| Case Report | ||
Open Vet. J.. 2022; 12(5): 774-781 Open Veterinary Journal, (2022), Vol. 12(5): 774–781 Case Report Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case reportNaoki Fukushima1, Nobuhisa Kakehi2, Kentarou Tahara3, Tsuyuko Watanabe3 and Eiichi Hirano4*1Panda Animal Hospital, Kobe, Japan 2Domestic Sales Department, Japan Bio Products Co., Ltd., Tokyo, Japan 3Medical Affairs Department, Japan Bio Products Co., Ltd., Tokyo, Japan 4Laennec Regenerative Medicine Research Center, Japan Bio Products Co., Ltd., Kurume, Japan *Corresponding Author: Eiichi Hirano. Laennec Regenerative Medicine Research Center, Japan Bio Products Co., Ltd., Kurume, Japan. Email: ehirano [at] placenta-jbp.co.jp Submitted: 07/07/2022 Accepted: 18/09/2022 Published: 15/10/2022 © 2022 Open Veterinary Journal
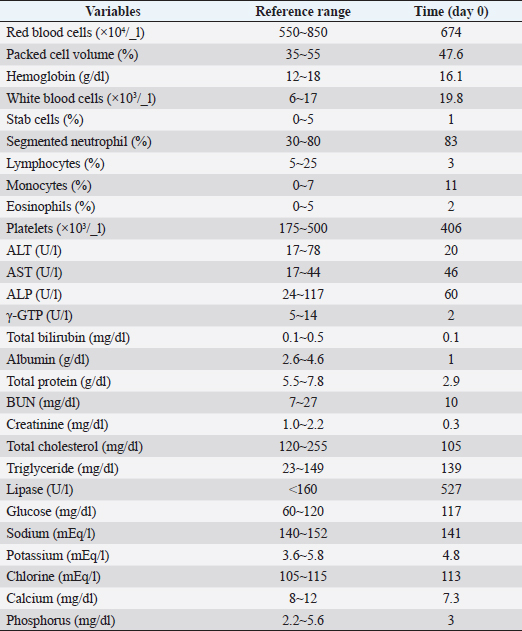
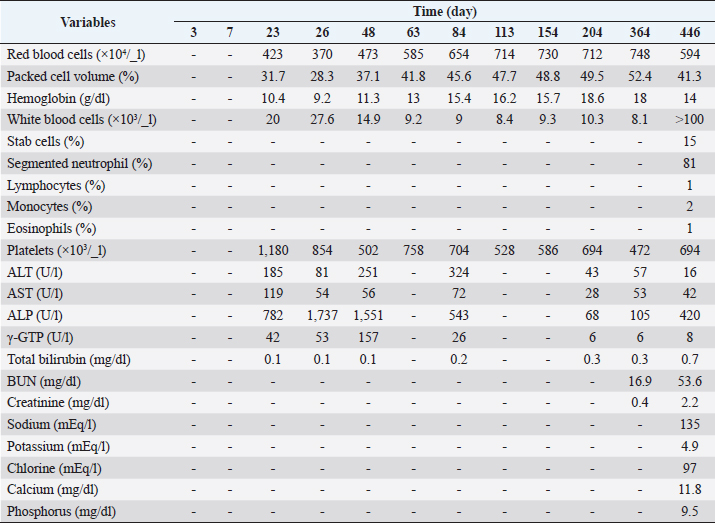
AbstractBackground: Protein-losing enteropathy (PLE) is characterized by leakage of serum proteins into the intestinal lumen, indicating hypoproteinemia. Immunosuppressive agents are the mainstay of treatment, but in many cases, patients are forced to taper off early owing to the induction of liver damage. Case Description: An 8-year-old, non-spayed female Chihuahua presented with diarrhea and ascites effusion lasting 2 weeks. Based on the results of radiography and blood tests, a diagnosis of PLE was made. Prednisolone (3 mg/kg semel in die [SID]) and MitoMax (200 mg/day) were administered, but ascites accumulation and diarrhea did not improve. Thus, azathioprine (2 mg/kg/day) was added, but there was no improvement, and liver damage developed. The liver injury did not improve immediately, but diarrhea and ascites effusion improved after serum total protein and serum albumin levels increased after they had decreased. Subsequent tapering of prednisolone from 3 mg/kg SID to 1 mg/kg SID, combined with MitoMax (200 mg/day) and equine placenta extract (eqPE) (2 ml/day), resulted in no recurrence of ascites or diarrhea. Conclusion: In canine PLE with prolonged diarrhea and ascites effusion, supplementation with eqPE may be considered a reasonable additional therapeutic strategy. Keywords: Canine protein-losing enteropathy, Ascites, Diarrhea, Placenta extract supplementation, Case report. IntroductionProtein-losing enteropathy (PLE) is a syndrome that occurs in several conditions of varying etiologies that often involve the lymphatic system, including lymphangiectasis and lymphangitis in dogs (Craven and Washabau, 2019). The pathophysiology of the lymphatic disease is not fully understood, making the management of this disease challenging. The mechanism of intestinal protein loss in PLE is similar in dogs and humans, regardless of the underlying cause. In humans, PLE is usually associated with genetically susceptible primary intestinal lymphangiectasia (IL) and idiopathic lymphatic obstruction. In dogs, PLE is more often associated with inflammatory bowel disease (IBD) and less often with IL, although it has not been proven which condition is the true cause. In addition, PLE is life-ending in 54.2% of dogs, compared to <20% of published disease-related deaths in dogs with IBD (Craven and Washabau, 2019), suggesting that PLE is not simply a pathophysiological continuum in the IBD spectrum. Although diet is the basis of management in humans, immunosuppressive therapy is often used in dogs for causes of PLE, such as lymphangiectasis, lymphangitis, and cryptic disease. However, currently, there is no scientific, extrapolative, or evidence-based support for autoimmune or immune-mediated mechanisms. Here, we report the case of a dog with PLE who presented with long-term ascites effusion and diarrhea and developed severe hepatic injury due to the addition of azathioprine because the prednisolone treatment did not improve her symptoms. Although