| Case Report | ||
Open Vet. J.. 2021; 11(4): 662-666 Open Veterinary Journal, (2021), Vol. 11(4): 662–666 Case Report Transient third-degree atrioventricular block during anesthesia in a catHiroshi Sunahara*, Kenji Tani, Yuki Nemoto, Kazuhito Itamoto, Harumichi Itoh, Munekazu Nakaichi,Toshie Iseri and Hiro HorikirizonoJoint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan *Corresponding Author: Hiroshi Sunahara. Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan. Email: sunahara [at] yamaguchi-u.ac.jp Submitted: 21/05/2021 Accepted: 17/10/2021 Published: 16/11/2021 © 2021 Open Veterinary Journal
AbstractBackground: Third-degree atrioventricular block (AVB) is usually permanent, with transient cases being rare. Cats with transient third-degree AVB. It had been not reported in detail. Case Description: A 9.3-year-old, male shorthair cat was evaluated for possible nervous disease resulting from otitis interna. Under propofol and isoflurane anesthesia, this cat developed a third-degree AVB. Isoproterenol was administered by continuous infusion to increase its heart rate. During recovery, heart rate returned to sinus bradycardia together with first-degree AVB without medical treatment. The cause of transient AVB was not observed at the examination. Conclusion: The case of this cat suggests that anesthesia can result in a transient third-degree AVB. Keywords: Transient third-degree atrioventricular block, Anesthesia, Cat. IntroductionThird-degree atrioventricular block (AVB) is defined as a complete disturbance of conduction between the sinus node and ventricles (Tilley, 1992; Kittleson and Kienle, 1998). Third-degree AVB is usually permanent, with transient cases being rare. In human beings and dogs, transient third-degree AVB has been reported with various non-cardiac diseases (Kittleson and Kienle, 1998; Rester and Bennett, 2005; Rotstein et al., 2008; Argun et al., 2018). In those cases, the third-degree AVB improved by treating the underlying disease. Although one previous report of a cat developed transient third-degree AVB, details about the transition from third-degree AVB to sinus rhythm were not reported in that study (Kellum and Stepien, 2006). Therefore, the cause of transient third-degree AVB in that case and the appropriate therapy for it were not clear. We experienced the case of a cat diagnosed with third-degree AVB during anesthesia and subsequently spontaneously transitioned from third-degree to first-degree AVB at the time of recovery. To the best of authors’ knowledge, there are no previous reports of the development of transient third-degree AVB due to anesthetic drugs in a cat. This is the first report describing the development of transient third-degree AVB due to anesthesia in detail. Case DetailsA 9.3-year-old, 4.59 kg, neutered male domestic shorthair cat had developed nystagmus, torticollis, and inappetence for 1½ months. At its referral to a veterinary hospital, the cat was suspected of having nervous disease resulting from otitis interna, and steroids and antibiotics were administered. However, its neurological symptoms did not improve. Therefore, it was referred to Yamaguchi University Animal Medical Center for further examination of nystagmus and torticollis. The client had not recognized seizures, collapse, or claudication. Preventive measures for fleas and ticks were regularly performed. Physical examination revealed bradycardia, but no heart murmur, fever (39.1°C), and arthritis. Blood tests revealed elevated values of leukocytes (250.5 × 102/μl) and serum amyloid A (155.3 μg/ml). Blood tests were negative for the feline immunodeficiency virus and feline leukemia virus. Since the cardiac silhouette enlargement on radiography, the cat was referred by the primary veterinarian to the department of cardiovascular medicine (Sunahara) for echocardiography. Echocardiography revealed enlargement of the left atrium [(LA)/Ao=1.6] and left ventricle (left ventricular internal diameter in diastole=1.94 cm). Other mechanical problems suggestive of myocarditis were not observed. There was no decline in local myocardial motility. Although electrocardiography (ECG) was not performed at the time of echocardiography, atrial and ventricular wall motion seemed normal. There were no findings suggestive of myocarditis at echocardiography. The cat was anesthetised to definitively determine the cause of nystagmus and torticollis. Following propofol administration for anesthesia induction and isoflurane inhalation for maintenance, the cat’s heart rate decreased to 75 beats/minute on the biomonitor. The biomonitor screen also showed that the rate of P waves was 115 beats/minute, and the rate of R waves was 75 beats/minute (Fig. 1). P waves were not associated with QRS complexes. Hence, the cat was diagnosed with third-degree AVB, and isoproterenol was administered by continuous infusion to increase its heart rate.
