| Review Article | ||
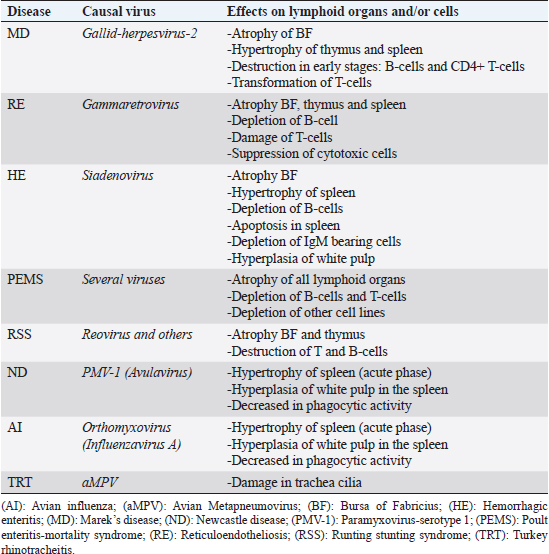
Open Vet. J.. 2019; 9(4): 349-360 doi: 10.4314/ovj.v9i4.13 Open Veterinary Journal, (2019), Vol. 9(4): 349–360 Review Article DOI: http://dx.doi.org/10.4314/ovj.v9i4.13 Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A reviewKhaled Kaboudi*Department of Poultry Farming and Pathology, National Veterinary Medicine School, University of Manouba, 2020 Sidi Thabet, Tunisia *Corresponding Author: Khaled Kaboudi. Department of Poultry Farming and Pathology, National Veterinary Medicine School, University of Manouba, 2020 Sidi Thabet, Tunisia. Email: khaled.kaboudi [at] enmv.uma.tn Submitted: 19/08/2019 Accepted: 30/11/2019 Published: 25/12/2019 © 2019 Open Veterinary Journal AbstractImmunosuppression is characterized by a dysfunction of humoral and/or cellular immune response leading to increase of susceptibility to secondary infections, increase of mortality and morbidity, poor productivity, and welfare and vaccination failures. Humoral immune response depression is due to perturbation of soluble factors, as complement and chemokines in innate immunity and antibodies or cytokines in adaptive immunity. At the cellular immune response, immunosuppression is the consequence of the dysfunction of T-cells, B-cells, heterophils, monocytes, macrophages, and natural Killer cells. Immunosuppression in turkeys can be caused by numerous, non-infectious, and infectious agents, having variable pathological and molecular mechanisms. Interactions between them are very complex. This paper reviews the common viruses inducing clinical and sub-clinical immunosuppression in turkeys, and enteric and neoplastic viruses in particular, as well as the interactions among them. The evaluation of immunosuppression is currently based on classical approach; however, new technique such as the microarray technology is being developed to investigate immunological mediator’s genes detection. Controlling of immunosuppression include, in general, biosecurity practices, maintaining appropriate breeding conditions and vaccination of breeders and their progeny. Nevertheless, few vaccines are available against immunosuppressive viruses in turkey’s industry. The development of new control strategies is reviewed. Keywords: Cellular immunity, Humoral immunity, Immunosuppression, Lymphocyte, Turkey. IntroductionThe development of breeding techniques exposes constantly poultry to immunosuppressive stressors and infectious diseases. The stress response is very complex, which involved varied biological mechanisms. In fact, birds use behavioral, physiological, metabolic, and immunological reactions. Animals adapt theses natural reaction to resist and survive (Shini et al., 2010). The environment, an essential composite of the life of poultry, can influence significantly the efficiency of physiological response and the homeostasis balance (Horning et al., 2003). Stress factors induce functional and morphological changes in birds (Horning et al., 2003). Moreover, poor immune response is usually associated to stressor factors related to management conditions (confinement, climatic and seasonal fluctuation, and poor feeding), parasitic infestation, and infectious diseases. Economic impact of the immunosuppression in poultry industry is well illustrated. A significant deterioration of performances is confirmed in affected flocks, compared to normal animals (Calcagni and Elenkov, 2006). In addition, affected birds respond poorly to vaccines and are more susceptible to secondary infections, causing mortality increase. Several noninfectious immunosuppressive factors cause severe metabolic and functional modifications. Nutritional stress, housing, vaccination, transportation from hatch to farm, tardive feed intake, defect brooding, and mycotoxicosis are considered as potential stress factors (Glaser and Kiecolt-Glaser, 2005). Ammonia and heat stress impose cellular count changes in birds, with a decrease in circulating heterophils and lymphocytes. Viral infections are very important and frequent cause of immunosuppression in poultry, particularly in turkeys. The severity degree of the immune system damage, the persistence of the immunosuppression and the type of affected immune response varied according to the causing virus. Infection may lead immunosuppression by destroying immune cells or inducing balance disorder. Interactions between innate and acquired immunity, cell mediated, and humoral mediated immune responses are affected in general. Perturbation of secretion and functional role of the immune modulator soluble factors, such as cytokines, are reported (Bi et al., 2018). Some of the immunosuppressive viruses in turkeys are hemorrhagic enteritis virus (HEV), Marek’s disease virus (MDV), Reticuloendotheliosis virus (REV), Orthoreovirus, Metapneumovirus, influenza virus, and Poult Enteritis Mortality Syndrome (PEMS). Clinically, immunosuppression may induce non-specific signs depending on age, the causing agent, the vaccine statute, and the management conditions. In general, increased incidence of secondary infections, poor feed conversion, growth rate delay, poor response to vaccines, and increased carcass condemnation rates have been reported in affected flocks (Sahin et al., 2015). Resistance to the immunosuppression is interesting all Professionals of poultry industry are interested in better handling of immunosuppression. Thus, integrated strategy based on good management conditions, respect of the animal needs by a good feeding and adapted vaccines against major infectious diseases must be performed. Genetic resistance, as an intrinsic property, has been explored to select natural resistant poultry strain. Genetic selection for resistance to immunosuppressive diseases has shown promising results (Hoerr, 2010; Cazaban, 2015). However, this tool developed to ameliorate bird resistance, should not interfere with animals behavior and their welfare. Moreover, genetic resistance should not increase the susceptibility to other pathogens. That is why, selection of resistant birds should consider the interaction between genetic factors, homeostasis balance, and animal welfare, which represent a current problem in poultry industry. The aims of this article are to analyze the current knowledge in viral immunosuppressive diseases in turkeys. The means used to detect decrease in immune response and possible measures control of immunosuppression are reviewed and discussed. Immunosuppressive virusesEnteric virusesEnteritis is a main problem in poultry, associated to considerable direct and indirect economic losses. Several enteric viruses have been identified in commercial flocks of turkeys worldwide. Enteric diseases may be occur in all age groups, nevertheless, they are predominantly affect young birds in the three first weeks of age, where infections appear more severe (Nuñez and Piantino Ferreira, 2013; Mettifogo et al., 2014). Enteric viruses increase susceptibility of affected birds to secondary infections and others immunosuppressive diseases. Several viruses are incriminated in the enteric diseases in commercial turkeys. Interaction between them is very complex, including many other management, feeding, and infectious factors. Because of the various etiologies, clinical signs are in general nonspecific, including diarrhea, increased mortality, and poor performances. Gross pathology showed gastrointestinal lesions, associated to liver, pancreatic, and lymphoid damage. These symptoms and lesions are considered to be the main enteric