| Short Communication | ||
Open Vet. J.. 2022; 12(5): 688-692 Open Veterinary Journal, (2022), Vol. 12(5): 688–692 Short Communication Diagnostic study of trypanosomiasis of cats in Mosul, IraqNadia Hamid Mohammed1*, Dhiyaa Abdullah Moosa2 and Mohammed Abdel-Mohsen Altaliby21Department of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq 2Department of Internal and Preventive medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Nadia Hamid Mohammed. Department of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: nadiahamid [at] uomosul.edu.iq Submitted: 20/04/2022 Accepted: 14/08/2022 Published: 16/09/2022 © 2022 Open Veterinary Journal
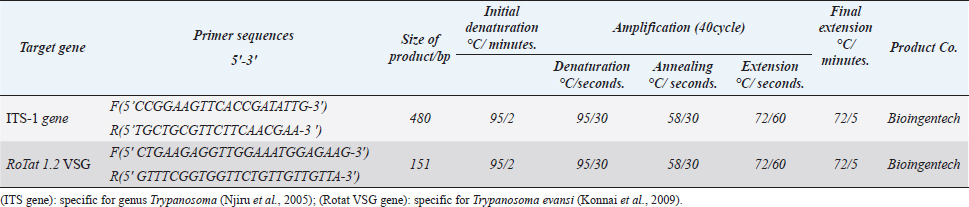
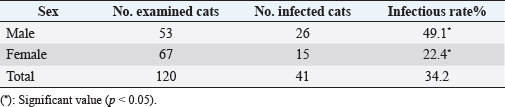
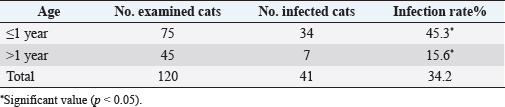
AbstractBackground: Trypanosomiasis is a zoonotic parasitic disease endemic in Iraq but with limited information about its occurrence in cats. Aim: This study was designed to detect Trypanosoma spp. in cats using microscopic examination by Giemsa stain and conventional polymerase chain reaction (PCR) technique in Mosul, Iraq. Methods: A total of 120 blood samples from cats were microscopically examined using Giemsa stain. Only 35 positive blood samples were examined by the conventional PCR technique. Hematological changes were also reported. Results: The infection rate of Trypanosoma spp. was 34.2% (41 out of 120). Results of conventional PCR technique for the positive 35 blood samples indicated 31.4% as Trypanosoma spp. and 20% Trypanosoma evansi. This study showed that the infection in younger cats was significantly more than in older cats, with significant differences between females and males. Affected cats suffered from fever, dullness, pale mucous membranes, emaciation, muco-purulent ocular discharge, anorexia, incoordination, and anemia. Results of the blood picture indicated increase in total leukocyte count and decrease in hemoglobin concentration, packed cell volume, and total red blood cells. Conclusion: Trypanosoma spp. and T. evansi infection in Mosul of Iraq is reported for the first time in cats, and younger cats were more affected than older cats. Keywords: Trypanosomiasis, Cats, Trypanosoma spp, Trypanosoma evansi, PCR. IntroductionTrypanosomiasis is considered as one of the most common and important diseases that affect human and many animals including cats (Panigrahi et al., 2015). Flies such as Stomaxys, Tabanus, and Triatomids bugs spread the disease, and some recent resources showed that the disease can be transmitted by ingestion of dead animals infected recently with trypanosomosis (Nwoha, 2013). Clinical signs start to appear on affected cats after 2–3 weeks of flies’ bite, initially with a local skin inflammatory reaction (Chancre) appearing at the site of biting. The size of Chancre is depending on several factors such as the immune status of the affected cat, virulence of Trypanosoma spp., and inoculation dose of Trypanosoma species. The parasites are dividing and multiplying inside Chancre and then enter the adjacent lymph nodes then the lymph vessels and finally the bloodstream (parasitemia) (Aloba et al., 2022). Clinical signs are varies depending on the severity of the disease and Trypanosoma spp. which include anorexia, anemia, fever, enlarged superficial lymph nodes, conjunctivitis as well as edema of limbs (Zenad and Radhy, 2020). The disease has three forms; acute, subacute, and chronic, where the acute form is highly fatal. The disease is common in cats as they are more exposed to vectors (Solikhah, 2021). There are many techniques used for the diagnosis of Trypanosoma spp. including wet smear. The ideal way to diagnose Trypanosoma parasite in cats is using a thin blood smear stained with one of Romanowsky stains as Giemsa stain and