| Short Communication | ||
Open Vet. J.. 2021; 11(3): 394-400 Open Veterinary Journal, (2021), Vol. 11(3): 394–400 Short Communication Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogsShoma Mikawa1, Akira Matsuda1*, Yasuyuki Kamemori2, Satoru Asanuma3 and Hitoshi Kitagawa11Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan 2Okayama University of Science Specialized Training College, Japan 3Purebox Co., Ltd.,Okayama, Japan *Corresponding Author: Akira Matsuda. Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan. Email: a-matsuda [at] vet.ous.ac.jp Submitted: 04/02/2021 Accepted: 07/07/2021 Published: 08/08/2021 © 2021 Open Veterinary Journal
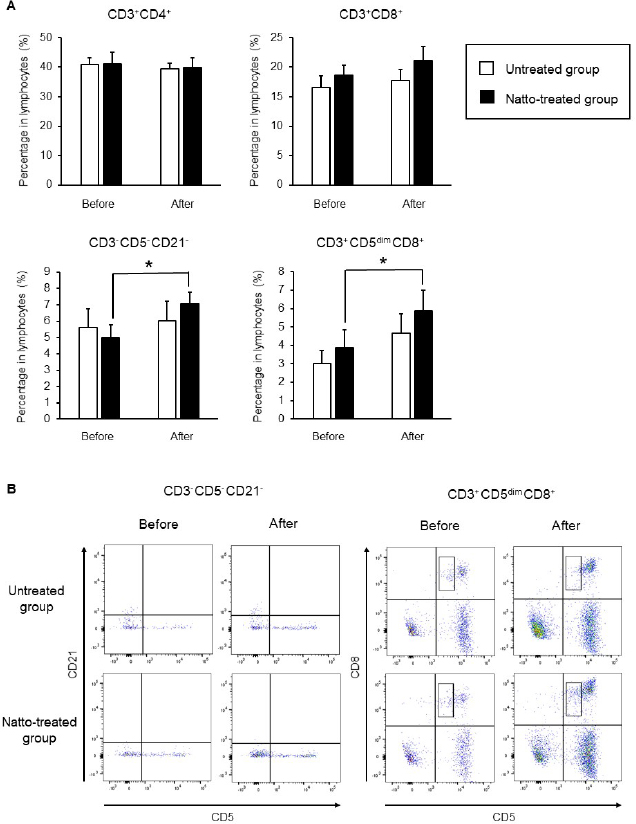
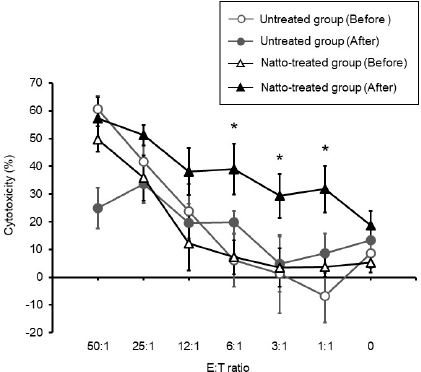
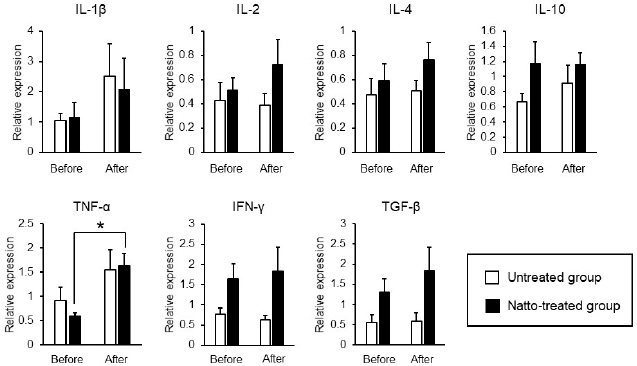
AbstractBackground: Probiotics are known for their ability to enhance cellular immunity, including the activation of macrophages, natural killer (NK) cells, and cytotoxic T lymphocytes. Natto is a Japanese traditional probiotic food made by fermenting soybean with bacteria Bacillus subtilis var. natto. Components of natto include spores of B. subtilis natto, poly-γ-glutamic acid, and levan, which have demonstrated their immunoadjuvant and anti-allergic effects through various in vitro and in vivo studies. However, it remains unclear whether oral administration of natto can modulate the immune activity in animals. Aim: This study aimed to investigate the effects of oral administration of natto on the immune system of dogs. Methods: Eight dogs were randomly divided into two groups: a natto-treated group and an untreated group. The dogs in the natto-treated group were fed with 10 g/head/day of a freeze-dried natto product in addition to a usual amount of regular dry food for 14 days, whereas the dogs in the untreated group were fed with the regular dry food alone. To determine cellular immune activity, the cell surface antigen analysis of peripheral blood lymphocytes and cytotoxicity analysis of peripheral blood mononuclear cells were carried out before and after the natto administration period. Additionally, a relative expression of inflammatory cytokines in peripheral blood monocytes after the introduction of antigen-stimulation was also examined. Results: At the end of the administration period, a proportion of NK cells (CD3− CD5− CD21− cells and CD3+ CD5dim CD8+ cells) in peripheral blood lymphocytes were found to be significantly increased, and the cytotoxic effect of the peripheral blood mononuclear cells on canine tumor cells were greatly enhanced in the natto-treated group, but not in the untreated group. The expression of TNF-α in peripheral blood mononuclear cells following an antigen-stimulation was increased considerably in the dogs after administration of natto. Conclusion: We conclude that oral administration of natto activated the cytotoxic activity of peripheral NK cells in dogs, and a daily intake of natto might be helpful in augmenting cellular immune activity. Keywords: Bacillus subtilis natto, Cellular immune, Dog, Fermented soy bean, Natural killer cell. IntroductionProbiotics, or foods that contain them, constitute live microorganisms with beneficial effects on human and animal health. Recently, various probiotic products have been developed, and their