, and P. karlingii inferred by maximum likelihood method based on the general time-reversible model. The percentage of trees in which the associated taxa clustered together is indicated next to the branches, and the branches are drawn to scale with the lengths measured as the number of substitutions per site. The dog was anesthetized for an MRI of the head with T1-weighted (T1W), T2-weighted (T2W), gradient echo (GRE), fluid-attenuated inversion recovery (FLAIR), and T1W post gadodiamide (Omniscan, GE Healthcare AS, Oslo, Norway) (50 mg/kg) contrast sequences. Findings included a marked patchy hyperintensity (T1W, T2W, and FLAIR) of the right-sided soft-tissue structures of the head with marked heterogeneous contrast enhancement rostral to the right eye extending caudally to the level of the temporomandibular joint and ventrally to the level of the mandible. Marked right-sided meningeal enhancement extended to the occipital and frontal lobes. Ill-defined T2W and FLAIR hyperintense and T1W hypointense contrast-enhancing regions were present in the right piriform and temporal lobes. Concurrently, there was a marked mass effect causing deviation of the falx cerebri to the left and compression of the right lateral ventricle. In addition, there were T2W and FLAIR hyperintense and non contrast-enhancing regions in the right caudate nucleus (Fig. 2). A cerebellomedullary cisternal cerebrospinal fluid (CSF) sample was obtained. Cell count analysis was within normal limits. A differential cell count revealed the presence of increased numbers of nondegenerate neutrophils (31%) and eosinophils (8%). There was a moderate increase in microprotein (CSF microprotein concentration 63 mg/dl [rr < 25 mg/dl]). No fungal structures were identified on a GMS stain of a concentrated preparation of CSF. The owner elected humane euthanasia.
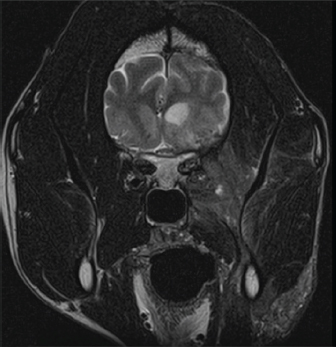
Fig. 2. Representative axial T2-weighted MRI image demonstrates patchy hyperintensity of the soft tissues of the right side of the head with heterogeneous contrast enhancement extending ventrally to the level of the mandible. The right-sided meningeal enhancement is present. Ill-defined hyperintense regions in the right temporal lobe display marked mass effect causing deviation of the falx cerebri to the left and compression of the right lateral ventricle.
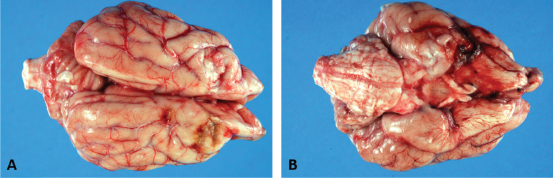
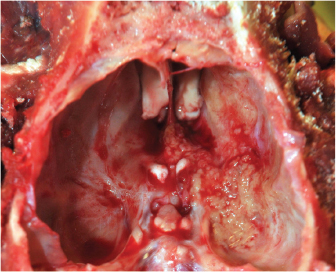
Fig. 3. Dorsal (A) and ventral (B) gross views of the brain showing focally extensive thrombosis of the right ventral cerebral artery resulting in an ischemic encephalopathy to the regions supplied by this artery and the loss of tissue which manifested as the depressed areas of malacia on the dorsal view. Necropsy findings included granulomatous myositis with fibrosis overlying the ventral aspect of the right masseter muscle, extending into the buccal submucosa and concurrent moderate multifocal chronic granulomatous lymphadenitis of the maxillary lymph nodes. Two lesions consistent with encephalomalacia were identified in the cerebral cortex: the first, a 1.5 cm × 1 cm, irregular, focal tan-brown depressed soft area within the right rostral and dorsal cerebral cortex just caudal to the median raphe, and the second, a 1 cm × 1 cm area associated with the left rostral cerebral cortex (Fig. 3). Severe, multifocal malacia was present in both the dorsal right hippocampus and right caudate nucleus. A