examined under microscope (magnification of 1,000×) (Solikhah et al., 2019). In addition, for accurate diagnosis, polymerase chain reaction (PCR) technique is considered as a very good procedure for the diagnosis of trypanosomiasis (Njiru et al., 2005). The current study was aimed to provide information about the trypanosomiasis of cats in Mosul city of Iraq using microscopical examination and molecular methods. Material and MethodsAnimals and samples collectionA total number of 120 blood samples were collected from the cephalic vein of young cats (≤1 year) and old cats (>1 year), 53 males and 67 females, from different regions in Mosul city of Iraq. All blood sample were microscopically examined using blood smears stained with Giemsa (Soulsby, 1986; Konnai et al., 2009; Prasad et al., 2015; Salvioni et al., 2021). Positive samples were stored at −20°C until PCR tested. Clinical signs were recorded for each cat. Molecular examinationGenomic DNAs from 35 positive blood samples were extracted by using column pure blood genomics DNA mini kits (Alanazi, 2018). Applied biological materials by using conventional PCR technique was performed for the detection of Trypanosoma spp. and Trypanosoma evansi (Table 1). Hematological examinationHematological examination was used for hemoglobin concentration (HB), packed cell volume (PCV), total red blood cells (TRBC), MCV, MCHC, and total leukocyte count (TLC). Parasitemia were calculated according to Da Silva et al. (2010) and Al-thuwaini (2021). Statistical analysisStatistical analysis done by using chi-square test in IBM-SPSS statistics version 19 program (Leech et al., 2007). Ethical approvalThe ethical approval was issued by the Institutional Animal Care and Use Committee (UM.VET.2021.16) of the Faculty of Veterinary Medicine, University of Mosul on the 2nd of September 2021. Results and DiscussionThe microscopic examination of blood smears stained with Giemsa in this study showed that the infection rate of Trypanosoma spp. was 34.2% (41 out of 120 cats). The infection rate was significantly (p < 0.05) higher in males (49.1%) compared with that in females (22.4%) (Table 2). The highest rate of infection was reported in younger cats (≤1 years). Statistical analysis showed significant differences between ages (Table 3). All 35 microscopically positive samples were examined by PCR technique and showed that the infection rate of Trypanosoma spp. was 31.4% (11 out of 35 cats), and with T. evansi was 20% (7 out of 35 cats). Positive bands were at 480 bp for Trypanosoma spp. and 151 bp for T. evansi (Figs. 1 and 2). The infected cats with Trypanosoma spp. were suffering from pale mucous membranes, fever, dullness, anorexia, emaciation, muco-purulent ocular discharge, incoordination and anemia (Fig. 3). In addition, there was decrease in TRBC, HB, PCV, with a macrocytic hypochromic anemia type and increase in TLC due to increase of lymphocytes, eosinophils, basophils, monocytes and increase in parasitemia (Table 4). The infectious rate of Trypanosoma infection in the current study was higher than that reported in other studies such as in Southern Louisiana and US with infection rates of 7.3% and 11.4%, respectively (Zecca et al., 2020; Eric et al., 2021). The difference of the infection rate of Trypanosoma spp. might be due to different efficacy of the control programs and sensitivity of diagnostic tests (Alanazi, 2018). The results of molecular methods are more sensitive and many researchers revealed Trypanosoma spp. using the PCR technique (Faraj et al., 2015; Eric et al., 2021). Table 1. PCR protocol used to detect Trypanosoma spp. and T. evansi in DNA samples.
Table 2. The infections rate of Trypanosome spp. according to the sex.
Table 3. Infections rate with Trypanosoma spp according to the age.
