Open Veterinary Journal, (2021), Vol. 11(3): 500–507
Original Research
10.5455/OVJ.2021.v11.i3.23
Evaluation of immunochromatography test for detection of four enteropathogens in the feces of sheep and goats in Kuwait
Maha S. AlAzemi1 , Qais A. H. Majeed1
, Qais A. H. Majeed1 , Attia Samy2,3, Adawia A. Henedi4
, Attia Samy2,3, Adawia A. Henedi4 , Wessam Youssef 5,6, and Nadra-Elwgoud M. I. Abdou7,8*
, Wessam Youssef 5,6, and Nadra-Elwgoud M. I. Abdou7,8* 
1Department of Science, College of Basic Education, PAAET, Adailiya, Kuwait
2Virology lab, Veterinary Laboratories, PAAFR, Rabia, Kuwait
3Department of Virology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
4Parasitology lab. Veterinary Laboratories, PAAFR, Rabia, Kuwait
5Department of Biotechnology, Animal Health Research Institute, Dokki, Egypt
6Molecular Biology Lab. Veterinary Laboratories, PAAFR, Rabia, Kuwait
7GCC Early Warning Center for Transboundary Animal Diseases, PAAFR, Rabia, Kuwait
8Department of Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
*Corresponding Author: Nadra-Elwgoud M. I. Abdou. GCC Early Warning Center for Transboundary Animal Diseases, PAAFR, Rabia, Kuwait. Email: veterinarylaboratories [at] gmail.com
Submitted: 17/04/2021 Accepted: 01/09/2021 Published: 17/09/2021
© 2021 Open Veterinary Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Abstract
Background: Diarrhea in newborn small ruminants continues to be the cause of significant financial loss in sheep and goat farms worldwide. Commercial immunochromatographic (IC) assays have been designed and evaluated to be used for the diagnosis of diarrhea in cattle; however, there are no trials to use rapid tests in small ruminants.
Aim: This study was carried out in Kuwait to evaluate the performance of the rapid immunochromatography test (BoviD-4, BioNote, Inc, Korea) for diagnostics of Cryptosporidium, rotavirus A (RVA), bovine coronavirus (BCoV), and Escherichia coli K99 (E. coli K99) in fecal samples of sheep and goats.
Methods: A total of 85 samples were examined using BoviD-4, and the results were compared with that of polymerase chain reaction for Cryptosporidium, RVA, and BCoV, whereas for E. coli K99 it was by isolation and identification as reference tests.
Results: The kappa test agreement results between the BoviD-4 and reference tests were 0.870 (perfect), 0.783 (substantial), 0.728 (substantial), and 0.281 (fair) for the detection of E. coli K99, Cryptosporidium, RVA, and BCoV, respectively. The sensitivity of BoviD-4 kit was 91.2%, 80.0%, 90.0%, and 37.5% and the specificity was 88.2%, 96.0%, 96.4%, and 92.2% for Cryptosporidium, RVA, E. coli K99, and BCoV, respectively.
Conclusion: The Bovid-4 kit can be used as a rapid pen-side test for Cryptosporidium spp., E. coli K99, and RVA in the field. Nonetheless, care must be taken while interpreting the BCoV results of the kit.
Keywords: Diarrhea, Sheep, Goats, Immunochromatography assay, Kuwait.
Introduction
Sheep and goats are the most prominent and traditional livestock in Kuwait, with an estimated population of 684,231 sheep and 209,686 goats in 2018 (FAOSTAT, 2018). Diarrhea (scours) is a condition in which feces are discharged from the bowel with increased frequency and liquidity. Although there are continuous improvements in management practices and treatment strategies to prevent scours, it is still the most widely recognized health problem affecting neonatal small ruminants (Holland, 1990; Martella et al., 2015). Diarrhea accounted for 46% of lamb mortality in a study at the U.S. Sheep Experiment Station (Schoenian, 2007), while in Jordan, it was responsible for 51.6% of lambs and kids’ neonatal mortalities (Aldomy and Abu Zeid, 2007). In Kuwait, Abdou et al. (2021) studied the risk factors of diarrhea in sheep and goats. They found that 34.4% (191/556) of the examined sheep and goats suffered from diarrhea. Moreover, pre-weaned lambs and kids reared in the large herds were more susceptible to diarrhea.