placenta extract improves liver function and equine placenta extract (eqPE) has not been used in any case of PLE, we administered a supplement of eqPE to improve liver function in this case. Case DetailsAn 8-year-old, non-spayed, female Chihuahua weighing 2.6 kg presented with diarrhea and abdominal distension lasting 2 weeks. This dog had no specific medical history. Table 1 displays the blood test findings at the time of the initial visit, showing an increased white blood cell count, segmented neutrophils, and increased monocytes. However, the lymphocyte count was decreased. The red blood cell, packed cell volume, stab cell, eosinophil, and platelet counts were within the reference ranges (Table 1). An increase in aspartate aminotransferase (AST) and a decrease in gamma-glutamyl transpeptidase (γ-GTP) were observed. Alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin levels were within the reference ranges. Albumin and total protein levels were markedly decreased and approximately half or less than the reference values. Regarding renal function, the creatinine level was decreased, while the blood urea nitrogen (BUN) level was within the reference range. Cholesterol levels were decreased, but triglyceride levels were within the reference values. Lipase activity was markedly increased, and its value was approximately three times higher than the reference value. There were no abnormalities in the blood glucose levels. The calcium level was decreased, whereas sodium, potassium, chloride, and phosphorus levels were within the reference values. In addition to the aforementioned blood test results, radiography and physical examinations revealed an accumulation of ascites, leading to a diagnosis of PLE. Prednisolone was administered at a dose of 3 mg/kg semel in die (SID). MitoMax, a lactic acid Pediococcus acidilactici and Saccharomyces boulardii-based supplement, was also administered at 200 mg SID. Approximately 400 ml of ascitic fluid was removed. Table 2 shows the blood test findings over the course of this case, particularly the blood cell counts and liver function values over time. 4 days (day 4) after the start of prednisolone and MitoMax, diarrhea did not improve, and the patient still showed ascites accumulation. Approximately 400 ml of ascites fluid was removed. Based on these findings, azathioprine was started at 2 mg/kg SID, in addition to prednisolone (3 mg/kg) and MitoMax (200 mg daily). 14 days (day 23) after azathioprine administration (2 mg/kg daily), diarrhea did not improve and ascites remained present. Approximately 350 ml of ascites fluid was drained. Blood test results at this point of time showed decreased red blood cell, packed cell volume, and hemoglobin values below the reference values. However, the white blood cell and platelet counts were elevated above the reference values, with the platelet count approximately 2.9 times higher than the reference value. Liver function values were much higher for ALT, AST, ALP, and γ-GTP; they were approximately 2.4-, 2.7-, 6.7-, and 3.0-folds higher than the reference values, respectively. Based on these results, it was inferred that azathioprine administration was ineffective in this case and was inducing hepatic dysfunction. Therefore, azathioprine administration was discontinued, and 2 ml of eqPE SID was administered as a supplement to improve liver function to ameliorate worsening liver dysfunction. Prednisolone (3 mg/kg SID) and MitoMax (200 mg SID) were continued as before. 