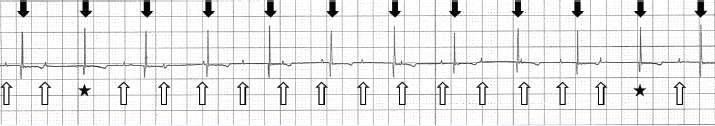
Fig. 1. Lead II trace on the biomonitor during anesthesia. The cat’s P waves are marked by upward arrows. The P-P intervals were consistent (0.52 seconds). The P waves that were followed by QRS complexes are indicated by asterisks. The rate of QRS complexes was 75 beats/minute (downward arrows) and their R-R intervals were consistent (0.8 seconds). QRS morphology was normal (duration, 0.04 seconds). Paper speed=25 mm/second; 1 cm=1 mV.
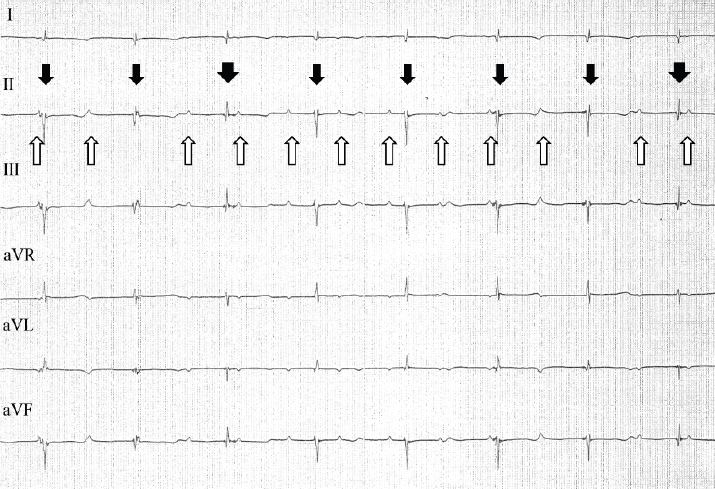
Fig. 2. ECG tracings from a 6-lead ECG examination of the cat during anesthesia and administration of isoproterenol. The cat’s P waves are marked by upward arrows; the P-P intervals were consistent (0.4 seconds). Multiple QRS complexes with narrow negative waves (thin downward arrows) and narrow positive waves (wide downward arrows) were observed. However, both were consistent (0.74 seconds). Paper speed=50 mm/second; 1 cm=1 mV. Under the administration of isoproterenol, ECG tracings revealed an increase in the rate of P waves to 150 beats/minute, with the waves appearing at regular intervals (Fig. 2). The rate of R waves was 81 beats/minute; the R-R intervals were also regular. However, P-R intervals were inconsistent, and P waves were not associated with QRS complexes. Multiple QRS complex morphologies were observed on the ECG tracings. Some QRS complexes appeared narrow and had negative waves. Since the R-R intervals of these QRS complexes indicated a rate of 85 beats/minute, in addition to narrow QRS complexes, these QRS complexes were considered to represent escape rhythm. Other QRS complexes appeared narrow and had positive waves. Positive QRS complex waves occurred at a lower frequency than the negative waves. However, R-R intervals between negative and positive QRS complexes were the same as the interval between negative QRS complexes. These narrow QRS complexes that resulted in the same heart rates suggested junctional rhythm originating from a location higher in the ventricle.