Infectious causes such as Escherichia coli K99 (E. coli K99), rotavirus (RVA), bovine coronavirus (BCoV), and Cryptosporidium spp. are frequently identified as causative agents of diarrhea in 1-month-old lambs and kids (Sargison, 2004; Skirnisson and Hansson, 2006; Aldomy and Abu Zeid, 2007; Schoenian, 2007; Abdou et al., 2021). To obtain a definitive diagnosis, fecal samples must be tested in microbiological and parasitological laboratories. The traditional techniques, such as microscopic examination and culture methods, are laborious and some of them have low sensitivity (Stensvold and Nielsen, 2012; Ahmed and Chowdhary, 2013). Therefore, rapid and reliable tests are required to diagnose etiology, and subsequently, early intervention (treatment/prevention strategy), which minimizes losses (Sargison, 2004). Using immunological techniques such as immunochromatography tests (IC) has been a significant advancement in the rapid detection of antigens of diarrhea-causing pathogens in cattle (Bartels et al., 2010; Cho et al., 2012). Cryptosporidium spp., RVA, BCoV, and E. coli K99 are the essential pathogens causing diarrhea in cattle; they are also incriminated as causative agents of diarrhea in sheep and goats (Traven et al., 1999; Dhama et al., 2009; Helmy et al., 2013; Shabana et al., 2013; Papp et al., 2014; Shabana et al., 2017; Amer, 2018; Majeed et al., 2018; Abdou et al., 2021). Therefore, using these rapid IC tests to identify those enteric pathogens in sheep and goats deserves attention.
This study was conducted to assess the diagnostic accuracy of BoviD-4, a rapid IC kit used to diagnose four diarrhea-causing pathogens in cattle, to be utilized in small ruminants. For assessment of the rapid IC test, their results were compared with the results of a real-time polymerase chain reaction (qPCR) for Cryptosporidium spp., reverse transcription real-time PCR (RT-qPCR) for both RVA and BCoV, while isolation and identification techniques were used for E. coli K99 as they are the highly sensitive and specific tests for diagnosis (Mackenzie et al., 1998; Caccio, 2004; Klein et al., 2009; Soltan et al., 2016).
Materials and Methods
Sampling
During 2016, 85 diarrheal fecal samples collected from small ruminants were procured from submission to the Veterinary Laboratories, PAAFR, Kuwait. The samples were received in a sterile screw-capped bottle containing 5–10 g of feces and were transported to the laboratory at the earliest in an icebox. A request was sent along with each sample filled with the sampled animal’s information such as species, sex, age, and health status of the animal and the farm’s geographic location, and the date of sampling. The sample selection criteria were; collected from animals that did not receive any medication, a sufficient amount of sample, and no more than three samples from one farm.
Rapid IC test BoviD-4
All the 85 samples were tested by the rapid test BoviD-4 (BioNote, Gyeonggi-do, Republic of Korea). The kit includes tubes with a diluent, swab sticks, disposable droppers, and BoviD-4 Ag test devices (Fig. 1). An appropriate amount of feces was collected using a swab stick. The swab was then inserted into the dilution tube and stirred well until the sample entirely dissolved into the diluent. Before discarding the swab, it was squeezed against the wall of the tube; the tube was covered and left for 30 minutes for sedimentation. The supernatant was taken using a disposable dropper, and one drop was added into each sample hole of the BoviD-4 Ag test device. The samples flowed across the result windows; if the samples did not appear within 1 minute, another drop of the supernatant was added to the sample hole. The results were interpreted after 5–10 minutes; the sample was considered negative if only the control line “C” appeared and positive if both “C” and “T” lines appeared (Fig. 2). The result was considered invalid if line “C” did not appear and the sample was retested.
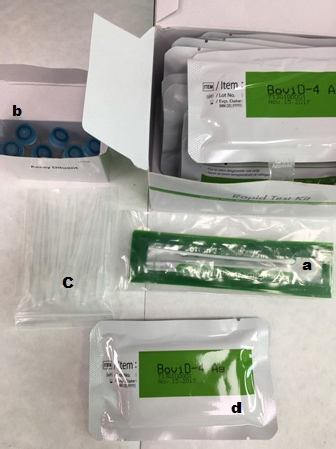
Fig. 1. Kit content: (a) swab stick, (b) tubes with diluent, (c) disposable droppers, and (d) BoviD-4 Ag test devices.
DNA extraction
The DNA extraction kit was used to isolate DNA from fecal samples using genesig®Easy LysoBead Direct-to-PCR Extraction Kit, PrimerdesignTM Ltd, Camberley, UK. It works well with all processes that yield high-quality DNA. 100 μl of the diarrheic fecal sample was added directly to the LysoBead tube, and 10 μl genesig Easy Internal extraction control DNA was added and vortexed at maximum speed for 5 minutes; it was then heated at 80°C for 2.5 minutes. Then, it was centrifuged at <5,000 g for 1 minute, and finally 200 μl of the solution was transfered to a fresh tube. The precise method of DNA extraction is described in the handbook of the manufacturer’s kit. The DNA extraction kit works well with all processes that yield high-quality DNA. A yield of 7.5–30 μg can be expected with minimal PCR inhibitors. 30 μg of the DNA template was used in the downstream application.