preventive or therapeutic effects on multiple diseases have been reported (Dang et al., 2020). Probiotic products have been proven to be effective in enhancing the cellular immune responses characterized by the activation of macrophages, natural killer (NK) cells, and antigen-specific cytotoxic T lymphocytes (Ashraf and Shah, 2014). Research on the immunomodulatory effects of probiotics involves extensive studies on Bifidobacterium lactis (HN019), a probiotic organism. It has been reported that the organism can increase the number and enhance the activity of T lymphocytes, polymorphonuclear cells, and NK cells in the human peripheral blood. Natto is a traditional Japanese probiotic food made by fermenting soybeans with Bacillus subtilis var. natto (Weng et al., 2017). Natto contains functional components such as natto kinase, poly-γ-glutamic acid, and levan, which possess thrombolytic, immunoadjuvant, and anti-allergic activities (Sumi et al., 1987; Xu et al., 2006; Lee et al., 2014). Additionally, natto-containing foods reportedly increase Bifidobacterium species in the intestines of mammals (Fujisawa et al., 2006; Mitsui et al., 2006). In addition, Gong et al. (2018) demonstrated that oral administration of B. subtilis natto spores enhanced cellular immunity in mice. Although these facts suggest that the supplementation of natto may have beneficial effects on canine health, especially cellular immunity, the effect of oral administration of natto is unknown in dogs. Recently, multicolor flow cytometry has been used for the analysis of canine lymphocyte subsets. Similar to those in humans, specific cell surface molecules such as CD4 (for helper T cells) and CD8 (for cytotoxic T cells) are widely accepted as biomarkers in dogs (Friedenberg et al., 2018; Withers et al., 2018). NK cells are non-T, non-B lymphocytes that play a pivotal role in the innate immune system (Michael et al., 2013). NK cells can kill cancer cells and virus-infected cells without prior activation through direct cytolytic activity and via the production of cytokines (Shin et al., 2013; Lee et al., 2018). Although the expression of specific surface molecules on human cells (CD56 and CD16) and murine cells (NK 1.1 and NKp 46) allow the identification of NK cells, these molecules have reportedly been unfavorable for the identification of canine NK cells (Otani et al., 2002; Hammond et al., 2009). In humans, CD3− CD5− CD21− and CD3+ CD5dim CD8+ cells are referred to as non-T, non-B cells, and NK cells, respectively. However, these cells have been identified as NK cells in dogs (Huang et al., 2008; Michael et al., 2013). A difference in the functional activity of CD3− CD5− CD21− and CD3+ CD5dim CD8+ cells was reportedly due to their degree of maturation (Lee et al., 2018). Hence, a change in the population of these cell types may be used to indicate cellular immune activity. In the present study, we examined whether oral administration of natto could activate or suppress the canine immune system. The results of this study could encourage the use of oral administration of natto for adjunctive therapy in dogs with some diseases, such as infectious diseases, neoplastic diseases, and autoimmune diseases. Materials and MethodsSelection of dogsA total of eight healthy dogs (six miniature