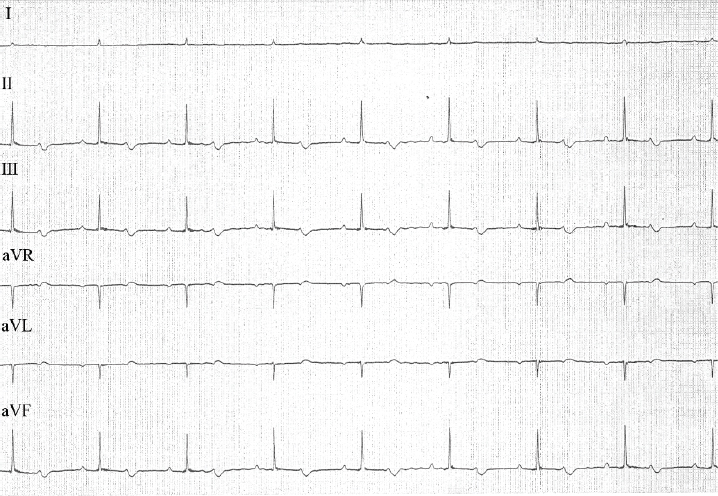
Fig. 3. ECG tracings from a 6-lead ECG examination of the cat during recovery from anesthesia. P waves were associated with QRS complexes. P-P intervals and R-R intervals were of the same duration (0.7 seconds). P-Q intervals were prolonged (duration, 0.16 seconds). QRS complex morphology revealed narrow, positive, enlarged complexes (amplitude, 1.7 mV; duration, 0.04 seconds). Paper speed=50 mm/second; 1 cm=1 mV. During recovery from anesthesia, the P waves gradually connected with the QRS complexes, finally ultimately associating with them (Fig. 3). The cat’s heart rate at this time was 85 beats/minute. Since the P-Q intervals were prolonged (duration, 0.16 seconds), the cat was diagnosed with first-degree AVB. Evaluation of QRS-complex morphology revealed enlarged narrow and positive waves, suggesting a left ventricular load pattern. During recovery, the cat’s heart rate returned to sinus bradycardia together with first-degree AVB without medical treatment. Magnetic resonance imaging (MRI) revealed meningoencephalitis, and computed tomography (CT) revealed fluid accumulation in the tympanic bulla. CT of the head, thorax, and abdomen did not reveal other diseases, such as hypothyroidism, which can also cause bradycardia. DiscussionThird-degree AVB is defined as a complete conduction disturbance between the sinus node and ventricles (Tilley, 1992; Kittleson and Kienle, 1998). It is usually permanent, with transient cases being rare. In both human beings and dogs, transient third-degree AVB was reported in cases of myocarditis due to Lyme disease (Levy and Duray, 1988; Kittleson and Kienle, 1998). Transient third-degree AVB in human beings has also been reported secondary to conditions other than Lyme disease (Rester and Bennett, 2005; Rotstein et al., 2008; Argun et al., 2018). Although there is a previous report of a cat that developed transient third-degree AVB in a study of third-degree AVB in cats, details of the transition from third-degree AVB to first-degree AVB were not reported in that study (Kellum and Stepien, 2006). This is the first case report describing the development and transition of transient AVB in detail. It has been previously reported that infectious myocarditis (viral, bacterial, protozoan, etc.) can induce arrhythmia and conduction disorders in both dogs and cats (Levy and Duray, 1988; Pisani et al., 1997; Meurs et al., 1998; Breitschwerdt et al., 1999; Bradley et al., 2000; Dvir et al., 2004). In this cat, although echocardiography was performed without ECG before anesthesia, there did not seem to be any atrioventricular conduction disturbance during echocardiography. Additionally, this cat did not have typical findings of infective endocarditis, and myocardial abnormalities were also not observed. Although myocarditis caused by parvovirus has been reported in dogs, parvovirus-induced myocarditis in cats has not been reported; this cat was also negative for feline leukemia virus (Pisani et al., 1997). Although Lyme disease, which can cause myocarditis and subsequent third-degree AVB, is caused by ticks, this cat received preventive therapy for ticks (Levy and Duray, 1988). In a previous report of Lyme disease in human beings in Japan, Yamaguchi Prefecture, where this cat resided, was declared free of Lyme disease. Therefore, the probability of this cat being infected with Lyme disease was low (National Institute of Infectious Diseases, 