firm thrombus was present in the rostral right cerebral artery of the right cerebrum (Fig. 3). Finally, along the right ventral calvarium, presumptive chronic multifocal, focally extensive proliferative osteitis was identified with compression and replacement of portions of the right optic nerve and chiasm (Fig. 4). No other gross lesions consistent with L. giganteum infection were identified outside the CNS and soft tissues of the head in a complete necropsy. Microscopically, the right mandibular mass and regional lymph nodes had multifocal to coalescing granulomatous inflammation with coalescing necrosis surrounded by thick rims of macrophages, epithelioid macrophages, and moderate numbers of lymphocytes and plasma cells extending into the right masseter muscle, mandibular buccal mucosa, and submucosa. Clear hyphal outlines that did not stain with hematoxylin and eosin (ghost hyphae) were evident within some of the areas of necrosis. The hyphae were 10–20 µm in diameter, argyrophilic with GMS histochemical staining, poorly septate with bulbous dilations, and had nonparallel walls. The tissue mass along the ventral calvarium revealed severe, multifocal, and focally extensive granulomatous osteomyelitis with osseous resorption and remodeling, myositis, and arteritis with multifocal vascular thrombosis and compression and replacement of the portions of the right optic nerve and right optic chiasm. Ghost fungal hyphae consistent with those described above were present within all areas of inflammation, fibrosis, and vascular walls and thrombi (Fig. 5). Within the brain, there was subacute to chronic, multifocal to coalescing, severe, granulomatous, lymphoplasmacytic, and neutrophilic meningoencephalitis predominantly of the right telencephalon and diencephalon associated with arteritis and arterial thrombosis of the right cerebral artery. Moderate chronic multifocal encephalomalacia was present bilaterally within the rostral cerebrum (Fig. 6). Numerous intralesional hyphae were identified in all lesions. Culture yielded a hyphal organism with morphologic features consistent with an oomycete, and PCR sequencing was consistent with the previously obtained L. giganteum isolate from this dog. DiscussionThe genus Lagenidium is a member of the class Oomycete in the Sar clade that is inclusive of stramenopiles, alveolates, and Rhizaria (Adl et al., 2012). Most Lagenidium species are considered nonpathogenic to mammals, but lagenidiosis has been recognized as a disease in dogs, cats, and people (Dunbar and Wamsley, 2009; Grooters, 2003; 2014; 2016; Grooters et al., 2003; Mendoza and Vilela, 2013; Rajeev et al., 2012; Reinprayon et al., 2013; Spies et al., 2016). Dogs infected with L. giganteum forma caninum typically develop progressive, ulcerated, cutaneous, or subcutaneous nodules of the extremities, mammary region, or trunk with regional lymphadenopathy. Dogs infected with lagenidiosis can also develop occult lesions within the thorax or abdomen, which can include the great vessels, abdominal lymph nodes, lungs, pulmonary hilus, and mediastinum, with sudden death, a possibility secondary to erosion of the great vessels and subsequent intracavitary hemorrhage (Grooters, 2016; Grooters et al., 2003; Mendoza and Vilela, 2013; Rajeev et al., 2012).
Fig. 4. Gross view demonstrating granulomatous osteomyelitis with the replacement of portions of the right optic nerve and optic chiasm.