poodles and two mixed-breed) were selected from the training course at the Okayama University of Science Specialized Training College for this study. Their mean age and mean body weight were 2.38 ± 0.73 years and 5.88 ± 1.32 kg, respectively. The dogs were housed in individual cages and walked regularly. All the dogs were fed a commercial dry food (Wish HAS II, Purpose Co., Ltd., Kyoto, Japan) at a dose of 20 g/kg/day as a regular diet. Freeze-dried nattoA commercially available freeze-dried natto product (Onaka natto, Purebox, Co., Ltd., Okayama, Japan) was used in this study. The product is guaranteed to contain 245.3 ± 26.4 mg of poly-γ-glutamate, 8.5 ± 2.1 mg of polyamine, 63.7 ± 8.1 mg of levan, 241.0 ± 29.1 IU of nattokinase, 1498 ± 74 μg of vitamin K2, and 34.4 ± 1.5 mg of dipicolinic acid per 100 g. The product contains 100 billion B. subtilis natto per 100 g. Experimental protocolThe dogs were randomly divided into two groups: a natto-treated group (n=4) and an untreated group (n=4). The dogs in the natto-treated group were fed with freeze-dried natto product at a dose of 10 g/dog along with the regular diet once daily and continued for 14 days by referencing to past reports (Fujisawa et al., 2006; Oh et al., 2014). According to the previous study, the dogs in the untreated group were fed a regular diet only (Oh et al., 2014). Five milliliters of blood samples were collected from the jugular veins before (day 0) and after (day 14) the feeding schedule. These blood samples were immediately transferred to the heparin tubes and preserved at 4°C until further examination. The heparinized whole blood samples were used to separate peripheral blood mononuclear cells (PBMCs) using Ficoll-Paque Plus (GE Healthcare Life Sciences, Amersham Place, England). The blood was diluted (1:1) using Phosphate-Buffered Saline (PBS), layered onto the Ficoll-Paque Plus, and centrifuged at 400 g for 40 minutes at room temperature. A PBMC layer at the interface of the two phases of the centrifuged product was collected using a Pasteur pipette and washed twice with PBS. These collected PBMCs were used to analyse cell surface antigens, real-time polymerase chain reaction (PCR), and cytotoxicity assays. Analysis of the cell surface antigens on PBMCsPBMCs were firstly incubated with the Canine Fc Receptor Binding Inhibitor Purified (Life Technologies, Carlsbad, CA, USA) for 20 minutes. After that, the PBMCs were incubated with fluorescein isothiocyanate (FITC)-conjugated mouse anti-canine CD3 monoclonal antibody (mAb) (Bio-Rad, Hercules, CA, USA), R-phycoerythrin (RPE)-conjugated rat anti-canine CD4 mAb (Bio-Rad), and Alexa Fluor® 647-conjugated rat anti-canine CD8 mAb (Bio-Rad) for analysis of helper T cells (CD3+ CD4+ cells) and cytotoxic T cells (CD3+ CD8+ cells), and incubated with FITC-conjugated mouse anti-canine CD3 mAb, RPE-conjugated mouse anti-canine CD21 mAb (Bio-Rad), Alexa Fluor® 647-conjugated rat anti-canine CD8 mAb, and PerCP-eFlour® 710-conjugated rat anti-canine CD5 mAb (Life Technologies) for analysis of NK cells (CD3− CD5− CD21− cells and CD3+ CD5dim CD8+ cells). Flow cytometry analysis was carried out using the BD LSR Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Lymphocytes were gated according to forward and side scatter, and the