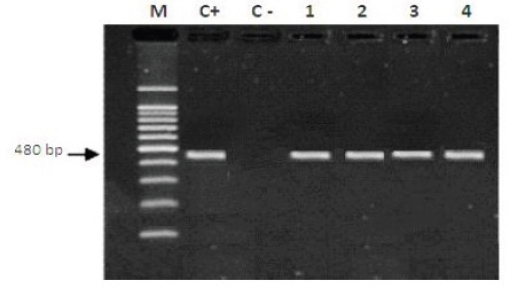
Fig. 1. Gel electrophoresis by using (ITS1) showing M: molecular size marker 100–1,200 base pair. Lane C+: Trypanosoma sp. DNA positive control. Lane C−: negative PCR control. Lanes 1–4: template DNA of Trypanosoma sp. at 480 bp isolated from cats.
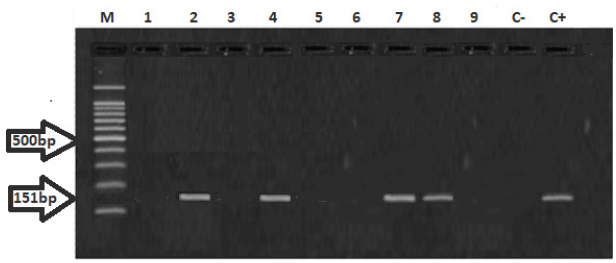
Fig. 2. Gel electrophoresis using RoTat VSG of T. evansi. M: molecular size marker 100–1,200 base pair. Lane C+: T. evansi DNA positive control. Lane C −: negative PCR control. Lanes 2,4,7,8: template DNA of T. evansi isolated from cats at 151 bp. Lanes 1,3,5,6,9: negative DNA samples.
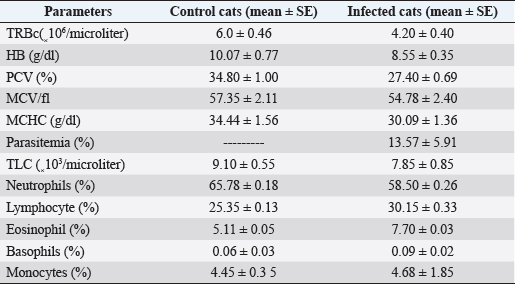
Fig. 3. Cat suffers from some clinical trypanosomiasis such as pale mucous membranes, fever, dullness, anorexia, emaciation, muco-purulent ocular discharge, incoordination, and anemia. Table 4. Hematologic parameters of control and infected cats with trypanosomiasis in 10 cats.
On the other hand, the infection rate was lower in older ages as that could be due to the older animals may produce better immunity against the parasite infection (Eric et al., 2021). The current study showed that there was an increase in the infection rate in males than females. These results are similar to the results of Faraj et al. (2015) and in contrast with Lauricella et al. (2010) who reported that both males and females were affected in similar rates. The clinical signs observed in infected cats were similar to other studies (Gurtler et al., 2007). Change in blood values were observed in affected cats compared to control cats. There was a decrease in TRBC, HB concentration and PCV that causes anemia, similar to others studies done on cats (Da silva et al., 2009). The increase in WBCs was due to increase in lymphocytes, eosinophils, basophils, and monocytes, and decrease of neutrophils was reported, as reported in other studies (Al-Badrani, 2012; Marwa and Alobaidii, 2022). The reason for this decrease could be a result of secondary bacterial infection (Da silva et al., 2009). However, Chaudhary and Iqbal (2000) found increase in the levels of neutrophils in camels infected with T. evansi. Author contributionsNHM and MAA: designed, photographed, and supervised this study. NHM and DAM: collected laboratory samples, conducted the practical part of the study and assisted in data analysis. NHM and MAA: contributed to the drafting of the manuscript. All authors contributed to the conduct of the study and discussed the results to a satisfactory scientific conclusion. AcknowledgmentsThe authors would like to thank the University of Mosul, College of Veterinary Medicine. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAlanazi, A.D. 2018. Parasitological and molecular detection of canine trypanosomiasis from Riyadh province, Saudi-Arabia. J. Parasitol. 