syndrome that is why laboratory investigations consist on the use of essential tool to confirm etiological agents (Alavarez et al., 2014; Mettifogo et al., 2014). In turkeys, the most important enteric viral diseases are represented by hemorrhagic enteritis (HE), runting stunting syndrome (RSS) and PEMS (Table 1). Hemorrhagic enteritis and other adenovirusesHE is an acute disease of turkeys caused by Siadenovirus (group II Aviadenovirus), immunosuppressive virus, which infect essentially animals at 4 weeks of age and older. Depression, bloody droppings, heterogeneity of the flock, and increased mortality characterize this disease. In field outbreaks, mortality varied from 0.1% to 60% (Gross and Moore, 1967). Virus replication occurs essentially in spleen, considered to be the major site (Saunders et al., 1993; Pierson and Fitzgerald, 2013). However, Enzyme Linked Immunosorbent Assay (ELISA), Immunofluorescent (IF), and Polymerase Chain Reaction (PCR) are used to confirm the presence of infected cells in many other tissues, such as intestine, bursa of Fabricius, caecal tonsils, thymus, liver, kidney, leukocytes, and lungs (Silim and Thorsen, 1981; Fasina and Fabricant, 1982; Fitzgerald et al., 1992; Trampel et al., 1992; Hussain et al., 1993; Suresh and Sharma, 1996). Primarily viral replication occurs in B cells and macrophages. Other cells target are represented by adherent mononuclear macrophages and non-adherent mononuclear cells (van den Hurk, 1990) bearing IgM (Suresh and Sharma, 1995; 1996). Virulent HEV strains are capable to induce apoptosis in spleen cells, due to the induction of interleukine-6 (IL-6) secretion in the spleen (Rautenschlein et al., 2000b). Activation of macrophages leads to cytokines (IL-6, interferon type I and II, and TNF) production. Immunosuppressive is the consequence of the nitric acid production, stimulated by the interferon-II (IFN-II) (Dhama et al., 2017). Transient immunosuppression has been reported during clinical phase of the disease, with considerable depletion of IgM-bearing B cells (Rautenschlein et al., 2000b). Vaccination failures are observed in infected turkeys. A significant decrease in hemagglutination inhibition antibody titers is detected in turkeys infected with virulent HEV. Moreover, depression in phytohemagglutinin (PHA) is also described in inoculated birds (Nagaraja et al., 1985). Secondary bacterial infections may extend the course of illness and increase mortality for an additional 2–4 weeks (Dhama et al., 2017). Increased predisposition to enteropathogenic Escherichia coli infection (Larsen et al., 1985; van den Hurk et al., 1994; Giovanardi et al., 2014) and clostridial dermatitis (Thachil and Nagaraja, 2013) has been well documented. Resistance of the virus outside, poor hygiene conditions, and short down time between flocks, contribute to the persistence of the HE (Pierson and Fitzgerald, 2013). Due to hemorrhage, carcasses appear pale. Gross pathology showed hemorrhagic intestinal mucosa, with the presence of natural coagulated blood. Spleen is characteristically enlarged, marbled, and friable. In dead birds, spleen may be smaller and pale because of blood loss and subsequent splenic contraction (Gross, 1967; Carlson et al., 1974; Fujiwara et al., 1975; Itakura and Carlson, 1975). Histological findings are more apparent in lymphoreticular and gastrointestinal systems. Hyperplasia of the white pulp, lymphoid necrosis and intranuclear inclusion body within lymphoreticular body cells are the most described microscopic modifications (Saunders et al., 1993). Table 1. Immunosuppressive effects of main viral diseases in turkeys.
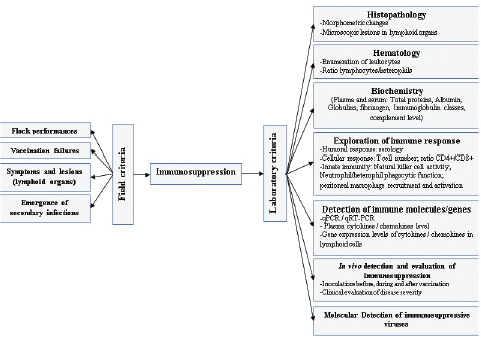
Histopathological changes are more evident in the duodenum, where congestion, hemorrhage, and hetetrophils infiltration and epithelium villus degeneration, consist the major observations. Less severe lesions cans be also find in the gizzard, the proventiculus, the caeca tonsils and the bursa of Fabricius (Saunders et al., 1993; Pierson and Fitzgerald, 2013). Intranuclear inclusions have been detected in many tissues, such as liver, pancreas, bone marrow, renal tubular epithelium, and lung (Gross, 1967; Carlson et al., 1974; Fujiwara et al., 1975; Itakura and Carlson, 1975; Meteyer et al., 1992; Trampel et al., 1992; Hussain et al., 1993). Many other fowl adenoviruses (FAV) are considered as immunosuppressive agent in turkey. Adenovirus responsible of inclusion body hepatitis (IBH) can induce atrophy of the bursa, the thymus and the spleen, that occurs following challenges involving serotypes 1, 4, and 8 (Singh et al., 2006; Schonewille et al., 2008). Virulent strains show affinity to lymphocytes and consequently cause impairment of the humoral and cellular responses. Effects on immune system are more severe when associated to aflatoxins (Shivachandra et al., 2003). Several FAV strains are capable of increasing the susceptibility of the bids to E. coli infections (Rosenberger et al., 1985). Vaccination failures again ND and avian influenza (subtype H9) is reported in animals inoculated by FAV serotype 4 (Niu et al., 2017). Runting stunting syndromeRSS is a multifactorial syndrome affecting young chickens and turkeys, in general under 2 weeks of age. Increased mortality, remarkable heterogeneity, aqueous diarrhea, and increased mortality are the most important alert clinical signs (de Wit et al., 2011). Etiology of this syndrome is not known exactly, however, many viruses are isolated from affected birds. Reoviruses, Astroviruses, Rotaviruses, Coronaviruses, Enterovirus-like, and Adenoviruses are detected in RSS outbreaks (Simmons et al., 1972; Kouwenhoven et al., 1978; Kisary et al., 1984; McNulty et al., 1984; Songserm et al., 2000; Baxendale and Mebatsion, 2004; Otto et al., 2006; Smyth, 2017). In addition, nutritional and management factors could contribute to RSS in poultry. Virulent strains of avian Reovirus cause atrophy of lymphoid tissues and interfere with humoral immunity (Kang et al., 2012). Reoviruses can replicate in monocytes but not in lymphocytes. Thus, the lymphoid atrophy is not caused by Reovirus tropism specifically for lymphocytes in contrast to other more specific viruses, such as infectious bursal disease virus, chicken anemia virus, and MDV. Lesions observed in the bursa of Fabricius are dominated by atrophy, edema, hyperplasia of the epithelium, and lymphoid depletion (Nili et al., 2007). Spleen cells collected from birds infected with avian Reovirus (Strain S1133) exhibited a significantly reduced response to the mitogens PHA (Sharma et al., 1994). Poult enteritis-mortality syndromePEMS is an infectious, transmissible, and multifactorial disease. It occurs, especially, in young turkeys aged from 1 week to weeks. Affected flocks appear with high heterogeneity, diarrhea, and increase in mortality, which can exceed 2% per week. Market growth rate depression, emergence of secondary bacterial, and mycosis infections and vaccination failure against major classical diseases are frequently observed. Initially, etiology of the PEMS was attributed to toxins. Nevertheless, later, many viruses was detected in field outbreaks, such as Coronavirus and Astrovirus, associated to others infectious agents (Salmonella, Escherichia coli, Campylobacter), nutritional and management factors (Barnes and Guy, 2003). Initial clinical signs consist of increase in water consumption and decrease in feed ingestion followed by emission of watery and brown droppings. Poults consume litter fragments and feathers. Increased