2019). CT and MRI examination showed no other systemic inflammatory disease other than meningoencephalitis and fluid accumulation in the tympanic bulla. Additionally, third-degree AVB developed following anesthesia induction, continuing during anesthesia maintenance, and finally transitioning from third-degree AVB to first-degree AVB at the time of anesthesia recovery. Therefore, this cat was diagnosed as third-degree AVB caused by anesthesia and not meningoencephalitis or fluid accumulation in the tympanic bulla. Although meningoencephalitis and fluid accumulation in the tympanic bulla might have promoted the AVB, the probability of third-degree AVB caused by meningitis is low. Our experience suggests that veterinarians should be aware of the probability of the development of third-degree AVB at the time of anesthesia in cases with suspected meningoencephalitis and fluid accumulation in the tympanic bulla. Since this cat’s heart rate improved slightly with isoproterenol, the recommended initial therapy for third-degree AVB, isoproterenol might have contributed to the transient nature of the third-degree AVB in this cat (Kittleson and Kienle, 1998). However, the development of third-degree AVB with anesthesia, transitioning to first-degree AVB at the time of anesthesia recovery, suggests that when third-degree AVB develops during anesthesia, as in this cat, anesthesia should be discontinued to reverse third-degree AVB. ConclusionIn cats, transient third-degree AVB can be caused by anesthesia, with the block improving by terminating anesthesia. In such cases, isoproterenol, which is used for treating transient third-degree AVB under anesthesia, might help to improve the heart rate. This is the first report describing transient third-degree AVB in detail. However, more data need to be accumulated to determine the etiology of transient third-degree AVB in such cases. Conflict of interestThe authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article. ReferencesArgun, M., Baykan, A., Özyurt, A., Pakokku, Ö., Üzüm, K. and Narin, N. 2018. Syncope due to complete atrioventricular block and treatment with a transient pacemaker in acute rheumatic fever. Turk. Pediatr. Ars. 53, 197–199. Bradley, K.K., Bergman, D.K., Woods, J.P., Crutcher, J.M. and Kirchhoff, L.V. 2000. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J. Am. Vet. Med. Assoc. 15, 1853–1857. Breitschwerdt, E.B., Atkins, C.E., Brown, T.T., Kordick, D.L. and Snyder, P.S. 1999. Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J. Clin. Microbiol. 37, 3618–3626. Dvir, E., Lobetti, GR., Jacobson, L.S., Pearson, J. and Becker, P.J. 2004. Electrocardiographic changes and cardiac pathology in canine babesiosis. J. Vet. Cardiol. 6, 15–23. Kellum, H.B. and Stepien, R.L. 2006. Third-degree atrioventricular block in 21 cats (1997–2004). J. Vet. Intern. Med. 20, 97–103. Kittleson, M.D. and Kienle, R.D. 1998. Diagnosis and treatment of arrhythmias (dysrhythmias). In Small animal cardiovascular medicine, 1st ed. Eds., Kittleson, M.D. and Kienle, R.D. St. Louis, MO: Mosby, pp: 489–494. Levy, S.A and Duray, P.H. 1988. Complete heart block in a dog seropositive for Borrelia burgdorferi. Similarity to human Lyme carditis. J. Vet. Intern. Med. 2, 138–144. Meurs, K.M., Anthony, M.A., Slater, M. and Miller, M.W. 1998. Chronic Trypanosoma cruzi infection in dogs: 11 cases (1987–1996). J. Am. Vet. Med. Assoc. 