104(5), 539–543. Al-Badrani, B. 2012. Clinical and hematological study of Trypanosoma brucci and Trypanosoma congolense in cattle in Mosul City, Iraq. Res. Opin. Anim. Vet. Sci. 2(2), 92–97. Aloba, W.A., Zaki, D. and Hasan, M.H. 2022. Detection of Dirofilaria immitis antigenia cats in Mosul city. Iraqi. J. Vet. Sci. 36(1), 57–60. Al-thuwaini, T.M. 2021. The relationship of hematological parameters with adaptation and reproduction in sheep a review study. Iraqi. J. Vet. Sci. 35(3), 575–580. Chaudhary, Z.I. and Iqbal, J. 2000. Incidence, biochemical and haematological alterations induced by natural trypanosomosis in racing dromedary camels. Acta. Tropica. 77, 209–213. Da silva, A.S., Costa, M.M., Wolkmer, P., Zanette, R.A., Faccio, L., Gressler, L.T., Dorneless, T.E.A., Santurio, J.M., dos Anjos Lopes, S.T. and Monteiro, S.G. 2009. Trypanosoma evansi hematologic changes in experimentally infected cats. Exper. Parasitol. 123, 31–34. Da Silva, A.S., Wolkmer, P., Costa, M.M., Tonin, A.A., Eilers, T.L., Gressler, LT., Otto, M.A., Zanette, R.A., Santurio, J.M., Lopes, STA. and Monteiro, S.G. 2010. Biochemical changes in cats infected with Trypanosome evansi. Vet. Parasit. 171(1–2), 48–52. Eric, D., Hans, D., Tu, W., Duhon, B., Wolfson, W., Balsamo, G. and Herrera, C. 2021. Shelter cats host infections with multiple Trypanosome cruzi discrete typing units in southern Louisiana. Vet. Res. 52(1), 53. Faraj, A.A., Hadi, A.M. and Al-Amery, A.M. 2015. Prevalence trypanosomiasis of stray dog in Baghdad city, Iraq. Int. J. Rec. Sci. Res. 6(11), 7206–7208. Gurtler, R.E., Cecere, M.C., Lauricella, M.A., Cardinal, M.V., Kitron, U. and Cohen, J.E. 2007. Domestic dogs and cats as sources of Trypanosome cruzi infection in rural northwestern Argentina. Parasitology 134(1), 69–82. Konnai, S., Mekata, H., Mingala, C.N., Abes, N.S., Gutierrez, C.A., Herrera, J.R.V., Dargantes, A.P., Witola, W.W., Cruz, L.C., Inoue, N., Onuma, M. and Ohashi, K. 2009. Development and application of quantitative real-time PCR for the diagnosis of surra in water buffaloes. Infect. Gene. Eval. 9, 449–452. Lauricella, M.A., Sinagra, A.J., Paulone, I., Riarte, A.R. and Segura, E.L. 2010. Natural Trypanosoma cruzi. C infection in dogs of endemic areas of the Argentine Republic. Rev. Inst. Med. Trop. Sao Paulo. 31(2), 63–70. Leech, N.L., Barrett, K.C. and Morgan, G.A. 2007. SPSS for intermediate statistics: use and interpretation. New York, NY: Lawrence Erlbaum Asso, pp: 1–97. Marwa, S.M. and Alobaidii, W.A. 2022. Molecular detection of Trypanosoma species in sheep and goats in Mosul city. Iraqi. J. Vet. Sci. 36(2), 445–449. Njiru, Z.K., Constantine, C.C., Guya, S., Crowther, J., Kiragu, J.M., Thompson, R.C.A. and Davila, A.M.R. 2005. The uses ITS1 rDNA PCR in detection of pathogenic African trypanosomes. Parasitol. Res. 95, 186–192. Nwoha, R.I.O. 2013. A review on trypanosomosis in dogs and cats. African. J. Biotechnol. 