lymphocyte subsets were then analyzed. Cytotoxicity assayCytotoxicity assay of PBMCs was carried out according to the methods described previously with some modifications (Huang et al., 2008; Michael et al., 2013; Lee et al., 2018). PBMCs were used as effector cells, and canine thyroid adenocarcinoma (CTAC) cells were used as the target tumor cells. CTAC cells were obtained from KAC Co., Ltd. (Kyoto, Japan). They were cultured at 37°C using RPMI 1,640 (Fujifilm Wako Chemicals, Tokyo, Japan) containing 10% fetal bovine serum (FBS) (Biosera, Nuaille, France), 20 IU/ml penicillin, and 10 μg/ml streptomycin. For the cytotoxicity assay, CTAC cells (2 × 104 cells/well) were cultured in 96-well plates in triplicate overnight. The next day, the CTAC cells were incubated with PBMCs at effector-to-target ratios (E:T) of 25:1, 12:1, 6:1, 3:1, and 1:1 in 200 μl of a serum-free medium ALyS505NK-AC1000 (Cell Science and Technology Institute, Miyagi, Japan). After 16 hours of incubation at 37°C, the plates were washed thrice with PBS solution to remove the PBMCs. The remaining target cells were detected by a 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8) assay using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Absorption at 450 nm (A450) was measured using a microplate reader (SH-1,300, Corona Electric, Ibaraki, Japan). Calculation of cytotoxicity percentage was conducted using the following formula: 100% − 100 × (A450 of effector cell − treated target cells)/(A450 of target cells). Real-time PCRPBMCs were incubated in RPMI 1,640 containing 10% FBS, 25 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA), and 1 μg/ml of ionomycin (Sigma) at 37°C for 6 hours. After incubation, total RNA was extracted from the cells using a NucleoSpin Plus kit (Takara Bio Inc., Shiga, Japan). The extracted RNA was then reverse-transcribed into cDNA with the PrimeScript II 1st strand cDNA synthesis kit (Takara Bio). Real-time PCR was carried out with the TB Green Premix Ex Taq II (Takara Bio) in the presence of 0.2 μM each of the forward and reverse primers for interleukin (IL)-1β (5ʹ-CAAGTCTCCCACCAGCTCTGTA-3ʹ and 5ʹ-GGGCTTCTTCAGCTTCTCCAA-3ʹ), IL-2 (5ʹ-CCTCAACTCCTGCCACAATGT-3ʹ and 5ʹ-TGCGACAAGTACAAGCGTCAGT-3ʹ), IL-4 (5ʹ-TAGCACTCACCAGCACCTTTGT-3ʹ and 5ʹ-CTTGACAGTCAGCTCCATGCA-3ʹ), IL-10 (5ʹ-AGGCTGCGACGCTGTCACC-3ʹ and 5ʹ-TGCGCTCTTCACCTGCTCCA-3ʹ), tumor necrosis factor (TNF)-α (5ʹ-GCTTCGCCGTCTCCTACCA-3ʹ and 5ʹ-TTGGCAAGGGCTCTTGATG-3ʹ), interferon (IFN)-γ (5ʹ-GCATTCCAGTTGCTGCCTACT-3ʹ and 5ʹ-ACCAGGCATGAGAAGAAATGCT-3ʹ), transforming growth factor (TGF)-β (5ʹ-CAAGTAGACATTAACGGGTTCAGTTC-3ʹ and 5ʹ-GGTCGGTTCATGCCATGAAT-3ʹ), and β-actin (5ʹ-TGTGGCCATCCAGGCTGTGC-3ʹ and 5ʹ-GTGGTCTCGTGGATACCGCA-3ʹ). The PCR amplification consisted of pre-denaturation (95°C, 10 seconds) and 40 cycles of denaturation (95°C, 10 seconds), annealing and extension (60°C, 30 seconds). Fluorescence intensity was measured in real time during the extension steps using the QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). Relative expression levels of the target genes were normalized to the endogenous reference (β-actin) and calculated by the 2−ΔΔCT method. Statistical analysisStatistical significance was analyzed by repeated measures analysis of variance, followed by Tukey’s test. The analysis was