RNA extraction
Genesig®Easy RNA Extraction Kit PrimerdesignTM Ltd, Camberley, UK, was used for RNA isolation from the examined fecal samples. 200 μl diarrheic fecal sample was mixed with 500 μl of sample preparation solution. The mixture was shaken vigorously for 1 minute, and then centrifuged at a low speed (500 g for 1 minute). Then, 200 μl of the supernatant was added into a 1.5 ml flip cap tube. Next, 200 μl lysis buffer, 20 μl proteinase K, 10 μl internal extraction control RNA, 5 μl of carrier RNA were added to the supernatant. The solution was mixed well by repeated pipetting up and down and incubated at room temperature for 15 minutes. Following the lysis incubation, 500 μl magnetic beads/binding buffer was added to the lysed sample, and mixed well by shaking, and kept for 5 minutes. The magnetic beads were separated from the sample by placing the tube into the magnetic separator. The solution was left for at least 2 minutes until all the beads were attracted to the magnet. Then, the supernatant was removed and discarded by pipetting.
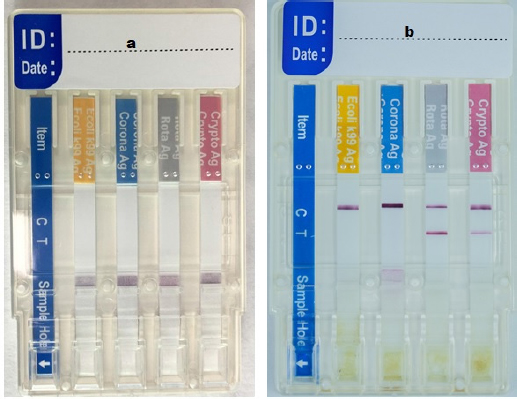
Fig. 2. The BoviD-4 Ag test device, (a) before adding the sample in the sample hole and (b) after adding a sample, showed a positive result for Cryptosporidim and RVA antigens (both “C” and “T” lines appeared) and negative for coronavirus and E. coli antigens (only the “C” line appeared).
For the washing step, 500 μl wash buffer 1 was added and the beads were shaken until they were resuspended completely. Then, the solution was left for 30 seconds. Alternatively, the beads were completely resuspended by repeated pipetting up and down. The magnetic beads were separated from the sample by placing the tube into the magnetic separator. The sample was left for at least 2 minutes until all the beads were attracted to the magnet. Then, the supernatant was removed and discarded by pipetting. This washing step was repeated twice using washing buffer 2 and then ethanol 80%. The magnetic bead pellets were air-dried for 10 minutes at room temperature with the tube lid open. 200 μl of the RNA solution buffer was added and the beads were shaken until they were resuspended completely. Then, there was a 30-second interval.
The magnetic beads were separated from the sample by placing the tube into a magnetic separator and the sample was left for at least 2 minutes until all the beads are attracted to the magnet.
Finally, the supernatant containing the purified RNA was transfered to a 0.5 ml flip cap tube for storage or use in downstream applications. The detailed method of RNA extraction is described in the handbook of the manufacturer’s kit. The RNA extraction kit works well with all processes that yield high-quality RNA. A yield of 7.5–30 μg can be expected with minimal PCR inhibitors. 20 μg of the RNA template was used in the downstream application.
Real-time PCR analysis
The following PCR kits were employed to detect enteropathogens:
Cryptosporidium oocyst wall protein (COWP)
COWP gene genesig® standard real-time PCR kit (PrimerdesignTM Ltd, Camberley, UK) is a qPCR detection protocol for the presence of COWP in fecal samples. All DNA extracted from fecal samples directly and DNA extracted from strains (positive control) used for validation were included in this qPCR to detect the presence of COWP in fecal samples. Non-template control (NTC) was used as a negative control. This assay was carried out in a 20 μl final reaction volume tube. The reaction mixture consisted of 10 μl of oasig or Precision PLUS 2 × qPCR Master Mix, 1 μl of Cryptosporidium primer/probe mix, 4 μl of RNase/DNase free water, and 5 μl of DNA template. The qPCR tube was tightly closed, vortexed, centrifuged, and placed into the block/rotor of the light thermocycler. Thermocycling conditions were as follows: 1 cycle at 95°C for 2 minute, followed by 50 cycles at 95°C for 10 seconds and 60°C for 1 minute. The fluorophore used in qPCR was FAM.