3 days (day 26) after the start of administering eqPE (2 ml SID) in addition to prednisolone (3 mg/kg SID) and MitoMax (200 mg SID), diarrhea was still present, but ascites disappeared. Blood test results showed that red blood cell, packed cell volume, and hemoglobin values were further decreased compared to those before treatment with eqPE; the white blood cell count was further increased compared to that before treatment with eqPE. ALT and AST levels decreased from 185 to 81 U/l and from 119 to 54 U/l, respectively, compared with those before treatment with eqPE, showing a recovery trend, but they still did not reach the reference values. Conversely, ALP and γ-GTP levels increased from 782 to 1,737 U/l and from 42 to 53 U/l, respectively, compared to those before the administration of eqPE. The total bilirubin level did not change after the administration of eqPE. 23 days (day 48) after the disappearance of ascites, the patient continued to receive prednisolone, MitoMax, and eqPE, and the diarrhea was confirmed to have improved. The blood test results showed that red blood cell and hemoglobin values increased from 3,700 to 4,730 × 103/ml and from 9.2 to 11.3 g/dl, respectively, compared to those at the time of ascites resolution, while the packed cell volume count increased from 28.3% to 37.1%, which was within the standard range. The platelet count decreased from 854 to 502 × 103 /_l compared with the time of ascites’ disappearance, showing a recovery trend, and it was close to the reference range. The ALT level increased from 81 to 251 U/l, reversing the recovery trend, and the AST level was slightly elevated from 54 to 56 U/l; both were outside the reference values. The ALP level decreased from 1,737 to 1,551 U/l, but the γGTP level increased from 53 to 157 U/l. No changes were observed in the total bilirubin level. After the 16th day (day 63) after diarrhea improved, the patient continued to receive prednisolone, MitoMax, and eqPE, and there was no recurrence of diarrhea or ascites. The red blood cell and packed cell volume counts continued to increase after day 63, reaching maximum values of 7,480 × 103/ml and 52.4%, respectively, but decreased to 5,940 × 103/ml and 41.3%, respectively, on day 446, which is the last visit date. Although the hemoglobin level showed a slight increase and temporarily deviated from the reference range, they remained within the reference values on day 446. The white blood cell count was normal but was much higher than the reference value on day 446. At this time, the number of stab cells was three times the reference value. The segmented neutrophil count was also outside the reference values but was only slightly elevated. The lymphocyte count was below the baseline level on day 446. The monocyte and eosinophil counts were normal on day 446. The platelet count was elevated and outside the reference range, temporarily showing a normal value on day 364. However, it was temporarily outside the reference range on day 446. The ALT level increased to 4.2 times the reference value on day 84, but then decreased to within the reference range; on day 446, it was very slightly below the reference value. The AST level was similarly 1.6 times the reference value on day 84, but then it decreased to within the reference range; on day 364, it was outside the reference value again, but on day 446 it was within the reference range. The ALP level increased to 4.6 times the reference value on day 84 but subsequently decreased to within the reference range and was again 3.6 times the reference value on day 446. The γ-GTP level increased to 1.9 times the reference value on day 84, but it subsequently decreased to within the reference range and remained within the reference range on day 446. The total bilirubin level was 1.4 times the reference value on day 446 but was otherwise normal. On days 364 and 446, creatinine levels were normal. In contrast, the BUN level was normal on day 364 but exceeded the reference range on day 446. On day 446, sodium, potassium, chlorine, and calcium levels were within the reference ranges, but the phosphorus level exceeded the reference value by 3.9 mg/dl. Table 1. Hematological and serum biochemical parameters at initial visit.