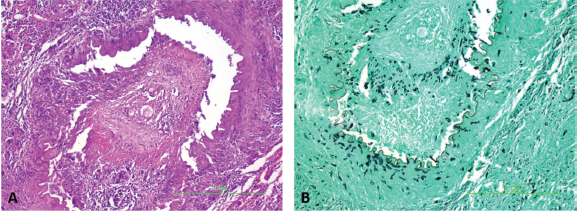
Fig. 5. Arteritis and periarteritis with arterial thrombosis (A; HE staining) with numerous intralesional ghost hyphae that are argyrophilic and appear black in the representative photomicrograph (B; GMS staining) (200×). This case report represents an uncommon presentation of lagenidiosis. Medical management with mefenoxam, minocycline, prednisone, and HBOT produced unknown effects given the rapid progression. This case suggests that the organism invaded through the ventral calvarium, gained access to local arteries producing thrombi with secondary acute ischemic encephalopathy and caused severe meningoencephalitis, resulting in the patient’s acute neurologic signs. The disparity between the clinical neurolocalization and necropsy findings is best explained by the diffuse inflammatory changes and vascular thrombi with secondary ischemic sequelae affecting more areas of the brain than observed. The acute blindness was likely caused by the encephalitis and ischemia in the brain, but lesions in the right optic nerve could have been contributory. The proprioceptive deficits on the left side are best explained by a right cortical lesion. The cause for the head tilt is unexplained; ischemic damage to the brainstem is possible and it is also possible that there was undetected asymmetric visual function that could have resulted in a compensatory head tilt. No causes of peripheral vestibular disease were identified on necropsy. The multiple foci of malacia in the brain away from the site of the lesion demonstrate the distant effects of the observed thrombosis.
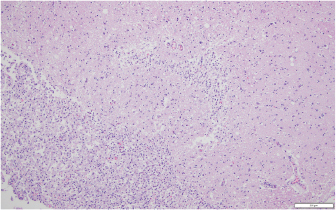
Fig. 6. Representative example of granulomatous encephalomalacia affecting the gray matter of the right cerebrum (HE staining), 100×. There were large numbers of macrophages, lymphocytes, plasma cells, fewer multinucleated giant cells, and scattered neutrophils within and surrounding areas of necrosis. The diversity of neurological signs does highlight the severity of the lesions and the inflammatory response. It further suggests that CNS involvement should now be considered as a possible presenting or progressive clinical sign of lagenidiosis. This novel presentation of lagenidiosis reinforces the organism’s ability to invade tissue and vasculature while causing a robust inflammatory reaction. A similar vascular invasion has been identified in cases of human pythiosis and canine gastrointestinal pythiosis, suggesting that these two pathogenic oomycetes share this feature (Chitasombat et al., 2018; Cooper et al., 1991). However, the great vessel infections reported in other cases of L. giganteum forma caninum were not identified in this case nor have such distant vascular lesions been reported in canine pythiosis (Grooters, 2003; 2014; 2016; Mendoza and Vilela, 2013). The patient’s rapid clinical deterioration and poor prognosis highlight the need for more rapid diagnostic screening and improved treatment options. Complete blood count and serum chemistries can be normal or may show eosinophilia, neutrophilia, hyperglobulinemia, and hypercalcemia (Dunbar and Wamsley, 2009; Grooters, 2014; 2016; Grooters et al., 2003; Rajeev et al., 2012). The patient in this report did not display an increase in globulins, presumably due to glucocorticoid administration, but did show a reduction in serum albumin, likely secondary to chronic inflammation. Cytologic examination of cutaneous lesions generally reveals pyogranulomatous and eosinophilic inflammation and occasionally the presence of broad hyphae with nonparallel walls or ghost hyphae with H and E stain (Grooters, 2003; 2014; 2016; Grooters et al., 2003; Rajeev et al., 2012). Identification of the organism without GMS or other special stains as with this case may prove elusive. The reason why the