carried out using EZR, which is a statistical software for R version 3.5.2. p values of < 0.05 were considered statistically significant. Ethical approvalThe study protocol was approved by the Institutional Animal Care and Use Committee of Okayama University of Science Specialized Training College. Results and DiscussionIn this study, we used the freeze-dried natto product that contained six functional components, namely poly-γ-glutamate, polyamine, levan, nattokinase, vitamin K2, and dipicolinic acid. Some of these components were reported to have immunoadjuvant or immunosuppressive effects (Xu et al., 2006; Kim et al., 2007; Lee et al., 2012, 2014). Analysis of cell surface antigens in this study revealed that the oral administration of natto did not affect the proportion of helper T cells (CD3+ CD4+ cells) and cytotoxic T cells (CD3+ CD8+ cells) in peripheral blood lymphocytes (Fig. 1A). On the contrary, a proportion of NK cells (CD3− CD5− CD21− cells and CD3+ CD5dim CD8+ cells) in the peripheral blood lymphocytes were significantly increased by oral administration of natto (Fig. 1A and 1B). A past study using spores of B. subtilis natto showed no increase in helper T cells, cytotoxic T cells, or NK cells in the murine spleen (Gong et al., 2018). Therefore, an increase in the peripheral NK cells observed in the natto-treated dogs might have been caused by the effect of not only B. subtilis natto but also the other components in the natto. In past studies, the oral administration of poly-γ-glutamate was reported to induce NK cell-mediated and dendritic cell-dependent anti-tumor effects in mice (Kim et al., 2007). Additionally, the oral administration of spores of B. subtilis natto in mice was reported to enhance in vitro cytotoxic activity of splenocytes against tumor cells (Gong et al., 2018). Our results demonstrate that PBMCs collected from the group treated with natto exhibited higher cytotoxicity than those collected from the untreated group (Fig. 2). Statistical significance was detected at 6:1, 3:1, and 1:1 of the E:T ratio. The results indicated that both B. subtilis natto and the functional components in natto induced and enhanced NK cell activity in dogs. The components of natto, which includes poly-γ-glutamate, polyamine, and B. subtilis natto, have demonstrated their immunosuppressive effects (Xu et al., 2006; Lee et al., 2012, 2014). Oral administration of poly-γ-glutamate was reported to ameliorate symptoms of atopic dermatitis in mice by suppressing the Th2-based immune response (Lee et al., 2014). Poly-γ-glutamate was also described to promote selective differentiation of Treg cells and suppress differentiation of Th17 cells (Lee et al., 2012). In addition, oral administration of B. subtilis natto alleviated the skin lesions induced by Dermatophagoides farinae body antigen in mice (Goto et al., 2011). These reports suggested the possible immunosuppressive effects and anti-allergic effects of natto. The results in this study showed neither suppressive effects on the expression of pro-inflammatory cytokines (IL-1β, IL-2, IL-4, TNF-α, and IFN-γ) nor promotive effects on the immunoregulatory cytokines (IL-10 and TGF-β) (Fig. 3). These results suggest that oral administration of natto has little immunosuppressive effect in dogs.