Table 2. Hematological and serum biochemical parameters after drugs and supplements administration.
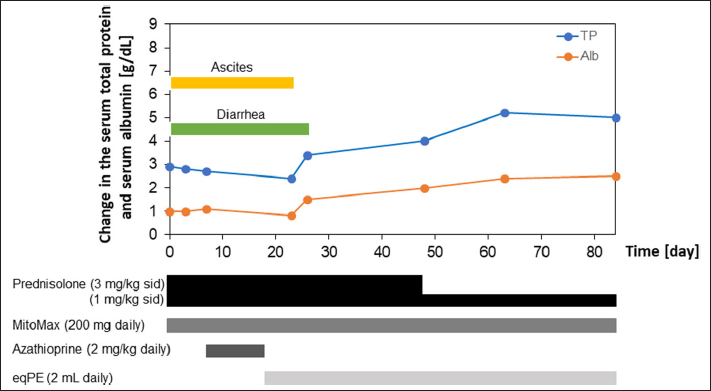
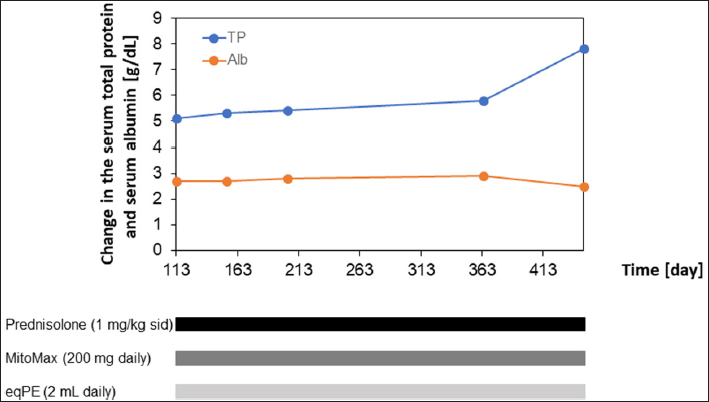
Figure 1 shows the variation in total protein and albumin levels and the types and amounts of drugs and supplements administered during the course of treatment between days 0 and 84 in this case. The fluctuations in total protein and albumin levels continued to decrease until day 23, when total protein and albumin levels reached their lowest values, which were only 44% and 31% of the reference values, respectively. However, after administration of eqPE (2 ml/day), these values began to increase rapidly, approaching normal by day 63 and only slightly outside the reference values by day 84. Azathioprine, which was added during treatment, was discontinued because of worsening liver function values after administration. The disappearance of ascites and improvement of diarrhea within a few days after administration of eqPE (2 ml daily) allowed prednisolone to be tapered down to one-third of the original dose. When these values approached normal, the dose of prednisolone was reduced by half, but no adverse effects were observed. Figure 2 shows the variation in total protein and albumin levels and the types and amounts of drugs and supplements during treatment between days 113 and 446 in this case. In this case, prednisolone (1 mg/kg SID), MitoMax (200 mg SID), and eqPE (2 ml SID) were administered. The total protein level increased sharply on day 446 but did not deviate from the reference range. The albumin level also remained normal, as did the total protein level, but was only slightly abnormal on day 446. No recurrence of diarrhea or ascites accumulation was observed until day 446, the last medical checkup. There have been no reports of re-treatment or death due to PLE recurrence since the last clinical date of this case. DiscussionIn the present case, drug treatment centered on immunosuppressants was administered. However, the dog developed liver damage, presumably caused by these drugs. As a result of the administration of eqPE to improve liver function, ascites and diarrhea were improved before liver function improved. To our best knowledge, this is the first reported case in which eqPE administration contributed to the clinical improvement of PLE in a dog. The dose of eqPE used in this study has not yet been determined. In other cases, we empirically confirmed that no adverse