15, 497–500. Pisani, B., Taylor, D.O. and Mason, J.W. 1997. Inflammatory myocardial diseases and cardiomyopathies. Am J Med. 102, 459–469. National Institute of Infectious Diseases. 2019. Lyme disease. Available via https://www.niid.go.jp/niid/ja/kansennohanashi/524-lyme.html (Accessed 3 March 2019). Rester, T.B. and Bennett, K.R. 2005. Kiss of the Benchuca Chagas’ disease presenting with transient third-degree atrioventricular block. J. Miss. State. Med. Assoc. 46, 369–372. Rotstein, A., Charrow, J. and Deal, B. 2008. Documented transient third-degree atrioventricular block and asystole in a child with familial dysautonomia. Pediatr. Cardiol. 29, 202–204. Tilley, L.P. 1992. Feline third degree AV block (complete block). In Essentials of canine and feline electrocardiography. Interpretation and treatment, 3rd ed. Eds., Tilley, L.P. Philadelphia, PA: Lea & Febiger, pp: 234–235. | ||
| How to Cite this Article |
| Pubmed Style Sunahara H, Tani K, Nemoto Y, Itamoto K, Itoh H, Nakaichi M, Iseri T, Horikirizono H. Transient third-degree atrioventricular block during anaesthesia in a cat. Open Vet. J.. 2021; 11(4): 662-666. doi:10.5455/OVJ.2021.v11.i4.16 Web Style Sunahara H, Tani K, Nemoto Y, Itamoto K, Itoh H, Nakaichi M, Iseri T, Horikirizono H. Transient third-degree atrioventricular block during anaesthesia in a cat. https://www.openveterinaryjournal.com/?mno=81810 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.16 AMA (American Medical Association) Style Sunahara H, Tani K, Nemoto Y, Itamoto K, Itoh H, Nakaichi M, Iseri T, Horikirizono H. Transient third-degree atrioventricular block during anaesthesia in a cat. Open Vet. J.. 2021; 11(4): 662-666. doi:10.5455/OVJ.2021.v11.i4.16 Vancouver/ICMJE Style Sunahara H, Tani K, Nemoto Y, Itamoto K, Itoh H, Nakaichi M, Iseri T, Horikirizono H. Transient third-degree atrioventricular block during anaesthesia in a cat. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 662-666. doi:10.5455/OVJ.2021.v11.i4.16 Harvard Style Sunahara, H., Tani, . K., Nemoto, . Y., Itamoto, . K., Itoh, . H., Nakaichi, . M., Iseri, . T. & Horikirizono, . H. (2021) Transient third-degree atrioventricular block during anaesthesia in a cat. Open Vet. J., 11 (4), 662-666. doi:10.5455/OVJ.2021.v11.i4.16 Turabian Style Sunahara, Hiroshi, Kenji Tani, Yuki Nemoto, Kazuhito Itamoto, Harumichi Itoh, Munekazu Nakaichi, Toshie Iseri, and Hiro Horikirizono. 2021. Transient third-degree atrioventricular block during anaesthesia in a cat. Open Veterinary Journal, 11 (4), 662-666. doi:10.5455/OVJ.2021.v11.i4.16 Chicago Style Sunahara, Hiroshi, Kenji Tani, Yuki Nemoto, Kazuhito Itamoto, Harumichi Itoh, Munekazu Nakaichi, Toshie Iseri, and Hiro Horikirizono. "Transient third-degree atrioventricular block during anaesthesia in a cat." Open Veterinary Journal 11 (2021), 662-666. doi:10.5455/OVJ.2021.v11.i4.16 MLA (The Modern Language Association) Style Sunahara, Hiroshi, Kenji Tani, Yuki Nemoto, Kazuhito Itamoto, Harumichi Itoh, Munekazu Nakaichi, Toshie Iseri, and Hiro Horikirizono. "Transient third-degree atrioventricular block during anaesthesia in a cat." Open Veterinary Journal 11.4 (2021), 662-666. Print. doi:10.5455/OVJ.2021.v11.i4.16 APA (American Psychological Association) Style Sunahara, H., Tani, . K., Nemoto, . Y., Itamoto, . K., Itoh, . H., Nakaichi, . M., Iseri, . T. & Horikirizono, . H. (2021) Transient third-degree atrioventricular block during anaesthesia in a cat. Open Veterinary Journal, 11 (4), 662-666. doi:10.5455/OVJ.2021.v11.i4.16 |