Fig. 1. Cell surface antigen analysis using flow cytometry. (A) Proportions of helper T cells (CD3+ CD4+ cells), cytotoxic T cells (CD3+ CD8+ cells), and NK cells (CD3− CD5− CD21− cells and CD3+ CD5dim CD8+ cells). (B) Representative plots of the detected NK cells. Before: before administration, after: after administration. Data represent mean ± SE. *p < 0.05 versus before administration.
Fig. 2. Cytotoxicity against CTAC cells mediated by PBMC. The cytotoxicity of the PBMCs collected from the untreated group of dogs had no significant change between before and after administration. On the contrary, there was a significant increase in the PBMC cytotoxicity of the natto-treated group after administration when compared to that before administration at E:T ratios of 6:1, 3:1, and 1:1. Before: before administration, after: after administration. Data represent mean ± SE. *p < 0.05 versus before administration.
Fig. 3. Expression levels of inflammatory cytokines in antigen-stimulated PBMCs. PBMCs were incubated in a culture medium containing 25 ng/ml of PMA and 1 μg/ml of ionomycin for 6 hours. Relative expression levels of the target genes were normalized against β-actin and calculated by the 2-ΔΔCT method. Values were standardized to those of a dog in the untreated group. Before: before administration, after: after administration. Data represent mean ± SE. *p < 0.05 versus before administration. Expression of TNF-α by the PBMCs had significantly increased after oral administration of natto in this study (Fig. 3). A past study demonstrated that levan induced the production of TNF-α in a murine macrophage cell line in vitro (Xu et al., 2006). Thus, the upregulation of TNF-α by the PBMCs, as observed in our results, might be caused by levan contained in the freeze-dried natto product. TNF-α is cytotoxic to tumor cells (Aggarwal, 2003). On the other hand, recent studies have revealed proinflammatory effects and paradoxical immunoregulative effects of TNF-α (Salomon et al., 2018). Owing to its pleiotropic effects, apparent implications of the elevated expression levels of TNF-α in PBMCs in our study remains unclear. Nevertheless, considering the enhanced cytotoxicity of NK cells due to TNF-α (Maki et al., 1998), the increased expression of TNF-α induced by the natto treatment might potentiate cellular immune activity in dogs. We conclude that oral administration of natto augments cellular immune activity in dogs through an elevation in the number of peripheral blood NK cells and an enhancement of their cytotoxic effects. Our results suggest that the administration of natto might be beneficial adjuvant therapy for dogs with neoplastic diseases. AcknowledgmentThe authors would like to thank the staff and students of Okayama University of Science Specialized Training College for the care they provided to the dogs. Conflict of interestThe authors declare that there is no conflict of interests. Authors’ contributionsAM, SA, and HK planed the experimental design of this study. SM, AM, and YK carried out the experiments. SM and AM analyzed the data. AM wrote the first draft. All authors wrote and revised the article and approved the final manuscript. ReferencesAggarwal, B.B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3(9), 745–756. Ashraf, R. and Shah, N.P. 2014. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 54, 938–956. Dang, X., Xu, M., Liu, D., Zhou, D. and Yang, W. 2020. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 15, e0228846. Friedenberg, S.G., Brown, D.L., Meurs, K.M. and Law, J.M. 2018. Lymphocyte subsets in the adrenal glands of dogs with primary hypoadrenocorticism. Vet. Pathol. 55, 177–181. Fujisawa, T., Shinohara, K., Kishimoto, Y. and Terada, A. 2006. Effect of miso soup containing Natto on the composition and metabolic activity of the human faecal flora. Microb. Ecol. Health. Dis. 18, 79–84. Gong, L., Huang, Q., Fu, A., Wu, Y.P., Li, Y., Xu, X., Huang, Y., Yu, D. and Li, W. 2018. Spores of two probiotic Bacillus species enhance cellular immunity in BALB/c mice. Can. J. Microbiol. 64, 41–48. Goto, K., Iwasawa, D., Kamimura, Y., Yasuda, M., Matsumura, M. and Shimada, T. 2011. Clinical and histopathological evaluation of Dermatophagoides farinae-induced dermatitis in NC/Nga mice orally administered Bacillus subtilis. J. Vet. Med. Sci. 73, 649–654. Hammond, J.A., Guethlein, L.A., Abi-Rached, L., Moesta, A.K. and Parham, P. 2009. Evolution and survival of marine carnivores did not require a diversity of killer cell Ig-like receptors or Ly49 NK cell receptors. J. Immunol. 182, 3618–3627. Huang, Y.C., Hung, S.W., Jan, T.R., Liao, K.W., Cheng, C.H., Wang, Y.S. and Chu, R.M. 2008. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukoc. Biol. 84, 1501–1510. Kim, T.W., Lee, T.Y., Bae, H.C., Hahm, J.H., Kim, Y.H., Park, C., Kang, T.H., Kim, C.J., Sung, M.H. and Poo, H. 2007. Oral administration of high molecular mass poly-γ-glutamate induces NK cell-mediated antitumor immunity. J. Immunol. 179, 775–780. Lee, K., Hwang, S., Paik, D.J., Kim, W.K., Kim, J.M. and Youn, J. 2012. Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 170, 66–76. Lee, S.H., Shin, D.J., Kim, Y., Kim, C.J., Lee, J.J., Yoon, M.S., Thanh Uong, T.N., Yu, D., Jung, J.Y., Cho, D., Jung, B.G., Kim, S.K. and Suh, G.H. 2018. Comparison of phenotypic and functional characteristics between canine non-B, non-T natural killer lymphocytes and CD3+ CD5dim CD21− cytotoxic large granular lymphocytes. Front. Immunol. 9, 1–15. Lee, T.Y., Kim, D.J., Won, J.N., Lee, I.H., Sung, M.H. and Poo, H. 2014. Oral administration of poly-γ-glutamate ameliorates atopic dermatitis in Nc/Nga mice by suppressing Th2-biased immune response and production of IL-17A. J. Invest. Dermatol. 134, 704–711. Maki, G., Krystal, G., Dougherty, F., Takei, F. and Klingemann, H.G. 1998. Induction of sensitivity to NK-mediated cytotoxicity by TNF-α treatment: possible role of ICAM-3 and CD44. Leukemia. 12, 1565–1572. Michael, H.T., Ito, D., McCullar, V., Zhang, B., Miller, J.S. and Modiano, J.F. 2013. Isolation and characterization of canine natural killer cells. Vet. Immunol. Immunopathol. 155, 211–217. Mitsui, N., Kajimoto, O., Tsukahara, M., Murasawa, H., Tamura, M., Nishimura, A., Kajimoto, Y. and Benno, Y. 2006. Effect of natto including Bacillus subtilis K-2 (Spore) on defecation and fecal microbiota, and safety of excessive ingestion in healthy volunteers. Jpn. Pharm. Ther. 34, 135–148. Oh, H.G., Kang, Y.R., Lee, H.Y., Kim, J.H., Shin, E.H., Lee, B.G., Park, S.H., Moon, D.I., Kim, O.J., Lee, I.A., Choi, J., Lee, J.E., Park, K.H. and Suh, J.W. 2014. Ameliorative effects of monascus pilosus-fermented black soybean (Glycine max L. Merrill) on high-fat diet-induced obesity. J. Med. Food. 17, 972–978. Otani, I., Niwa, T., Tajima, M., Ishikawa, A., Watanabe, T., Tsumagari, S., Takeishi, M. and Kanayama, K. 2002. CD56 is expressed exclusively on CD3+ T lymphocytes in canine peripheral blood. J. Vet. Med. Sci. 64, 441–444. Salomon, B.L., Leclerc, M., Tosello, J., Ronin, E., Piaggio, E. and Cohen, J.L. 2018. Tumor necrosis factor α and regulatory T cells in oncoimmunology. Front. Immunol. 