patient’s initial excisional biopsy was negative for staining hyphae remains unclear. Histologic features of Lagenidium sp. include large, poorly septate hyphae with right-angle branching of similar appearance but greater diameter (7–25 µm) than that of Pythium (Grooters, 2003; 2014; 2016; Grooters et al., 2003; Rajeev et al., 2012). Definitive diagnosis is obtained by performing culture and isolation of the causative organism, followed by PCR amplification and gene sequencing, as was performed on the reported patient’s isolate (Grooters, 2014; 2016; Rajeev et al., 2012; Spies et al., 2016). No definitive treatment has been described for lagenidiosis, and to the author’s knowledge, there is no report of any dog surviving a confirmed infection. Published treatment recommendations suggest that aggressive surgical resection with wide margins is the treatment of choice (Grooters, 2003; 2014; 2016). In this case, there was incomplete surgical resection due to the location of the lesion and the lack of definitive diagnosis. An earlier diagnosis may have improved surgical planning and prompted a more thorough diagnostic screening for the thoracic or abdominal extension as has been previously reported. In this case, there was an aggressive local invasion but not a distant extension of the disease as evidenced by the postmortem findings. An in vitro investigation of antifungal agents and mefenoxam against Lagenidium sp. identified mefenoxam as the most inhibitory of all agents tested (Brown et al., 2008). Terbinafine exerted weak effects (Brown et al., 2008). The azole antifungals, which impair ergosterol synthesis, appear to have little utility against oomycetes, which obtain sterols from the environment rather than synthesis (Brown et al., 2008; Grooters, 2014). Mefenoxam is an agricultural product without approval for medical use. However, this agent, along with itraconazole and terbinafine, has been reported to treat duodenal pythiosis (Hummel et al., 2011). In that case report, the dog achieved complete clinical remission in an 18-month follow-up period with no reported side effects though the mefenoxam was started after clinical remission and serologic improvement was noted. In this case report, the client consented to oral administration of mefenoxam (8 mg/kg PO q24h), a dose unlikely to cause toxicity (Novartis, 1997). Minocycline was also prescribed on the basis of favorable in vitro inhibition of P. insidiosum, but the dog received these drugs for only 3 days prior to euthanasia (Loreto et al., 2014). The use of prednisone was theorized to help control collateral damage from responding leukocytes, as oomycosis produces severe eosinophilic and granulomatous inflammation. This inflammation, likely mediated by Th2 cells, is responsible for severe tissue necrosis without destruction of the organism (Mendoza and Newton, 2005). HBOT, or the clinical use of oxygen at elevated pressure, is only rarely described in veterinary literature, and the applications are extrapolated from accepted human indications (Edwards, 2010; Grahl et al., 2012; John et al., 2005; Pagano et al., 2009; Tragiannidis and Groll, 2009). HBOT has been used adjunctively in animals for a wide array of diseases including CNS injury, severe soft tissue inflammation, ischemia, sepsis, and anaerobic infections (Edwards, 2010). Adjunctive HBOT has been employed for human zygomycosis, but the pathophysiological basis for its efficacy is unclear (John et al., 2005; Tragiannidis and Groll, 2009). Theorized benefits of HBOT include increased production of oxygen-based free radicals, reversal of growth-promoting lactic acidosis, restoration of phagocytosis, augmentation of the leukocyte oxidative burst, and increased tissue repair (Pagano et al., 2009; Tragiannidis and Groll, 2009). A previous study showed an