events have been observed in dogs and cats at a tolerable daily dose of 2 ml for up to 10 kg body weight and 4 ml for those weighing 10 kg or more. In the present case, since the dog weighed 2.6 kg, we administered 2 ml of eqPE SID and observed no notable adverse events, suggesting that the dosage was appropriate. However, more detailed dosage studies are needed to establish the appropriate dosage for eqPE. The white blood cell count was normal at the initial visit but increased after the start of treatment. However, it recovered within the normal range in parallel with the improvement in diarrhea following the disappearance of ascites. Thereafter, the leukocyte counts remained normal, but on day 446, the last day of treatment, the leukocyte counts became elevated, deviating from the reference range. Although the cause of the abnormally high white blood cell count on the last day has not yet been determined; segmented neutrophil, monocyte, and eosinophil counts were normal at that time, whereas lymphocytes were below the standard range and stab cell counts were above the standard range. These results suggest that the patient had some kind of infection, but since the dog was not seen again after 446 days, it was assumed to be due to transient inflammation. Regarding the low lymphocyte counts, the absence of a follow-up visit after 446 days suggests that they were caused by transient stress. Platelet counts were normal at the initial visit, but all platelet counts exceeded the reference values except during the period from the confirmation of the disappearance of ascites to the improvement of diarrhea and at the examination on day 364. However, the series of elevated platelet levels showed repetitive fluctuations of increasing and decreasing. Since there was no dehydration or persistent bleeding due to high platelet counts and the patient continued to require prednisolone after the resolution of ascites and improvement of diarrhea, it was suggested that the high platelet counts were due to chronic inflammation.
Fig. 1. Clinical course of treatment with prednisolone, MitoMax, azathioprine, and eqPE in this case. Administration of prednisolone (3 mg/kg SID) and MitoMax (200 mg SID) did not improve ascites accumulation and diarrhea; thus, azathioprine (2 mg/kg daily) was added, but no improvement was observed and hepatic injury developed. Therefore, azathioprine was discontinued, and eqPE (2 ml SID) was added. eqPE, equine placenta extract; SID, semel in die.