mortality is observed around 7–14 days of age with a high spike. At necropsy examination, emaciation, catarrhal enteritis, anemia, and spectacularly atrophy of all lymphoid organs are noted. Histopathological investigations showed lymphocyte depletion in lymphoid tissues (Jindal et al., 2010). Lymphocyte necrosis and severe depletion in the bursa of Fabricius occur in turkeys inoculated with enteric coronavirus and E. coli (Kang et al., 2012). Thymus atrophy is constantly associated with PEMS, in poults inoculated with Astrovirus (Grgić et al., 2011). Replication of Enterovirus in lymphoid tissues can induce lymphocyte necrosis and depletion of lymphoid organs, and subsequently reduction in lymphocyte subpopulations in circulating blood (Hoerr, 2010). Other enteric virusesCoronaviruses are incriminated in several poultry diseases, such as infectious bronchitis, enteric syndrome and PEMS. Turkey coronavirus (TCoV), the causative agent of Bluecomb disease, induces diarrhea, ruffled feathers, decreased feed and water consumption in addition to poor growth rate (Yu et al., 2000; Barnes and Guy, 2003). Villous atrophy and desquamation, catarrhal enteritis, and hemorrhagic infiltration are the major microscopic lesions observed in intestines. Immunosuppressive effect of TCoV consists of atrophy of the bursa of Fabricius. Histopathological examination shows that pseudo-stratified columnar epithelium is replaced by a stratified squamous epithelium, and intense heterophilic inflammation is seen within and underneath the epithelium (Guy, 2003). Parvoviruses are more common in goose and ducks with the most known affection in young geese, the Derzsy’s disease (Gough, 2008). Strains of these viruses belong to the subfamily of Dependovirinae. In turkeys, a distinct group has been detected in the subfamily of Parvovirinae, which has been recognized as a causative agent of naturally occurring enteric infections and possible neurologic troubles in turkeys and chickens (Zsak et al., 2008; Marusak et al., 2010). Genetic studies revealed differences between chicken and turkey’s parvovirus strains (Zsak et al., 2008; Domanska-Blicharz et al., 2012; Sharafeldin et al., 2017). The implication of parvoviruses in complex enteric diseases has been reported in chicken and turkeys since 1980s (Trampel et al., 1983; Kisary et al., 1984). In chickens, parvoviruses are isolated from RSS outbreaks. While in turkeys, the cited viruses are detected in field cases of PEMS and “Light Turkey Syndrome” (Mor et al., 2013). Neoplastic diseasesMarek’s diseaseMarek’s disease, most common avian neoplasm, is caused by serotype- 1 strains of Marek’s disease herpesvirus (MDV-1) (Schat and Nair, 2008). MDV-1 is an induced oncogenic and immunosuppressive virus in poultry. More frequent in chicken, the diagnosis of MD in turkeys represents a relatively unusual finding. Three MDV serotypes are currently recognized: serotype 1, which is divided into pathotypes, ranging from mild (m), virulent (v), and very virulent (vv) to very virulent plus (vv+) strains (Witter, 1997; Witter et al., 2005). Apathogenic strains (e.g., Rispens: CVI-988) in serotype 1 are used to prepare vaccines. Serotype 2, isolated from normally chickens and contains pathogenic strains, and serotype 3, isolated from turkeys, includes apathogenic strains, represented particularly by the Herpes Virus of Turkey strain, used as heterologous vaccine. Replication of MDV in lymphoid tissues is considered as early in B cells, with cytolysis effect. Transient immunosuppression is described from three to seven days post inoculation. The latent infection occurs in both B and T cells. The infection of T lymphocytes induces necrosis of cells and consequently an immunosuppression. Finally, MDV is capable of transforming T cells, which leads to lymphoid tumor formation and death (Calnek et al., 1998; Calnek, 2001; Heidari et al., 2010). Cytolysis of B and T cells, induced by virulent strains, is associated with severe lymphopaenia. Therefore, MDV induce immunosuppression, involving both humoral and cell-mediated immunity (Calnek et al., 1998; Biggs and Nair, 2012; Haq et al., 2013). This effect is correlated with massive apoptosis of CD4+CD8+ thymocytes, leading to thymic atrophy and reduction in circulating CD4+ lymphocytes (Morimura et al., 1996). The degree of immunosuppression is correlated to the virulence of viral strains (Hoerr, 2010; Couteaudier and Denesvre, 2014; Hu et al., 2014). Surveillance of immunosuppression induced by the MDV is not evident, because of the continuous circulation of other immunosuppressive agents. The detection of emerging forms with skin tumors, lymphomas and early forms represent practical, but inaccurate approach to evaluate MDV immunosuppression (Biggs and Naire, 2012; Couteaudier and Denesvre, 2014). ReticuloendotheliosisReticuloendotheliosis (RE) is an important immunosuppressive disease of turkeys and chickens. REV induces neoplastic disease, which may be similar to MD and lymphoid leucosis (LL). The disease sporadically appears to cause significant death and condemnation loss in commercial turkey flocks and is a potential contaminant of vaccine viruses, especially those of turkey origin. The immunosuppressive effect of REV is the consequence of lymphocytes T and B abrogation and the disturbance of endotheliocytes function (Etienne and Emerman, 2013). The disease causes atrophy of the bursa of Fabricius and thymus. Dysfunction of the spleen is reported (Payne and Venugopal, 2000). Tumors induced by the REV are associated to the B cells (Woźniakowski et al., 2018). Damage of T cells, reduction of lymphocyte T in peripheral blood, and suppression of splenic cells have been documented in chickens (Hrdlicková et al., 1994; Bi et al., 2018). The decreased number of T-cells might be responsible for the lower levels of IL8 and IL18 (Bi et al., 2018). Other clinical signs and lesions are described in RE outbreaks, including, neurological disorders, enteritis, spleen, and liver necrosis and ulceration of proventriculus (Woźniakowski et al., 2018). Respiratory virusesMany other viruses have been considered as immunosuppressive agent in turkey. Respiratory viruses have usually negative effect on immune system, such as Newcastle disease virus (NDV) avian influenza viruses (AIV), and avian Metapneumoviruses (aMPV). Newcastle disease (ND) is a worldwide disease causing severe economic losses. NDV can damage lymphoid tissues and decrease macrophage secretion and their phagocytosis role. Necrosis of lymphocytes and apoptosis of peripheral blood lymphocytes and mononuclear cells have been also reported (Cheville and Beard, 1972; Agoha et al., 1992; Lam, 1996). aMPV is the causal agent of turkey rhinotracheitis (TRT). Replication of this virus in epithelial cells the upper respiratory tract can impair the mucociliary functions and increase deeper bacterial infections, with E. coli and Ornithobacterium rhinotracheale (Majo et al., 1997; Jirjis et al., 2004). Being an immunosuppressive pathogen, aMPV is able to reduce reactions to phytohaemagglutinin and immune responses to sheep red cells in poults. Infected animals showed lower thymus weight (Timms et al., 1986). Furthermore, it has been shown that aMPV can interfere with HEV vaccines and subsequently, reduce immune response in turkeys (Chary et al., 2002). Evaluation of immunosuppressionEvaluation of immunosuppression is based on field criteria and laboratory investigations (Fig. 1). Yet, practical and evaluable methods are restricted for an accurate evaluation.