Veterinary RVA
The non-structural protein 5 (NSP5) genesig® standard real-time PCR kit (PrimerdesignTM Ltd, Camberley, UK) is a one-step reverse transcription RT-qPCR amplification protocol to detect NSP5 of RVA in feces. All RNA extracted from fecal samples directly and RNA extracted from strains (positive control) used for validation were included in this RT-qPCR to detect NSP5 of RVA presence in feces. NTC was used as a negative control. This assay was carried out in a 20 μl final reaction volume tube. The reaction mixture consisted of 10 μl of oasigTM OneStep or PrecisionTM OneStepPLUS 2 × RT-qPCR Mastermix, 1 μl of RVA primer/probe mix, 4 μl of RNase/DNase free water, and 5 μl of RNA template. The qPCR tube was tightly closed, vortexed, centrifuged, and placed into the block/rotor of the light thermocycler. Thermocycling conditions were as follows: 1 cycle at 55°C for 10 minutes, followed by 1 cycle at 95°C for 2 minutes, 50 cycles at 95°C for 10 seconds, and 60°C for 1 minute. The fluorophore used in RT-qPCR was FAM.
ViroReal® Kit BCoV
This is a one-step reverse transcription RT-qPCR to detect the nucleocapsid protein gene (N gene) of the BCoV (ingenetix GmbH, Vienna, Austria). All RNA extracted from fecal samples directly and RNA extracted from strains (positive control) used for validation were included in this RT-qPCR to detect the presence of nucleocapsid protein gene (N gene) of the BCoV. NTC was used as a negative control. This assay was carried out in a 20 μl final reaction volume tube. The reaction mixture consisted of 1 μl of BCoV Assay Mix, 5 μl of RNA Reaction Mix, 4 μl of Nuclease-free Water, and 10 μl of RNA template. The RT-qPCR tube was tightly closed, vortexed, centrifuged, and placed into the block/rotor of the light thermocycler. Thermocycling conditions were as follows: 1 cycle at 50°C for 15 minutes, followed by 1 cycle at 95°C for 20 seconds, 45 cycles at 95°C for 5 seconds, and 60°Cfor 1 minute. The fluorophore used in real-time PCR was FAM.
All the kits used for the detection of the pathogens were followed the manufacturers’ instructions. The results were analyzed directly on ExicyclerTM 96 Real-Time Quantitative Thermal Block (Bioneer, Daedeokgu, Daejeon, Republic of Korea).
E. coli K99 isolation and identification
A total of 85 samples were examined by isolation and identification techniques to test the presence of E. coli K99 and to compare with the results of the BoviD-4 kits. Feces samples were inoculated onto MacConkey agar. Agar plates were incubated at 37°C, and the bacterial growth was evaluated after 24 and 48 hours. Gram-negative microorganisms were selected using MacConkey agar and identified with biochemical tests (Ewing, 1986). The detection of K99 antigen was carried out by slide agglutination test as previously described (Bailey and Scott, 1990).
Statistical analysis
The evaluation of diagnostic tests (sensitivity, specificity, accuracy, positive predictive value, and negative predictive value) was computed with their 95% confidence interval (95% CI) using MedCalc®, while kappa (κ) test agreement along with the strength of agreement were calculated and judged by QuickCalcs, GraphPad Software®. κ-values were interpreted as no agreement (κ =< 0), slight (κ =0.00–0.20), fair (κ =0.21–0.40), moderate (κ =0.41–0.60), substantial (κ =0.61–0.80), and perfect (κ =0.81–1.00).
Ethical approval
The ethics committee of kuwait foundation for advancement of sciences approved this study (KFAS-Award Number 2012-1207-04). The samples were sent to the lab and there was no direct contact with the animals.