Fig. 2. Clinical course of treatment with prednisolone, MitoMax, and eqPE in this case. Prednisolone was tapered from 3 mg/kg SID to 1 mg/kg SID with MitoMax (200 mg SID) and eqPE (2 ml SID). eqPE, equine placenta extract; SID, semel in die. Hypocalcemia was observed in this patient at the initial visit but was confirmed to have improved as the patient showed normal calcium values on day 446, the last visit. However, the phosphorus level, which was normal at the initial visit, showed an above-normal value on day 446. Renal disease was suspected because of the high phosphorus level. At this time, the creatinine level was normal, but the BUN level was elevated above the reference range. Based on these results, renal disease was likely in this patient, but it has not yet been determined whether it was due to PLE, an adverse event caused by the long-term use of therapeutic agents, or simply aging. Additionally, a high BUN level increases the concern of lymphoma; however, the absence of concurrent hypercalcemia excludes the possibility of lymphoma. Placenta extract is obtained by hydrolyzing human or domestic animal placentas with hydrochloric acid alone or with hydrochloric acid and enzymatic digestion. They have been found to have numerous biological activities (Yamauchi et al., 2017; Bak et al., 2018; Ito et al., 2018; Bak et al., 2019; Yamauchi et al., 2019; Yoshimoto et al., 2019; Yamauchi et al., 2020; Igarashi et al., 2021) and are widely used as pharmaceuticals and health foods. Clinically, only human placenta extract is available as a drug, and it has been used in patients with hepatitis and liver cirrhosis in Japan and several other countries. A drastic increase in liver function values was observed on day 23 following eqPE administration in our case. Liver function values temporarily decreased, but they were not within the normal range and increased again to abnormal values, suggesting that improvement of liver function by eqPE had failed. However, the fact that diarrhea improved following the disappearance of ascites accumulation after the administration of eqPE and that the liver function values recovered to almost normal values in the subsequent outcome may not exclude the effectiveness of eqPE in improving liver function. Therefore, it was speculated that eqPE may be effective in improving liver function. It is possible that eqPE has the potential to ameliorate ascites accumulation and diarrhea, and its efficacy needs to be verified. Edema and ascites are associated with decreased liver function in patients with hepatitis, but findings have shown that the administration of placenta extract has improved these symptoms in other patients. If this is verified, it can be inferred that eqPE may contribute to the improvement of ascites via improvement in liver function. Prednisolone was used as an immunosuppressive agent in a dog with PLE showing chronic diarrhea and mild lymphangiectasia, but hepatotoxicity was observed; therefore, the dose had to be tapered to a less effective dose. However, a combination of azathioprine, tacrolimus, and cyclosporine showed good symptomatic improvement, and further titration of prednisolone did not worsen symptoms (Yuki et al., 2005). Although this case is similar to the present case, concerns exist that the concomitant use of immunosuppressant drugs may pose a risk of complications, e.g., infections, due to reduced immunity. Moreover, it has been noted that prednisolone is catabolic and may be harmful to dogs with negative energy balance (Dandrieux et al., 2013). In addition, prednisolone may exacerbate preexisting hypercoagulable conditions and induce signs, such as muscle weakness and lethargy (Goodwin et al., 2011). Thus, there are potential problems associated with the long-term use of prednisolone. Yet, in our case, the aforementioned risks may be presumed to be low because of the combination of immunosuppressive drugs and eqPE supplementation. Further, since PLE is mainly characterized by panhypoproteinemia associated with small intestinal disease, a homemade ultra-low-fat diet is effective for short-term treatment of PLE due to IL; however, this diet is low in calcium and vitamin D content and seems to make