12(46), 6432–6442. Panigrahi, P.N., Mahendran, K., Jena, S.C., Behera, P., Mahajan, S., Arjun, K. and Dey, S. 2015.Trypanosome evansi infection in a German shepherd dog-Apparent successful treatment using serial low dose of diminazena aceturate. Vet. Parasit. Reg. 1(2), 70–74. Prasad, K.L., Kondaiah, P.M., Rayulu, V.C. and Srilatha, C. 2015. Prevalence of canine trypanosomiasis in certain areas of Andhra Pradesh. J. Parasite. Dis. 39(2), 238–240. Salvioni, O.D., Fraenkel, S., Tintel, M.J., Arze, V.P., Centurion, N.R., Rolon, M. and Gomez, V. 2021. First report of the presence of Trypanosome evansi in dogs from Paraguay applying molecular techniques. Brazilian. J. Vet. Med. 43, 10–20. Solikhah, T. 2021. Aloevera and virigin coconut oil (VCO) accelerate healing process in domestic cat (felis domesticus) suffering from scabies. Iraqi. J. Vet. Sci. 35(4), 699–704. Solikhah, K.P.U., Gibson, W. and Ezeokonkwo, R.C. 2019. Identification of Trypanosome brucci gambiense in naturally infected dogs in Nigeria. Parasit. Vectors. 12, 420–427. Soulsby, E.J.L. 1986. Helminths, arthropods, protozoa of domesticated animals, 7th ed. London, UK: Baillier, pp: 430–431. Zecca, I.B., Hodo, C.L., Slack, S., Auckland, L., Rodgers, S., Killets, K.C., Saunders, A.B. and Hamer, S.A. 2020. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Feliscatus). Vet. Parasitol. 27(8), 109014. Zenad, M.M. and Radhy, A.M. 2020. Clinical serological and antigenic study of feline panlenkopenia virus in cats in Baghdad. Iraqi. J. Vet. Sci. 34(2), 435–439. | ||
| How to Cite this Article |
| Pubmed Style Mohammed NH, Moosa DA, Altaliby MA. Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Vet. J.. 2022; 12(5): 688-692. doi:10.5455/OVJ.2022.v12.i5.13 Web Style Mohammed NH, Moosa DA, Altaliby MA. Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. https://www.openveterinaryjournal.com/?mno=819 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.13 AMA (American Medical Association) Style Mohammed NH, Moosa DA, Altaliby MA. Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Vet. J.. 2022; 12(5): 688-692. doi:10.5455/OVJ.2022.v12.i5.13 Vancouver/ICMJE Style Mohammed NH, Moosa DA, Altaliby MA. Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 688-692. doi:10.5455/OVJ.2022.v12.i5.13 Harvard Style Mohammed, N. H., Moosa, . D. A. & Altaliby, . M. A. (2022) Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Vet. J., 12 (5), 688-692. doi:10.5455/OVJ.2022.v12.i5.13 Turabian Style Mohammed, Nadia Hamid, Dhiyaa Abdullah Moosa, and Mohammed Abdel-mohsen Altaliby. 2022. Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Veterinary Journal, 12 (5), 688-692. doi:10.5455/OVJ.2022.v12.i5.13 Chicago Style Mohammed, Nadia Hamid, Dhiyaa Abdullah Moosa, and Mohammed Abdel-mohsen Altaliby. "Diagnostic study of trypanosomiasis of cats in Mosul, Iraq." Open Veterinary Journal 12 (2022), 688-692. doi:10.5455/OVJ.2022.v12.i5.13 MLA (The Modern Language Association) Style Mohammed, Nadia Hamid, Dhiyaa Abdullah Moosa, and Mohammed Abdel-mohsen Altaliby. "Diagnostic study of trypanosomiasis of cats in Mosul, Iraq." Open Veterinary Journal 12.5 (2022), 688-692. Print. doi:10.5455/OVJ.2022.v12.i5.13 APA (American Psychological Association) Style Mohammed, N. H., Moosa, . D. A. & Altaliby, . M. A. (2022) Diagnostic study of trypanosomiasis of cats in Mosul, Iraq. Open Veterinary Journal, 12 (5), 688-692. doi:10.5455/OVJ.2022.v12.i5.13 |