Results
Rapid IC test performance in the detection of the four pathogens
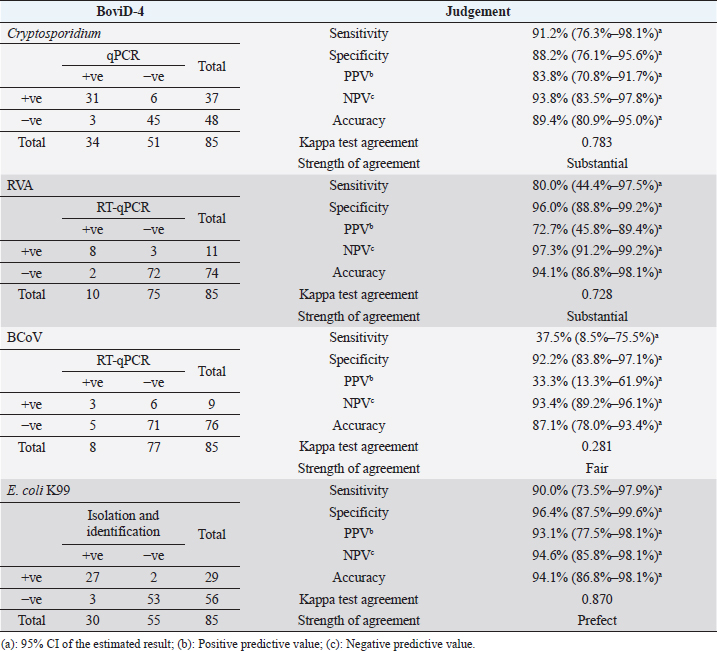
The rapid test showed a relatively high sensitivity for the diagnosis of Cryptosporidium and RVA (91.2% and 80.0%, respectively), but the sensitivity was low for BCoV (37.5%). The specificity of the rapid test was high for the detection of RVA (96.0%) and relatively good for both Cryptosporidium and BCoV (88.2% and 92.2%, respectively). The sensitivity and specificity for identifying E. coli K99 were high (90.0% and 96.4%, respectively), whereas the accuracy of the test was 89.4% for Cryptosporidium, 94.1% for both E. coli K99 and RVA, and 87.1% for BCoV. The results of the κ test agreement were prefect (0.870) in detecting E. coli K99, substantial for both Cryptosporidium and RVA (0.783 and 0.728, respectively), while it was fair (0.281) for BCoV (Table 1). Negative predictive values were high for the detection of all pathogens, while positive predictive values (PPVs) were low for both RVA and BCoV (72.7% and 33.3%, respectively) and high for Cryptosporidium and E. coli K99 as shown in Table 1.
Prevalence of enteric pathogens and coinfections detected by rapid IC (BoviD-4)
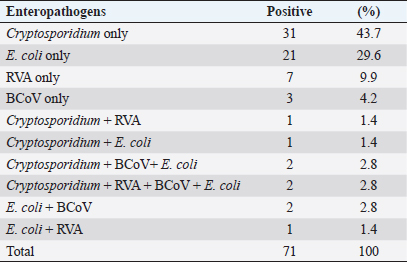
Examination of fecal samples of small ruminants revealed that single infections with Cryptosporidium, E. coli K99, RVA, and BCoV were diagnosed in 43.7%, 29.6%, 9.9%, and 4.2%, respectively. Mixed infections with all the pathogens were detected in 2.8%, and three pathogens (Cryptosporidium, BCoV, and E. coli) were found in 2.8% of the examined samples. Coinfections with two enteropathogens also were reported, as shown in Table 2.
Table 1. Evaluation of rapid IC test (BoviD-4) in comparison with real-time PCR for Cryptosporidium, RVA, and BCoV, while for E. coli K99 it was compared with isolation and identification.
Discussion
Diarrhea is one of the most critical health problems, especially in young animals (Tzipori, 1985 Holland, 1990). In the Gulf area, few studies investigated diarrhea in sheep and goats. Shabana et al. (2017) investigated viral and bacterial enteropathogens of diarrheic sheep and goats in Medina, Saudi Arabia. They reported that E. coli was the most prevalent agent in sheep and goats (34.7% and 30.7%, respectively). RVA was more prevalent than coronavirus among the surveyed animals, at a rate of 30% and 18.4%, respectively. The prevalence of bacterial and viral enteropathogens was significantly higher in the 0–12 months age group compared to the older age groups (Shabana et al., 2017). However, E. coli K99, Cryptosporidium spp., and Eimeria spp. were the most predominant enteric pathogens identified as a risk factor of diarrhea in sheep and goats in Kuwait (Abdou et al., 2021). In addition, young pre-weaned animals in intensive rearing farms were more susceptible to diarrhea (Abdou et al., 2021).
Rapid and accurate diagnosis has significant implications on the results of epidemiological studies and optimal control of a disease. Therefore, field and pen-side tests are fundamental for the veterinary practitioner as long as it is simple, sensitive, and reliable to make quick decisions on treatment and preventive measures. Cryptosporidium, RVA, BCoV, and E. coli K99 are common diarrhea-causing pathogens in cattle (Bartels et al., 2010) and small ruminants (Schoenian, 2007; Abdou et al., 2021). Commercial rapid IC assays have been developed for antigen detection of these four pathogens in the feces of cattle, and their sensitivity and specificity have been compared with the results of PCR, ELISA, or conventional methods as reference tests (Klein et al., 2009; Cho et al., 2012). This study is the first trial to assess the diagnostic performance of commercially rapid tests (BoviD-4 kit) to observe the presence of those enteric pathogens’ antigens in the examined fecal samples of small ruminants.