nutritional balance challenging. Recently, it was reported that the oral administration of active vitamin D3 improved hypocalcemia within 28 days in a dog with diarrhea, pancytopenia, and recurrent transient seizures suspected to be secondary to nutritional hyperparathyroidism after 18 months of treatment with immunosuppressive drugs and a very low-fat diet (Tamura et al., 2022). Furthermore, the administration of a very low-fat diet and active vitamin D3 successfully reduced the dose of immunosuppressive drugs by approximately one-fourth. In the present case, the dosage of immunosuppressive agents was successfully reduced to one-third after approximately 40 days of eqPE administration. Since dietary fat restriction has been suggested as an effective treatment for dogs with IL that do not respond to prednisolone treatment or dogs with recurrent clinical signs and hypoalbuminemia when the prednisolone dose is reduced (Okanishi et al., 2014), synergistic effects may be expected when combined with eqPE, and it is expected that new treatment methods will be developed in the future. In our case, ALT and ALP levels continued to increase temporarily after eqPE administration and were outside the reference range, but they subsequently continued to decrease and remained within the reference range. These results suggest that eqPE contributed to the improvement of liver function, not immediately but slowly. In contrast, albumin and total protein levels, which affect the prognosis of PLE, increased rapidly after eqPE administration and almost reached the reference ranges. These results suggested that eqPE contributed to the normalization of albumin and total protein levels somewhat immediately in this case. The difference in the speed of action may be because eqPE is composed of various components, including amino acids and peptides, and the active ingredients may have been different from each other and may have been multiple components. The active ingredient of eqPE in this case has not been determined and is a subject for future research. In conclusion, this study showed improvements after eqPE administration in a PLE dog with prolonged diarrhea and ascites effusion. The effect of eqPE was delayed, not immediate. No notable adverse events were observed during the long-term use of eqPE, which contributed to prednisolone tapering. In canine PLE with prolonged diarrhea and ascites effusion, supplementation with eqPE may be considered a reasonable additional therapeutic strategy. However, more detailed studies, including more cases, are required to confirm our findings. AcknowledgmentsWe are sincerely grateful to all the co-medical staff, the dog, and the dog’s owner. We also appreciate Editage (www.editage.jp) for English language editing. Conflict of interestNobuhisa Kakehi, Tsuyuko Watanabe, Kentarou Tahara, and Eiichi Hirano are employees of the Japan Bio Products Co., Ltd. Naoki Fukushima has no competing interests. Author contributionsNF conceived and designed the clinical study and analyzed and interpreted the data. NK, TW, and KT analyzed and interpreted the data. EH analyzed and interpreted the data, and wrote the manuscript. ReferencesBak, D.H., Na, J., Choi, M.J., Lee, B.C., Oh, C.T., Kim, J.Y., Han, H.J., Kim, M.J., Kim, T.H. and Kim, B.J. 2018. Anti-apoptotic effects of human placental hydrolysate against hepatocyte toxicity in vivo and in vitro. Int. J. Mol. Med. 42(5), 2569–2583. Bak, D.H., Na, J., Im, S.I., Oh, C.T., Kim, J.Y., Park, S.K., Han, H.J., Seok, J., Choi, S.Y., Ko, E.J., Mun, S.K., Ahn, S.W. and Kim, B.J. 2019. Antioxidant effect of human placenta hydrolysate against oxidative stress on muscle atrophy. J. Cell. Physiol. 234(2), 1643–1658. Craven, M.D. and Washabau, R.J. 2019. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 33(2), 383–402. Dandrieux, J.R.S., Noble, P.J.M., Scase, T.J., Cripps, P.J. and German, A.J. 2013. Comparison of a chlorambucil-prednisolone combination with an azathioprine-prednisolone combination for treatment of chronic enteropathy with concurrent protein-losing enteropathy in dogs: 27 cases (2007–2010).J. Am. Vet. Med. Assoc. 