9, 444. Shin, D.J., Park, J.Y., Jang, Y.Y., Lee, J.J., Lee, Y.K., Shin, M.G., Jung, J.Y., Carson, W.E., Cho, D. and Kim, S.K. 2013. Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet. Immunol. Immunopathol. 153, 249–259. Sumi, H., Hamada, H., Tsushima, H., Mihara, H. and Muraki, H. 1987. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 43, 1110–1111. Weng, Y., Yao, J., Sparks, S. and Wang, K.Y. 2017. Nattokinase: an oral antithrombotic agent for the prevention of cardiovascular disease. Int. J. Mol. Sci. 18, 523. Withers, S.S., Moore, P.F., Chang, H., Choi, J.W., McSorley, S.J., Kent, M.S., Monjazeb, A.M., Canter, R.J., Murphy, W.J., Sparger, E.E. and Rebhun, R.B. 2018. Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs. Dev. Comp. Immunol. 87, 64–74. Xu, Q., Yajima, T., Li, W., Saito, K., Ohshima, Y. and Yoshikai, Y. 2006. Levan (β-2, 6-fructan), a major fraction of fermented soybean mucilage, displays immunostimulating properties via Toll-like receptor 4 signalling: induction of IL-12 production and suppression of T-helper type 2 response and immunoglobulin E production. Clin. Exp. Allergy. 36, 94–101. | ||
| How to Cite this Article |
| Pubmed Style Mikawa S, Matsuda A, Kamemori Y, Asanuma S, Kitagawa H. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J.. 2021; 11(3): 394-400. doi:10.5455/OVJ.2021.v11.i3.10 Web Style Mikawa S, Matsuda A, Kamemori Y, Asanuma S, Kitagawa H. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. https://www.openveterinaryjournal.com/?mno=52074 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i3.10 AMA (American Medical Association) Style Mikawa S, Matsuda A, Kamemori Y, Asanuma S, Kitagawa H. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J.. 2021; 11(3): 394-400. doi:10.5455/OVJ.2021.v11.i3.10 Vancouver/ICMJE Style Mikawa S, Matsuda A, Kamemori Y, Asanuma S, Kitagawa H. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J.. (2021), [cited January 25, 2026]; 11(3): 394-400. doi:10.5455/OVJ.2021.v11.i3.10 Harvard Style Mikawa, S., Matsuda, . A., Kamemori, . Y., Asanuma, . S. & Kitagawa, . H. (2021) Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Vet. J., 11 (3), 394-400. doi:10.5455/OVJ.2021.v11.i3.10 Turabian Style Mikawa, Shoma, Akira Matsuda, Yasuyuki Kamemori, Satoru Asanuma, and Hitoshi Kitagawa. 2021. Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Veterinary Journal, 11 (3), 394-400. doi:10.5455/OVJ.2021.v11.i3.10 Chicago Style Mikawa, Shoma, Akira Matsuda, Yasuyuki Kamemori, Satoru Asanuma, and Hitoshi Kitagawa. "Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs." Open Veterinary Journal 11 (2021), 394-400. doi:10.5455/OVJ.2021.v11.i3.10 MLA (The Modern Language Association) Style Mikawa, Shoma, Akira Matsuda, Yasuyuki Kamemori, Satoru Asanuma, and Hitoshi Kitagawa. "Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs." Open Veterinary Journal 11.3 (2021), 394-400. Print. doi:10.5455/OVJ.2021.v11.i3.10 APA (American Psychological Association) Style Mikawa, S., Matsuda, . A., Kamemori, . Y., Asanuma, . S. & Kitagawa, . H. (2021) Enhancement of natural killer cell activity by oral administration of a fermented soybean product in dogs. Open Veterinary Journal, 11 (3), 394-400. doi:10.5455/OVJ.2021.v11.i3.10 |