Table 2. Prevalence of four pathogens and co-infections detected by rapid IC test (BoviD-4) in fecal samples of 85 sheep and goats.
The identification efficacy of Cryptosporidium spp. using BoviD-4 kit was substantial (sensitivity=91.2%, specificity=88.2%, and κ value=0.783) when compared with qPCR. The results in this study nearly agreed with that of Cho et al. (2012) who reported that the rapid commercial kit (Enterichek) performance was excellent when matched with multiplex PCR for detection of Cryptosporidium spp. in feces from diarrheic calves (κ value=0.840, sensitivity=81.5%, and specificity=98.6%). Meanwhile, Klein et al. (2009) contrasted the results of FASTest CRYPTO Strip (IC strip test) with that of sedimentation–flotation technique for the presence of Cryptosporidium oocysts in feces of examined calves, the kit’s strength of agreement was almost perfect (sensitivity=100%, specificity=94.6%, and κ value=0.908).
Most of the commercial IC lateral flow kits were labeled with monoclonal antibodies to diagnose Cryptosporidium parvum antigens. Cryptosporidium spp., were detected from small ruminants’ fecal samples and identified in the Centers for Disease Control and Prevention, Atlanta, as C. parvum, Cryptosporidium xiaoi, and Cryptosporidium ubiquitum (Majeed et al., 2018). The samples containing the three species of Cryptosporidium were included in this study and gave positive results with the rapid test, indicating a cross-reaction between C. parvum and other species. Similarly, cross-reactivity between different Cryptosporidium spp. was reported elsewhere (Agnamey et al., 2011; Chalmers et al., 2011; OIE, 2018); however, C. parvum antibodies-based IC assays did not show cross-reactivity with some protozoa, helminths, bacteria, or viruses (Garcia et al., 2003; Cheun et al., 2013). Even in this study, no positive results of the rapid test were observed in the fecal samples containing Eimeria species. The results of this study and other studies (papers in preparation) showed that BoviD-4 kits are useful for detecting different Cryptosporidium spp. in fecal samples from sheep, goats, cattle, and camels and this rapid commercial assay is specific for Cryptosporidium, but not for C. parvum in particular. However, when rapid assays detected Cryptosporidium in the fecal samples from diarrheic sheep and goats in Kuwait, we expected the occurrence of C. parvum, which is the most pathogenic and prevalent species (Majeed et al., 2018).
The observed agreement of BoviD-4 was perfect when compared with isolation and identification of E. coli K99 (κ value=0.870, sensitivity=90%, and specificity=96.4%). Previous investigators obtained nearly the same results when IC tests confronted with multiplex PCR (Cho et al., 2012) or cultivation and PCR (Dieguéz et al., 2012).
To assess the rapid IC assays for the viral enteric pathogens, BCoV and RVA, RT-qPCR is considered the gold standard test. The sensitivities of the rapid test were very low for BCoV (37.5%) and high for RVA (80%), whereas the specificities were high for both BCoV and RVA (92.2% and 96.0%, respectively). Other investigators observed nearly the same findings when evaluating the rapid IC strip assays, when compared to antigen ELISA and RT-PCR, respectively, indicating a higher specificity and lower sensitivities in diagnosing BCoV using this strip kit (Luginbühl et al., 2005; Klein et al., 2009). The strength of agreement for the BoviD-4 test in detecting BCoV was fair (κ-value=0.281); however, a substantial agreement was observed in the diagnosis of RVA by the rapid IC test (κ value=0.728). The previous study (Cho et al., 2012) observed a slight and moderate agreement for both BCoV and RVA, respectively (κ value=0.095 and 0.521, respectively). However, a substantial agreement for RVA (κ-value=0.688) and moderate for BCoV (κ-value=0.600) were previously reported (Luginbühl et al., 2005).
Although the sensitivity of these rapid tests for detection of RVA was not as good as Cryptosporidium and E. coli K99, they can be used in the diagnosis of this virus in fecal samples during the acute stage of diarrhea when a large number of the virus is expected to shed (Luginbühl et al., 2005; Cho et al., 2012). However, the rapid test was less optimal in detecting BCoV in the fecal samples, as shown in previous studies and in the current study. Therefore, it is recommended that when rapid kits test the presence of BCoV, the results should also be confirmed by another laboratory test (Cho et al., 2012).
In this study, Cryptosporidium spp. and E. coli were the most frequently detected enteropathogens among sheep and goats. This result agreed with previous studies in Kuwait (Majeed et al., 2018, Abdou et al., 2021) and Saudi Arabia (Shabana et al., 2017). Mixed infections are common in gastroenteritis which may increase disease severity (Sargison, 2004).