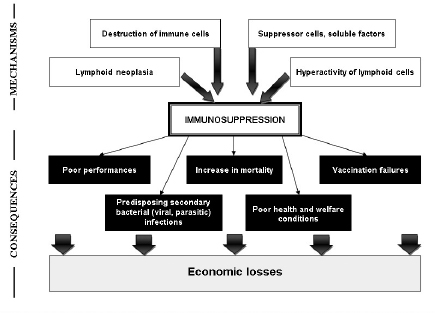
Fig. 1. Evaluation criteria of the immunosuppression in turkeys. Field criteria consist on global evaluation of the flock health statute. However, these criteria are nonspecific and allow for only first orientation. In general, immunosuppression leads to degradation of performances, with poor feed conversion, decrease in growth rate, heterogeneity, low weight, and increased mortality. Due to the influence of viruses on host immune system, vaccination failures are declared. Depressed animals are more susceptible to develop bacterial and parasitic secondary infections, which are accompanied by increased mortality (Fig. 2). The various symptoms and lesions induced by immunosuppressive viral diseases can help to establish clinical suspicion, which need laboratory investigations to confirm it. Many laboratory tests are useful in order to evaluate immunosuppression in turkeys. The global approach can be summarized in four main criteria (Dohm and Saif, 1984):
Pathomorphological examination of lymphoid organs is easily practicable. The use of quantitative indices may largely contribute to more rapid and correct diagnosis. Lymphoid organs masses (bursa, thymus, and spleen) contribute to objective evaluation (Halouzka and Jurajda, 1991; Sellaoui et al., 2012). Histopathological investigation is an important tool for evaluating severity of immunosuppression and discriminating between several diseases (Pope, 1991).
Fig. 2. Mechanisms and consequences of the immunosuppression in turkeys. Detection of specific causal agents by viral isolation and molecular detection is a practical approach. Serology may be an easy test for the diagnosis. Evaluation of cellular immune competency is used in vitro as well as in vivo (Fadly et al., 1982). Hematological investigations may provide heterophil:lymphocyte ration, as significant indicator of stress (Huff et al., 2005; Cotter, 2015) and immunocompetency (Hocking et al., 2002). In vitro lymphocyte proliferation response to mitogen is widely used to evaluate the integrity of cell-mediated immunity. Lymphocyte proliferation responses of spleen cells are higher in turkeys infected by TCoV than in non-infected animals, with increase of CD4+ subpopulation of T lymphocytes (Loa et al., 2001). The lymphoproliferative response to phytohemagglutinin phosphate is performed as an indicator of a T-cell-induced delayed-type hypersensitivity reaction. A mononuclear phagocytic system function assessment is used to study the degree of clearance from the blood circulation in commercial turkeys (Cheema et al., 2007). Determining cytokines levels to evaluate cellular immune response is well developed in mammals due to available commercial systems, especially ELISA tests and RT-PCR (Wigley and Kaiser, 2003). For avian species, IFN-gamma can be quantified by currently available ELISA test in chickens (Lambrecht et al., 2000) and in turkeys (Lawson et al., 2001). Microarray technology is performed to evaluate cellular immune response in poultry, by detecting genes involved in antiviral and pro-inflammatory cytokine responses (Kapczynski et al., 2013). Moreover, innate and adaptive immune responses can be explored by real-time RT-PCR to investigate changes in the gene expression of cytokines interleukin (IL) and chemokines (Gadde et al., 2011). Evaluation of immunosuppression represents a delicate approach, which is not routinely applied in poultry pathology. Exploration of the different components of the immune system is based on several in vivo and in vitro methods. Due to the complexity of the immunosuppression etiology, the consequence of several intrinsic and extrinsic factor interactions, the use of combination of many techniques would help interpreting the data. Control of viral-induced immunosuppressionThe main objective is to prevent economic losses caused by viral immunosuppressive infection. Strategy control is based on different approaches including, good management, application of biosecurity standards, immunization of birds, and genetic selection. The good management has an important role in optimizing turkey’s performances and in maintaining bird health and welfare. Delivering constantly a good quality air, a high quality water and feed is essential and required at all stages of growth. Control of mycotoxins, as an immunosuppressive agent, must be continually performed. Litter management is a key of the pathogens control. Application of strict biosecurity measures is fundamental to prevent exposition to immunosuppressive viruses. Increasing bird’s resistance is a complement but essential tool through good vaccination, as the best tool inducing specific protection. Vaccines must be administrated properly for all animals in the same flock, in order to achieve uniform immune response. However, vaccines are not available for the main viral diseases in turkeys. The control of other immunosuppressive agents (bacteria, mycotoxins, parasites, and stress) must be considered to prevent vaccination failures. Currently, turkeys can be vaccinated against few immunosuppressive viral diseases, such as HE, ND, aMPV, and Reoviruses. Live vaccines used against HE are effective in preventing disease outbreaks. However, they are immunosuppressive, predisposing young animals to opportunistic infections and vaccination failures. Control of ND is based on different types of vaccines. Lives vaccines, worldwide used in poultry industry, can stimulate local protection. Inactivated vaccines required for a long lasting immunity. Currently, vectored vaccines, using glycoprotein F and administered in ovo or to 1-day old poults, are effective and safety Vaccination against aMPV is performed to prevent lesions in the upper respiratory tract and the decrease in egg production. Live vaccines are used in young poults, while inactivated vaccines are reserved to adults. The development of vaccination against Reoviruses interest chickens. In general, strain vaccine protected against viral arthritis with partial protection against RSS. Although TCoV was identified as the causative agent of Bluecomb disease of turkey poults over 50 years ago (28), vaccines are not available to control the disease. Recent assays of vaccination using protein spike are performed with promoted results (Chen