242(12), 1705–1714. Goodwin, L.V., Goggs, R., Chan, D.L. and Allenspach, K. 2011. Hypercoagulability in dogs with protein-losing enteropathy. J. Vet. Intern. Med. 25(2), 273–277. Igarashi, K., Sugimoto, K. and Hirano, E. 2021. Placental extract suppresses the formation of fibrotic deposits by tumor necrosis factor alpha and transforming growth factor beta-induced epithelial-mesenchymal transition in ARPE-19 cells. BMC. Notes. 14(1), 407. Ito, K., Yamada, R., Matsumoto, N. and Imamura, T. 2018. Evaluation of fibroblast growth factor activity exerted by placental extract used as a cosmetic ingredient. J. Cosmet. Dermatol. 17(5), 821–829. Okanishi, H., Yoshioka, R., Kagawa, Y. and Watari, T. 2014. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J. Vet. Intern. Med. 28(3), 809–817. Tamura, Y., Terakado, K., Neo, S., Igarashi, H. and Hisasue, M. 2022. Successful treatment with oral alfacalcidol supplementation for nutritional hypocalcaemia with protein-losing enteropathy in a dog. Vet. Rec. Case Rep. 10(1), e261 Yamauchi, A., Kamiyoshi, A., Koyama, T., Iinuma, N., Yamaguchi, S., Miyazaki, H., Hirano, E., Kaku, T. and Shindo, T. 2017. Placental extract ameliorates non-alcoholic steatohepatitis (NASH) by exerting protective effects on endothelial cells. Heliyon 3(9), e00416. Yamauchi, A., Tone, T., Sugimoto, K., Lim, H.S., Kaku, T., Tohda, C., Shindo, T., Tamada, K., Mizukami, Y. and Hirano, E. 2019. Porcine placental extract facilitates memory and learning in aged mice. Food. Sci. Nutr. 7(9), 2995–3005. Yamauchi, A., Tone, T., de-Toledo, A., Igarashi, K., Sugimoto, K., Miyai, H., Deng, D., Nakamura, J., Lim, H.S., Kaku, T., Hirano, E. and Shindo, T. 2020. Placental extract ameliorates liver fibrosis in a methionine- and choline-deficient diet-induced mouse model of non-alcoholic steatohepatitis. Biomed. Res. 41(1), 1–12. Yoshimoto, S., Ohagi, Y., Yoshida, M., Yanagi, H., Hibino, S., Ichihashi, M. and Ando, H. 2019. Placental extracts regulate melanin synthesis in normal human melanocytes with alternations of mitochondrial respiration. Exp. Dermatol. 28(1), 50–54. Yuki, M., Sugimoto, N., Higuchi, T., Takahashi, K., Otsuka, H. and Suzuki, K. 2005. A case of canine protein-losing enteropathy treated with immunosuppressive agents. J. Jpn. Vet. Med. Assoc. 58(4), 257–260. | ||
| How to Cite this Article |
| Pubmed Style Fukusima N, Kakehi N, Tahara K, Watanabe T, Hirano E. Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Vet. J.. 2022; 12(5): 774-781. doi:10.5455/OVJ.2022.v12.i5.24 Web Style Fukusima N, Kakehi N, Tahara K, Watanabe T, Hirano E. Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. https://www.openveterinaryjournal.com/?mno=79639 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.24 AMA (American Medical Association) Style Fukusima N, Kakehi N, Tahara K, Watanabe T, Hirano E. Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Vet. J.. 2022; 12(5): 774-781. doi:10.5455/OVJ.2022.v12.i5.24 Vancouver/ICMJE Style Fukusima N, Kakehi N, Tahara K, Watanabe T, Hirano E. Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 774-781. doi:10.5455/OVJ.2022.v12.i5.24 Harvard Style Fukusima, N., Kakehi, . N., Tahara, . K., Watanabe, . T. & Hirano, . E. (2022) Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Vet. J., 12 (5), 774-781. doi:10.5455/OVJ.2022.v12.i5.24 Turabian Style Fukusima, Naoki, Nobuhisa Kakehi, Kentarou Tahara, Tsuyuko Watanabe, and Eiichi Hirano. 2022. Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Veterinary Journal, 12 (5), 774-781. doi:10.5455/OVJ.2022.v12.i5.24 Chicago Style Fukusima, Naoki, Nobuhisa Kakehi, Kentarou Tahara, Tsuyuko Watanabe, and Eiichi Hirano. "Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report." Open Veterinary Journal 12 (2022), 774-781. doi:10.5455/OVJ.2022.v12.i5.24 MLA (The Modern Language Association) Style Fukusima, Naoki, Nobuhisa Kakehi, Kentarou Tahara, Tsuyuko Watanabe, and Eiichi Hirano. "Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report." Open Veterinary Journal 12.5 (2022), 774-781. Print. doi:10.5455/OVJ.2022.v12.i5.24 APA (American Psychological Association) Style Fukusima, N., Kakehi, . N., Tahara, . K., Watanabe, . T. & Hirano, . E. (2022) Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report. Open Veterinary Journal, 12 (5), 774-781. doi:10.5455/OVJ.2022.v12.i5.24 |