Conclusion
Using rapid IC tests to detect different enteropathogens in the feces of cattle and small ruminants in this study indicated that these tests showed high sensitivity and specificity for detection of Cryptosporidium and E. coli K99 and that it can be used as a field test for the diagnosis of diarrhea caused by these two pathogens in small ruminants. Also, the rapid IC test could be used for the diagnosis of RVA, especially in acute infection. Thus, the Bovid-4 kit can be used as a rapid pen-side test for Cryptosporidium spp., E. coli K99, and RVA in the field. Nonetheless, care must be taken while interpreting the BCoV positive results of the kit.
Acknowledgments
The authors would like to thank the Public Authority of Agriculture Affairs and Fish Resources (PAAFR) for providing facilities in their Veterinary Laboratories Department. They also sincerely thank the significant help and continuous support provided by Madam Maha Al-Batel, Former Head Manager of the Veterinary Laboratories Department.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the Kuwait Foundation for Advancement of Sciences (KFAS) [grant number 2012-1207-04].
Authors’ contribution
Abdou, N.-E. M. I., Attia, S., and Majeed, Q. A. H. designed the project. Attia, S., Abdou, N.-E. M. I., Henedi, A. A., and Youssef, W. performed the experiment. Abdou, N.-E. M. I. and AlAzemi, S. A. statistically analyzed the data. Abdou, N.-E M. I., AlAzemi, S. A., and Majeed, Q. A. H. wrote and edited the manuscript. All the authors read the manuscript and approved the contents.
References
Abdou, N.E.M.I., Majeed, Q.A.H., El-Azazy, O.M.E., Tahrani, L.M.A., AlAzemi, M.S. and Alajmi, A. 2021. Risk factors of diarrhea in small ruminants in Kuwait. Iran. J. Vet. Res. 22, 146–149.
Agnamey, P., Sarfati, C., Pinel, C., Rabodoniriina, M., Kapel, N., Dutoit, E., Garnaud, C., Diouf, M., Garin, J.F., Totet, A., Derouin, F. and Network., A.C.N. 2011. Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: a blind multicenter trial. J. Clin. Microbiol. 49, 1605–1607.
Ahmed, N.H. and Chowdhary, A. 2013. Comparison of different methods of detection of enteric pathogenic protozoa. Indian J. Med. Microbiol. 31, 154–160.
Aldomy, F. and Abu Zeid, N. 2007. Neonatal mortality of small ruminants in Jordan. Bulg. J. Vet. Med. 10, 195–199.
Amer, H. 2018. Bovine-like coronaviruses in domestic and wild ruminants. Anim. Health Res. Rev. 19, 113–124.
Bailey, W.R. and Scott, E.G. 1990. Diagnostic microbiology, 4th ed. In A text book for the isolation and identification of pathogenic microorganisms. St Louis, MO: C.V. Mosby Company.
Bartels, C.J., Holzhauer, M., Jorritsma, R., Swart, W.A. and Lam, T.J. 2010. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 93, 162–169.
Caccio, S.M. 2004. New methods for the diagnosis of Cryptosporidium and Giardia. Parassitologia 46, 151–155.
Chalmers, R.M., Campbell, B.M., Crouch, N., Charlett, A. and Davies, A. P. 2011. Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J. Med. Microbiol. 60, 1598–1604.
Cheun, H.I., Chung, B.S., Ma, D.W., Goo, B.L., Cho, S.H., Ji, M.J. and Lee, W.J. 2013. Development of a diagnostic kit to detect Cryptosporidium parvum and Giardia lamblia. Osong. Public. Health. Res. Perspect. 4, 146–151.
Cho, Y.I., Sun, D., Cooper, V., Dewell, G., Schwartz, K. and Yoon, K.J. 2012. Evaluation of a commercial rapid test kit for detecting bovine enteric pathogens in feces. J. Vet. Diagn. Invest. 24, 559–562.
Dhama, K., Chauhan, R.S., Mahendran, M. and Malik, S.V. 2009. Rotavirus diarrhea in bovines and other domestic animals. Vet. Res. Commun. 33, 1–23.
Dieguéz, F.J., González, A.M., Soilán, M., Eiras, C., Sanjuán, M.L. and Yus, E. 2012. Evaluation of immunochromatographic test strips for rapid diagnosis of neonatal calf diarrhoea. Cattle. Pract. 20, 1–4.
Ewing, W.H. 1986. Edwards and Ewing’s identification of Enterobacteriaceae, 4th ed. New York, NY: Elsevier Science Publishing Co., Inc.