association between prolonged courses of hyperoxygenation [>10 Atmospheres Absolute (ATA)] with improved survival in zygomycosis (John et al., 2005; Tragiannidis and Groll, 2009). Fungal pathogens may display adaptation to hypoxia or increased pathogenicity under low oxygen conditions, leading to the hypothesis that HBOT might reduce pathogenicity or induce fatal oxidative stress (Grahl et al., 2012). The effect of HBOT on in vitro or in vivo growth of pathogenic oomycetes is not reported, but HBOT was previously used as an adjunctive treatment for canine oomycosis (Shmalberg et al., 2015). It cannot be excluded that hyperoxia could increase Lagenidium growth. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionJustin Shmalberg was the clinician for the dog in this report, helped to draft the manuscript, performed a phylogenetic analysis of isolates, and revised the manuscript. Patrick S. Moyle helped to draft the initial manuscript. William F. Craft performed the necropsy, interpreted the histopathology, generated relevant figures, and helped to draft the manuscript. Stuart A. Walton helped to draft and revise the manuscript and provided critical review. ReferencesAdl, S.M., Simpson, A.G., Lane, C.E., Lukes, J., Bass, D., Bowser, S.S., Borwn, M.W., Burki, F., Dunthorn, M., Hampl, V., Heiss, A., Hoppenrath, M., Lara, E., Le Gall, L., Lynn, D.H., McManus, H., Mitchell, E.A., Mozley-Stanridge, S.E., Parfrey, L.W., Pawlowski, J., Rueckert, S., Shadwick, L., Schoch, C.L., Smirnov, A. and Spiegel, F.W. 2012. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59(5), 429–493. Brown, T.S., Grooters, A.M. and Hosgood, G.L. 2008. In vitro susceptibility of Pythium insidiosum and Lagenidium sp. to itraconazole, posaconazole, voriconazole, terbinafine, caspofungin, and mefenoxam. Am. J. Vet. Res. 69, 1463–1468. Chitasombat, M.N., Larbcharoensub, N., Chindamporn, A. and Krajaejun, T. 2018. Clinicopathological features and outcomes of pythiosis. Int. J. Infect. Dis. 71, 33–41. Cooper, R.C., Allison, N. and Boring, J.G. 1991. Apparent successful surgical treatment of intestinal pythiosis with vascular invasion in a dog. Canine Pract. 16, 9–12. Dunbar, M.D. and Wamsley, H.L. 2009. What is your diagnosis? Lymph node cytology from a dog. Vet. Clin. Pathol. 38, 91–92. Edwards, M.L. 2010. Hyperbaric oxygen therapy. Part 2: Application in disease. J. Vet. Emerg. Crit. Care. 20, 289–297. Grahl, N., Shepardson, K.M., Chung, D. and Cramer, R.A. 2012. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryotic Cell. 11, 560–570. Grooters, A.M. 2003. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet. Clin. Small Anim. 33, 695–720. Grooters, A.M. 2014. Pythiosis, lagenidiosis, and zygomycosis. In Canine and feline infectious diseases. ed., Sykes, J.E., St. Louis, MO: Saunders, pp: 668–678. Grooters, A.M. 2016. Miscellaneous fungal infections. In Textbook of Veterinary Internal Medicine St. Louis, MO: (Elsevier). (eds.), Ettinger, S.J, Feldman, E.C. and Cote, E., pp: 1044–1050. Grooters, A.M., Hodgin, E.C., Bauer, R.W., Detrisac, C.J., Znajda, N.R. and Thomas, R.C. 2003. Clinicopathologic findings associated with Lagenidium sp. infection in 6 dogs: initial description of an emerging oomycosis. J. Vet. Intern. Med. 17, 637–646. Hummel, J., Grooters, A., Davidson, G., Jennings, S., Nicklas, J. and Birkenheuer, A. 2011. Successful management of gastrointestinal pythiosis in a dog using itraconazole, terbinafine, and mefenoxam. Med. Mycol. 49, 539–542. John, B., Chamilos, G. and Kontoyiannis, D. 2005. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin. Microbiol. Infect. 11, 515–517. Loreto, E.S., Tondolo, J.S.M., Pilotto, M.B., Alves, S.H. and Santurio, J.M. 2014. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob. Agents Chemther. 