et al., 2018). The MD is well controlled by vaccination in chickens. Despite the appearance of natural disease in commercial turkeys flocks, vaccination is not performed. However, possible immunization of poults at the hatchery with Rispens strain is suggested (Blake-Dyke and Baigent, 2013). Cytokines may be used as therapeutic agents for viral diseases and for vaccines adjuvants (Wigley and Kaiser, 2003). IFN-α induces increase of antibody titer in turkeys immunized by NDV DNA vaccine (Rautenschlein et al., 2000a). While, addition of IFN-γ to NDV DNA vaccine, is accompanied by more rapid humoral response and increased protection to NDV challenge in turkeys and chickens vaccinated in ovo (Rautenschlein et al., 1999; Cardenas-Garcia et al., 2016). Given the limitations associated with vaccination, several assays of genetic selection have been performed. Genetic resistance to MD is well documented, with a special focus on major histocompatibility complex. Notable correlation is demonstrated between resistance of chickens to MD and B21 allele, which is accompanied by reduction of infected T-cells. Mapping genes suggested the presence of a resistance gene in the natural killer region within chromosome 1 (Bumstead, 1998), whereas birds with a B-19 haplotype suffer 100% mortality (Briles et al., 1977). Certain types of artificial selection, as higher growth rates, influence negatively immune competence in turkeys (Husby et al., 2011). Recently, insertion of transgenes that target AIV into the genomes of chickens allowed to limit virus spread, but this approach is incapable de prevent emergence of disease (Looi et al., 2018). Genetic selection birds for optimal immune response to used vaccines may complete optimizing natural resistance to viral diseases. Selection for enhanced innate immunity is possible because of the existence of toll-like receptors in chickens and turkeys. Possible interactions between adjuvants and immunogenetics may lead to develop novel vaccines. ConclusionImmunosuppression is a common condition in intensive breeding, where stress factors are diverse and constantly present. The pressure supported by the immune system of birds can have several origins: environmental, management, nutritional, infectious, and parasitic. Transient or permanent immunosuppression induces considerable economic losses in terms of performance, secondary infections, mortality, vaccination failures, condemnation in slaughterhouse, and poor animal welfare conditions. Viruses-induced immunosuppression in turkeys is a major cause of decrease in profitability. Despite the knowledge of many features of virus’s effects on immune system of birds, several molecular and immunological aspects are still unclear. The interaction between immunosuppressive viruses and other stressors is not yet well explained. On the other hand, some immune mechanisms, particularly related to cellular mediated immune response, due to viral infection are insufficiently explored and clarified. Diagnosis and evaluation of immunosuppression due to viral diseases are based on field and laboratory criteria. The role of avian veterinarians is fundamental in term of early detection of immunosuppression. They will be challenged with emergent viruses and immunosuppressive in particular, in turkey industry. Preventing of immunosuppression needs an integrate approach, where the research development and the field observations play an important role in the refining of turkey industry strategies for a better and efficient controlling programs. The maintaining of appropriate management, environmental, and nutritional conditions is essential to minimize stressors. Application of strict biosecurity and vaccination programs of breeders and their progenitor, against immunosuppressive and other major diseases are currently practical and feasible measures to prevent introduction and propagation of pathogens and enhance the quality of life for animals. In addition, development of new controlling methods, bases on novel generation of vaccines, administration of cytokines and genetic resistance, is still being tested despite the promoter results relative to increase in disease resistance of birds. Conflict of interestThe author declares that there is no conflict of interest. ReferencesAgoha, N.J., Akpavie, S.O., Durojaiye, O.A. and Adene, D.F. 1992. Pathogenicity of two strains of Newcastle disease virus in the grey-breasted helmet guinea fowl. Vet. Q. 14, 51–53. Alavarez, J.M., Ferreira, C.S.A. and Ferreira, A.J.P. 2014. Enteric viruses in turkey flocks: a historic review. Brazilian J. Poult. Sci. 16, 225–232. Barnes, H.J. and Guy, J.S. 2003. Poult enteritis-mortality syndrome. In Diseases of poultry, 11th ed. Eds., Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R. and Swayne, D.E. Ames: Iowa State University Press, pp: 1171–1180. Baxendale, W. and Mebatsion, T. 2004. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 33, 364–370. Bi, Y., Xu, L., Qiu, L., Wang, S., Liu, X., Zhang, Y., Chen, Y., Zhang, Y., Xu, Q., Chang, G. and Chen, G. 2018. Reticuloendotheliosis virus inhibits the immune response acting on lymphocytes from peripheral blood of chicken. Front. Physiol. 9, 4. doi:10.3389/fphys.2018.00004. Biggs, P.M. and Nair, V. 2012. The long view: 40 years of Marek's disease research and Avian Pathology. Avian Pathol. 41, 3–9. Blake-Dyke, C. and Baigent, S. 2013. Marek's disease in commercial turkey flocks. Vet. Rec. 173, 376. Briles, W.E., Stone, H.A. and Cole, R.K., 1977. Marek's disease: effects of B histocompatibility alloalleles in resistant and susceptible chicken lines. Science 195, 193–195. Bumstead, N. 1998. Genomic mapping of resistance to Marek's disease. Avian Pathol. 27, 78–81. Calcagni, E. and Elenkov, I. 2006. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. N. Y. Acad. Sci. 1069, 62–76. Calnek, B.W. 2001. Pathogenesis of Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 255, 25–55. Calnek, B.W., Harris, R.W., Buscaglia, C., Schat, K.A. and Lucio, B. 1998. Relationship between the immunosuppressive potential and the pathotype of Marek’s disease virus isolates. Avian Dis. 42, 124–132. Cardenas-Garcia, S., Dunwoody, R.P., Marcano, V., Diel, D.G., Williams, R.J., Gogal, R.M. Jr., Brown, C.C., Miller, P.J. and Afonso, C.L. 2016. Effects of chicken interferon gamma on Newcastle disease virus vaccine immunogenicity. PLoS One 11(7), e0159153. doi:10.1371/journal.pone.0159153 Carlson, H.C., Al-Sheikhly, F., Pettit, J.R. and Seawright, G.L. 1974. Virus particles in spleens and intestines of turkeys with hemorrhagic enteritis. Avian Dis. 18, 67–73. Cazaban, C. 2015. Immunosuppression in chickens - what is it? Int. Poult. Prod. 13, 13–14. Chary, P., Rautenschlein, S. and Sharma, J.M. 2002. Reduced efficacy of hemorrhagic enteritis virus vaccine in turkeys exposed to avian pneumovirus. Avian Dis. 46, 353–359. Cheema, M.A., Qureshi, M.A., Havenstein, G.B., Ferket, P.R. and Nestor, K.E. 2007. A Comparison of the Immune Response of 2003 Commercial Turkeys and a 1966 Randombred Strain When Fed Representative 2003 and 1966 Turkey Diets. Poult. Sci. 86(2), 241–248. Chen, Y.N., Wu, C.C., Bryan, T., Hooper, T., Schrader, D. and Lin, T.L. 2018. Pathogenicity, immunogenicity, protection efficacy, and spike protein gene sequence of a high-passage turkey coronavirus serially passaged in embryonated turkey eggs. Taiwan Vet. J. 44(4), 165–178. Cheville, N.F. and Beard, C.W. 1972. Cytopathology of Newcastle disease. The influence of bursal and thymic lymphoid systems in the chicken. Lab. Invest. 27, 129–143. Cotter, P.F. 2015. An examination of the utility of heterophil-lymphocyte ratio in assessing stress of caged hens. Poult. Sci. 94, 512–517. Couteaudier, M. and Denesvre, C. 2014. Marek's disease virus and skin interactions. Vet. Res. 45, 36. de Wit, J.J., Dam, G.B.T., van de Laar, J.M.A.M., Biermann, Y., Verstegen, I., Edens, F. and Schrier, C.C. 2011. Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Pathol. 40, 453–461. Dhama, K., Gowthaman, V., Karthik, K., Tiwari, R., Sachan, S., Kumar, M.A., Palanivelu, M., Malik, Y.S., Singh, R.K. and Munir, M. 2017. Haemorrhagic enteritis of turkeys—current knowledge, Vet. Q. 37(1), 31–42. Dohm, J.E. and Saif, Y.M. 1984. Criteria for evaluating immunosuppression. Avian Dis. 28, 305–310. Domanska-Blicharz, K., Jacukowicz, A., Lisowska, A. and Minta, Z. 2012. Genetic characterization of parvoviruses circulating in turkey and chicken flocks in Poland. Arch. Virol. 157(12), 2425–2430. Etienne, L. and Emerman, M. 2013. The mongoose, the pheasant, the pox, and the retrovirus. PLoS Biol. 11, e1001641. Fadly, A.M., Lee, L.F. and Bacon, L.D. 1982. Immunocompetence of chickens during early and tumorigenic stages of Rous-associated Virus-1 infection. Infect. Immun. 37(3), 1156–1161. Fasina, S.O. and Fabricant, J. 1982. Immunofluorescence studies on the early pathogenesis of hemorrhagic enteritis virus infection in turkeys and chickens. Avian Dis. 26, 158–163. Fitzgerald, S.D., Reed, W.M. and Burnstein, T. 1992. Detection of type II avian adenoviral antigen in tissue sections using immunohistochemical staining. Avian Dis. 36, 341–347. Fujiwara, H., Tanaami, S., Yamaguchi, M. and Yoshiro, T. 1975. Histopathology of hemorrhagic enteritis in turkeys. Nat. Inst. Anim. Hlth. Quart. 15, 68–75. Gadde, U., Chapman, H.D., Rathinam, T. and Erf, G.F. 2011. Cellular immune responses, chemokine, and cytokine profiles in turkey poults following infection with the intestinal parasite Eimeria adenoeides. Poult. Sci. 90, 2243–2250. Giovanardi, D., Lupini, C., Pesente, P., Rossi, G., Ortali, G. and Catelli, E. 2014. Longitudinal field studies of Avian Metapneumovirus and Turkey Hemorrhagic Enteritis Virus in turkeys suffering from colibacillosis associated mortality. Vet. Res. Commun. 38, 129–137. Glaser, R. and Kiecolt-Glaser, J.K. 2005. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 5(3), 243–251. Gough, R.E. 2008. Parvovirus infections. In Diseases of poultry. Ed., Saif, Y.M. Ames: Blackwell, pp: 397–404. Grgić, H., Yang, D. and Nagy, E. 2011. Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8 isolate. Virus Res. 56, 91–97. Gross, W.B. 1967. Lesions of hemorrhagic enteritis. Avian Dis. 11, 684–693. Gross, W.B. and Moore, W.E.C. 1967. Hemorrhagic enteritis of turkeys. Avian Dis. 11, 296–307. Guy, J.S., 2003. Turkey coronavirus enteritis. In Disease of poultry, 11th ed. Eds., Ames, Saif, Y.M., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E. Iowa city: Iowa State University Press, pp: 300–307. Halouzka, R. and Jurajda, V. 1991. Morphological expression of imunosuppression in poultry. Acta Vet. Brno. 60, 271–276. Haq, K., Schat, K.A. and Sharif, S. 2013. Immunity to Marek's disease: where are we now? Dev. Comp. Immunol. 41, 439–446. Heidari, M., Sarson, A.J., Huebner, M., Sharif, S., Kireev, D. and Zhou, H. 2010. Marek's disease virus-induced immunosuppression: array analysis of chicken immune response gene expression profiling. Viral Immunol. 23, 309–319. Hocking, P.M., Maxwell, M.H., Robertson, G.W. and Mitchell, M.A. 2002. Welfare assessment of broiler breeders that are food restricted after peak rate of lay. Br. Poult. Sci. 43, 5–15. Hoerr, F.J. 2010. Clinical aspects of immunosuppression in poultry. Avian Dis. 54(1), 2–15. Horning, G., Rasmussen, S., Permin, A. and Bisgaard, M. 2003. Investigations on the influence of helminth parasites on vaccination of chickens against Newcastle disease virus under village conditions. Trop. Anim. Health Prod. 35, 415–424. Hrdlicková, R., Nehyba, J. and Humphries, E.H. 1994. V-rel induce expression of three avian immuno-regulatory surface receptors more efficiently than c-rel. J. Virol. 68, 308–319. Hu, X., Xu, W., Qin, A., Wu, G., Qian, K., Shao, H. and Ye, J. 2014. Marek's disease virus may interfere with T cell immunity by TLR3 signals. Vet. Res. Commun. 38, 149–156. Huff, G.R., Huff, W.E, Balog, J.M., Rath, N.C., Anthony, N.B. and Nestor, K.E. 2005. Stress response differences and disease susceptibility reflected by heterophil to lymphocyte ratio in turkeys selected for increased body weight. Poult. Sci. 84(5), 709–717. Husby, A., Ekblom, R. and Qvarnström, A. 2011. Let's talk turkey: immune competence in domestic and wild fowl. Heredity (Edinb) 107(2), 103–104. Hussain, I., Choi, C.U., Rings, B.S., Shaw, D.P. and Nagaraja, K.V. 1993. Pathogenesis of hemorrhagic enteritis virus infection in turkeys. J. Vet. Med. 40, 715–726. Itakura, C. and Carlson, H.C. 1975. Electron microscopic findings of cells with inclusion bodies in experimental hemorrhagic enteritis of turkeys. Can. J. Comp. Med. 39, 299–304. Jindal, N., Patnayak, D.P., Chander, Y., Ziegler, A.F. and Goyal, S.M. 2010. Detection and molecular characterisation of enteric viruses from poult enteritis syndrome in turkeys. Poult. Sci. 89, 217–226. Jirjis, F.F., Noll, S.L., Halvorson, D.A., Nagaraja, K.V., Martin, F. and Shaw, D.P. 2004. Effects of bacterial coinfection on the pathogenesis of avian pneumovirus infection in turkeys. Avian Dis. 48, 34–49. Kang, K., El-Gazzar, M., Sellers, H.S., Dorea, F., Williams, S.M., Kim, T., Collett, S. and Mundt, E. 2012. Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathol. 41, 41–50. Kapczynski, D.L., Afonso, C.L. and Miller, P.J. 2013. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 41(3), 447–453. Kisary, J., Nagy, B. and Bitay, Z. 1984. Presence of parvoviruses in the intestine of chickens showing stunting syndrome. Avian Pathol. 13, 339–343. Kouwenhoven, B., Davelaar, F.G. and Van Walsum, J. 1978. Infectious proventriculitis causing runting in broilers. Avian Pathol. 7, 183–187. Lam, K.M. 1996. Newcasde disease virus-induced apoptosis in the peripheral blood mononudear cells of chickens. J. Comp. Pathol. 114, 63–71. Lambrecht, B., Gonze, M., Meulemans, G. and van den Berg, T.P. 2000. Production of antibodies against chicken interferon-γ: demonstration of neutralising activity and development of a quantitative ELISA. Vet. Immunol. Immunopathol. 74, 137–144. Larsen, C.T., Domermuth, C.H., Sponenberg, D.P. and Gross, W.B. 1985. Colibacillosis of turkeys exacerbated by hemorrhagic enteritis virus. Laboratory studies. Avian Dis. 29, 729–732. Lawson, S., Rothwell, L., Lambrecht, B., Howes, K., Venugopal, K. and Kaiser, P. 2001. Turkey and chicken interferon-γ, which share high sequence identity, are biologically cross-reactive. Dev. Comp. Immunol. 25, 69–82. Loa, C.C., Lin, T.L., Wu, C.C., Bryan, T., Thacker, H.L., Hooper, T. and Schrader, D. 2001. Humoral and cellular immune responses in turkey poults infected with Turkey Coronavirus. Poult. Sci. 80, 1416–1424. Looi, F.Y., Baker, M.L., Townson, T., Richard, M., Novak, B., Doran, T.J. and Short, K.R. 2018. Creating disease resistant chickens: a viable solution to Avian Influenza? Viruses 10(10), 561. doi:10.3390/v10100561 Majo, N., Gibert, X., Vilafranca, M., O'Loan, C.J., Allan, G.M., Costa, L., Pages, A. and Ramis, A. 1997. Turkey rhinotracheitis virus and Escherichia coli experimental infection in chickens: histopathological, immunocytochemical and microbiological study. Vet. Microbiol. 57, 29–40. Marusak, R.A., Guy, J.S., Abdul-Aziz, T.A., West, M.A., Fletcher, O.J., Day, J.M., Zsak, L. and Barnes, H.J. 2010. Parvovirus-associated cerebellar hypoplasia and hydrocephalus in day old broiler chickens. Avian Dis. 54, 156–160. McNulty, M.S., Allan, G.M., Connor, T.J., Mc Ferran, J.B. and McCracken, R.M. 1984. An entero-like virus associated with the runting syndrome in broiler chickens. Avian Pathol. 13, 429–439. Meteyer, C.U., Mohammed, H.O., Chin, R.P., Bickford, A.A., Trampel, D.W. and Klein, P.N. 1992. Relationship between age of flock seroconversion to hemorrhagic enteritis virus and appearance of adenoviral inclusions in the enteritis and renal tubule epithelia of turkeys. Avian Dis. 36, 88–96. Mettifogo, E., Nuñez, L.F.N., Chacon, J.L., Parra, S.H.S., Astolfi-Ferreira, C.S., Jerez, J.A., Jones, R.C. and Ferreira, A.J.P. 2014. Emergence of enteric viruses in production chickens is a concern for avian health. Sci. World J. 450423. doi:10.1155/2014/450423 Mor, S.K., Sharafeldin, T.A., Abin, M., Kromm, M., Porter, R.E., Goyal, S.M. and Patnayak, D.P. 2013. The occurrence of enteric viruses in light turkey syndrome, Avian Pathol. 42, 497–501. Morimura, T., Ohashi, K., Kon, Y., Hattori, M., Sugimoto, C. and Onuma, M. 1996. Apoptosis and CD8-down-regulation in the thymus of chickens infected with Marek’s disease virus. Arch. Virol. 141, 2243–2249. Nagaraja, K.V., Kang, S.Y. and Newman, J.A. 1985. Immunosuppressive effects of virulent strain of hemorrhagic enteritis virus in turkeys vaccinated against Newcastle disease. Poult. Sci. 64(3), 588–590. Nili, H., Jahantigh, M. and Nazifi, S. 2007. Clinical observation, pathology, and serum biochemical changes in infectious stunting syndrome of broiler chickens. Comp. Clin. Pathol. 16(3), 161–166. Niu, Y., Sun, Q., Zhang, G., Sun, W., Liu, X., Xiao, Y., Shang, Y. and Liu, S. 2017. Pathogenicity and immunosuppressive potential of fowl adenovirus in specific pathogen free chickens. Poult. Sci. 96, 3885–3892. Nuñez, L.F.N. and Piantino Ferreira, A.J. 2013. Viral agents related to enteric disease in commercial chicken flocks, with special reference to Latin America. World's Poult. Sci. J. 69, 853–864. Otto, P., Liebler-Tenorio, E.M., Elschner, M., Reetz, J., Löhren, U. and Diller, R. 2006. Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with Runting and Stunting Syndrome (RSS). Avian Dis. 50, 411–418. Payne, L.N. and Venugopal, K. 2000. Neoplastic diseases: Marek’s disease, lymphoid leukosis, and reticuloendotheliosis. Rev. Sci. Tech. Off. Int. Epiz. 19(2), 544-556. Pierson, F.W. and Fitzgerald, S.D. 2013. Hemorrhagic enteritis and related infections. In: Diseases of poultry, 13th ed. Eds., Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L. and Nair, V. Ames: Iowa State University Press editors, pp: 237–247. Pope, C.R. 1991. Pathology of lymphoid organs with emphasis on immunosuppression. Vet. Immunol. Immunopathol. 30, 31–44. Rautenschlein, S., Sharma, J.M., Winslow, B.J., McMillen, J., Junker, D. and Cochran, M. 2000a. Embryo vaccination of turkeys against Newcastle disease infection with recombinant fowlpox virus constructs containing interferons as adjuvants. Vaccine 18, 426–433. Rautenschlein, S., Subramanian, A. and Sharma, J.M. 1999. Bioactivities of a tumour necrosis-like factor released by chicken macrophages. Dev. Comp. Immunol. 23, 629–640. Rautenschlein, S., Suresh, M. and Sharma, J.M. 2000b. Pathogenic avian adenovirus type II induces apoptosis in turkey spleen cells. Arch. Virol. 145, 1671–1683. Rosenberger, J.K., Fries, P.A., Cloud, S.S. and Wilson, R.A. 1985. In vitro and in vivo characterization of avian Escherichia coli. II. Factors associated with pathogenicity. Avian Dis. 29, 1094–1107. Sahin, O., Kassem, I.I., Shen, Z., Lin, J., Rajashekara, G. and Zhan, Q. 2015. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 59, 185–200. Saunders, G.K., Pierson, F.W. and van den Hurk, J.V. 1993. Haemorrhagic enteritis virus infection in turkeys: A comparison of virulent and avirulent virus infections, and a proposed pathogenesis. Avian Pathol. 22, 47–58. Schat, K.A. and Nair, V. 2008. Marek’s disease. In Diseases of poultry, 12th edn. Eds., Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan and D.E. Swayne. Ames, IA: Black-well Publishing, pp: 452–514. Schonewille, E., Singh, A., Gobel, T.W., Gerner, W., Saalmuller, A. and Hess, M. 2008. Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Vet. Immunol. Immunopathol. 121, 130–139. Sellaoui, S., Alloui, N., Mehenaoui, S. and Djaaba, S. 2012. Evaluation of size and lesion scores of bursa cloacae in broiler flocks in Algeria. J. World Poult. Res. 2(2), 37–39. Sharafeldin, T.A., Singh, A., Abdel-Glil, M.Y., Mor, S.K., Porter, R.E. and Goyal, S.M. 2017. Prevalence of parvovirus in Minnesota turkeys. Poult. Sci. 96, 320–324. Sharma, J.M., Karaca, K. and Pertile, T. 1994. Virus-induced immunosuppression in chickens. Poult. Sci. 73, 1082–1086. Shini, S., Huff, G.R., Shini, A. and Kaiser, P. 2010. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 89, 841–851. Shivachandra, S.B., Sah, R.L., Singh, S.D., Kataria, J.M. and Manimaran, K. 2003. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet. Res. Commun. 27, 39–51. Silim, A. and Thorsen, J. 1981. Hemorrhagic enteritis: Virus distribution and sequential development of antibody in turkeys. Avian Dis. 25, 444–453. Simmons, D.G., Colwell, W.M., Muse, K.E. and Brewer, C.E. 1972. Isolation and characterization of an enteric reovirus causing high mortality in turkey poults. Avian Dis. 16, 1094–1102. Singh, A., Grewal, G.S., Maiti, N.K. and Oberoi, M.S. 2006. Effect of fowl adenovirus- 1 (IBH isolate) on humoral and cellular immune competency of broiler chicks. Comp. Immunol. Microbiol. Infect. Dis. 29, 315–321. Smyth, V.J. 2017. A Review of the Strain Diversity and Pathogenesis of Chicken Astrovirus. Viruses 9, 29. doi:10.3390/v9020029 Songserm, T., Pol, J.M.A., Van Roozelaar, D., Kok, G.L., Wagenaar, F. and Ter Huurne, A. 2000. A comparative study of the pathogenesis of malabsorption syndrome in broilers. Avian Dis. 44, 556–567. Suresh, M. and Sharma, J.M. 1995. Hemorrhagic enteritis virus induced changes in the lymphocyte subpopulations in turkeys and the effect of experimental immunodeficiency on viral pathogens. Vet. Immunol. Immunopathol. 45, 139–150. Suresh, M. and Sharma, J.M. 1996. Pathogenesis of type II avian adenovirus infection in turkeys: in vivo immune cell tropism and tissue distribution of the virus. J. Virol. 70, 30–36. Thachil, A.J. and Nagaraja, K.V. 2013. Effects of hemorrhagic enteritis virus infection in the development of clostridial dermatitis in turkeys. Proc. of American Association of Avian Pathologists, 150th American Veterinary Medical Association annual meetings, July 19–23. Chicago (IL), p: 54. Timms, L.M., Jahans, K.L. and Marshall, R.N. 1986. Evidence of immunosuppression in turkey poults affected by rhinotracheitis. Vet. Rec. 119, 91–92. Trampel, D.W., Kinden, D.A., Solorzano, R.F. and Stogsdill, P.L. 1983. Parvovirus-like enteropathy in Missouri turkeys. Avian Dis. 27, 49–54. Trampel, D.W., Meteyer, C.U. and Bickford, A.A. 1992. Hemorrhagic enteritis virus inclusions in turkey renal tubular epithelium. Avian Dis. 36, 1086–1091. van den Hurk, J., Allan, B.J., Riddell, C., Watts, T. and Potter, A.A. 1994. Effect of infection with hemorrhagic enteritis virus on susceptibility of turkeys to Escherichia coli. Avian Dis. 38, 708–716. van den Hurk, J.V. 1990. Efficacy of avirulent hemorrhagic enteritis virus propagated in turkey leukocyte cultures for vaccination against hemorrhagic enteritis in turkeys. Avian Dis. 34, 26–35. Wigley, P. and Kaiser, P. 2003. Avian cytokines in health and disease. Brazilian J. Poult. Sci. 5, 1–14. Witter, R.L. 1997. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 41, 149–163. Witter, R.L., Calnek, B.W., Buscaglia, C., Gimeno, I.M. and Schat, K.A. 2005. Classification of Marek’s disease viruses according to pathotype: philosophy and methodology. Avian Pathol. 34, 75–90. Woźniakowski, G., Frant, M. and Mamczur, A. 2018. Avian Reticuloendotheliosis in chickens—an update on disease occurrence and clinical course. J. Vet. Res. 62(3), 257–260. Yu, M., Ismail, M.M., Qureshi, M.A., Dearth, R.N., Barnes, H.J. and Saif, Y.M. 2000. Viral agents associated with poults enteritis and mortality syndrome: the role of a small round virus and turkey coronavirus. Avian Dis. 44, 297–304. Zsak, L., Strother, K.O. and Kisary, J. 2008. Partial genome sequence analysis of parvoviruses associated with enteric disease in poultry. Avian Pathol. 37, 435–441. | ||
| How to Cite this Article |
| Pubmed Style Khaled KABOUDI|. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Vet. J.. 2019; 9(4): 349-360. doi:10.4314/ovj.v9i4.13 Web Style Khaled KABOUDI|. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. https://www.openveterinaryjournal.com/?mno=61879 [Access: January 25, 2026]. doi:10.4314/ovj.v9i4.13 AMA (American Medical Association) Style Khaled KABOUDI|. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Vet. J.. 2019; 9(4): 349-360. doi:10.4314/ovj.v9i4.13 Vancouver/ICMJE Style Khaled KABOUDI|. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Vet. J.. (2019), [cited January 25, 2026]; 9(4): 349-360. doi:10.4314/ovj.v9i4.13 Harvard Style Khaled KABOUDI| (2019) Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Vet. J., 9 (4), 349-360. doi:10.4314/ovj.v9i4.13 Turabian Style Khaled KABOUDI|. 2019. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Veterinary Journal, 9 (4), 349-360. doi:10.4314/ovj.v9i4.13 Chicago Style Khaled KABOUDI|. "Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review." Open Veterinary Journal 9 (2019), 349-360. doi:10.4314/ovj.v9i4.13 MLA (The Modern Language Association) Style Khaled KABOUDI|. "Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review." Open Veterinary Journal 9.4 (2019), 349-360. Print. doi:10.4314/ovj.v9i4.13 APA (American Psychological Association) Style Khaled KABOUDI| (2019) Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Veterinary Journal, 9 (4), 349-360. doi:10.4314/ovj.v9i4.13 |