FAOSTAT. 2018. Food and agriculture organization of the United Nations (FAO) statistics division. Rome, Italy: FAOSTAT. Available via http://www.fao.org/faostat/en/#data/QA (Accessed 20 December 2020).
Garcia, L.S., Shimizu, R.Y., Novak, S., Carroll, M. and Chan, F. 2003. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J. Clin. Microbiol. 41, 209–212.
Helmy, Y.A., Krucken, J., Nockler, K., von Samson-Himmelstjerna, G. and Zessin, K.H. 2013. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet. Parasitol. 193, 15–24.
Holland, R.E. 1990. Some infectious causes of diarrhea in young farm animals. Clin. Microbiol. Rev. 3, 345–375.
Klein, D., Kern, A., Lapan, G., Benetka, V., Mostl, K., Hassl, A. and Baumgartner, W. 2009. Evaluation of rapid assays for the detection of bovine coronavirus, rotavirus A and Cryptosporidium parvum in faecal samples of calves. Vet. J. 182, 484–486.
Luginbühl, A., Reitt, K., Metzler, A., Kollbrunner, M., Corboz, L. and Deplazes, P. 2005. Field study of the prevalence and diagnosis of diarrhea- causing agents in the newborn calf in a Swiss veterinary practice area. Schweiz. Arch. Tierheilkd. 147, 245–252.
Mackenzie, A.M., Lebel, P., Orrbine, E., Rowe, P.C., Hyde, L., Chan, F., Johnson, W. and McLaine, P.N. 1998. Sensitivities and specificities of premier E. coli O157 and premier EHEC enzyme immunoassays for diagnosis of infection with verotxin (Shiga-like toxin)-producing Escherichia coli. The SYNSORB Pk Study investigators. J. Clin. Microbiol. 36, 1608–1611.
Majeed, Q.A.H., El-Azazy, O.M.E., Abdou, N.M.I., Al-Aal, Z.A., El-Kabbany, A.I., Tahrani, L.M.A., AlAzemi, M.S., Wang, Y., Feng, Y. and Xiao, L. 2018. Epidemiological observations on cryptosporidiosis and molecular characterization of Cryptosporidium spp. in sheep and goats in Kuwait. Parasitol. Res. 117, 1631–1636.
Martella, V., Decaro, N. and Buonavoglia, C. 2015. Enteric viral infections in lambs or kids. Vet. Microbiol. 181, 154–160.
OIE. 2018. Cryptosporidiosis. In Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: OIE, pp: 1678–1692.
Papp, H., Malik, Y.S., Farkas, S.L., Jakab, F., Martella, V. and Bányai, K. 2014. Rotavirus strains in neglected animal species including lambs, goats and camelids. Virusdisease 25, 215–222.
Sargison, N. 2004. Differential diagnosis of diarrhoea in lambs. In. Pract. 26, 20–27.
Schoenian, S. 2007. Diarrhea (scours) in small ruminants. Maryland small ruminants page. College Park, MD: Maryland University. Available via https://www.sheepandgoat.com/scours (Accessed 15 December 2020).
Shabana, I.I., Bouqellah, N.A. and Zaraket, H. 2017. Investigation of viral and bacterial enteropathogens of diarrheic sheep and goats in Medina, Saudi Arabia. Trop. Biomed. 34, 944–955.
Shabana, I.I., Zaraket, H. and Suzuki, H. 2013. Molecular studies on diarrhea-associated Escherichia coli isolated from humans and animals in Egypt. Vet. Microbiol. 167, 532–539.
Skirnisson, K. and Hansson, H. 2006. Causes of diarrhoea in lambs during autumn and early winter in an Icelandic flock of sheep. Icel. Agric. Sci. 19, 43–57.
Soltan, M.A., Tsai, Y.L., Lee, P.A., Tsai, C.F., Chang, H.G., Wang, H.T. and Wilkes, R.P. 2016. Comparison of electron microscopy, ELISA, real time RT-PCR and insulated isothermal RT-PCR for the detection of rotavirus group A (RVA) in feces of different animal species. J. Virol. Methods 235, 99–104.
Stensvold, C.R. and Nielsen, H.V. 2012. Comparison of microscopy and PCR for detection of intestinal parasites in Danish patients supports an incentive for molecular screening platforms. J. Clin. Microbiol. 50, 540–541.
Traven, M., Carlsson, U., Lunden, A. and Larsson, B. 1999. Serum antibodies to bovine coronavirus in Swedish sheep. Acta Vet. Scand. 40, 69–74.
Tzipori, S. 1985. The relative importance of enteric pathogens affecting neonates of domestic animals. Adv. Vet. Sci. Comp. Med. 29, 103–206.