58, 7534–7537. Mendoza, L. and Newton, J.C. 2005. Immnuology and immunotherapy of the infections caused by Pythium insidiosum. Med. Mycol. 43, 477–487. Mendoza, L. and Vilela, R. 2013. The mammalian pathogenic oomycetes. Curr. Fungal Infect. Rep. 7(3), 198–208. Novartis. 1997. Pesticide petition. Federal Register PP 6F4723, 62, 40083–40086. Pagano, L., Valentini, C.G., Caira, M. and Fianchi, L. 2009. Zygomycosis: current approaches to management of patients with hematological malignancies. Br. J. Haematol. 146, 597–606. Rajeev, S., Ilha, M.R., Harrison, J.M., Clifton, W.G., Grooters, A.M., Wickes, B.L. and Sutton, D.A. 2012. Lagenidium infection. J. Am. Vet. Med. Assoc. 241, 447–449. Reinprayon, U., Permpalung, N., Kasetsuwan, N., Plongla, R., Mendoza, L. and Chindamporn, A. 2013. Lagenidium sp. ocular infection mimicking ocular pythiosis. J. Clin. Microbiol. 51, 2778–2780. Shmalberg, J., Davies, W., Lopez, S., Shmalberg, D. and Zilberschtein, J. 2015. Rectal temperature changes and oxygen toxicity in dogs treated in a monoplace chamber. Undersea Hyperb. Med. 42, 95–102. Spies, C.F., Grooters, A.M., Levesque, C.A., Rintoul, T.L., Redhead, S.A., Glockling, S.L., Chen, C.Y. and de Cock, A.W. 2016. Molecular phylogeny and taxonomy of Lagenidium-like oomycetes pathogenic to mammals. Fungal Biol. 120, 931–947. Tragiannidis, A. and Groll, A.H. 2009. Hyperbaric oxygen therapy and other adjunctive treatments for zygomycosis. Clin. Microbiol. Infect. 15, 82–86. Vilela, R. and Mendoza, L. 2018. Human pathogenic Entomophthorales. Clin. Microbiol. Rev. 31(4), 1–40. Vilela, R., Humber, R.A., Taylor, J.W. and Mendoza, L. 2019. Phylogenetic and physiological traits of oomycetes originally identified as Lagenidium giganteum from mosquito larvae. Mycologia 111(3), 408–422. Vilela, R., Taylor, J.W., Walker, E.D. and Mendoza, L. 2015. Lagenidium giganteum pathogenicity in mammals. Emerg. Infect. Dis. 21(2), 290–297. | ||
| How to Cite this Article |
| Pubmed Style Moyle PS, Walton SA, Craft WF, Shmalberg JW. Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Vet. J.. 2020; 10(1): 31-38. doi:10.4314/ovj.v10i1.6 Web Style Moyle PS, Walton SA, Craft WF, Shmalberg JW. Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. https://www.openveterinaryjournal.com/?mno=51125 [Access: January 25, 2026]. doi:10.4314/ovj.v10i1.6 AMA (American Medical Association) Style Moyle PS, Walton SA, Craft WF, Shmalberg JW. Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Vet. J.. 2020; 10(1): 31-38. doi:10.4314/ovj.v10i1.6 Vancouver/ICMJE Style Moyle PS, Walton SA, Craft WF, Shmalberg JW. Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Vet. J.. (2020), [cited January 25, 2026]; 10(1): 31-38. doi:10.4314/ovj.v10i1.6 Harvard Style Moyle, P. S., Walton, . S. A., Craft, . W. F. & Shmalberg, . J. W. (2020) Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Vet. J., 10 (1), 31-38. doi:10.4314/ovj.v10i1.6 Turabian Style Moyle, Patrick S, Stuart A Walton, William F. Craft, and Justin W Shmalberg. 2020. Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Veterinary Journal, 10 (1), 31-38. doi:10.4314/ovj.v10i1.6 Chicago Style Moyle, Patrick S, Stuart A Walton, William F. Craft, and Justin W Shmalberg. "Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog." Open Veterinary Journal 10 (2020), 31-38. doi:10.4314/ovj.v10i1.6 MLA (The Modern Language Association) Style Moyle, Patrick S, Stuart A Walton, William F. Craft, and Justin W Shmalberg. "Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog." Open Veterinary Journal 10.1 (2020), 31-38. Print. doi:10.4314/ovj.v10i1.6 APA (American Psychological Association) Style Moyle, P. S., Walton, . S. A., Craft, . W. F. & Shmalberg, . J. W. (2020) Severe meningoencephalitis secondary to calvarial invasion of Lagenidium giganteum forma caninum in a dog. Open Veterinary Journal, 10 (1), 31-38. doi:10.4314/ovj.v10i1.6 |