| Case Report | ||
Open Vet. J.. 2021; 11(4): 667-671 Open Veterinary Journal, (2021), Vol. 11(4): 667–671 Case Report Herniorrhaphy in two newborn lambs with omphaloceleJalal M. Abdelhadi1* and Aiman H. Oheida21Department of Surgery and Theriogenology, Faculty Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Department of Anatomy, Histology and Embryology, Faculty Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Jalal M. Abdelhadi. Department of Surgery and Theriogenology, Faculty Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: abdelhadijalal [at] gmail.com Submitted: 20/05/2021 Accepted: 17/10/2021 Published: 17/11/2021 © 2021 Open Veterinary Journal
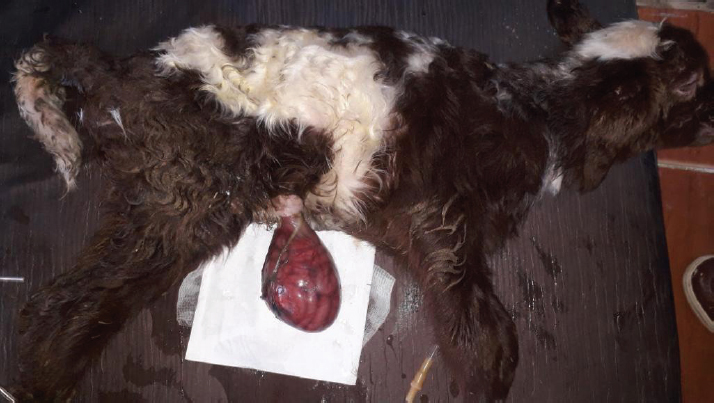
AbstractBackground: Omphalocele is an uncommon congenital defect in the ventral abdominal wall. Its etiology and pathogenesis are not certainly approved despite the numerous theories. Cases Description: Two newborn lambs with protruded membranous sacs from the umbilical region were presented. The herniated sac in both lambs contained loops of intestines and was covered by a thin membrane sac. The second lamb’s sac was disrupted, and its contents were severely congested. Physical and clinical examination of the cases revealed the congenital omphalocele. An immediate herniorrhaphy was performed for both after failing the gentle reduction of the sacs. All the preparing procedures and the surgical intervention were performed successfully. The first lamb recovered completely within 2 weeks after the operation. However, the second lamb died shortly after surgery. Conclusion: Omphalocele is an urgent case that needs instant treatment. Its prognosis is good unless the herniated sac is badly manipulated and its contents are proportionately large in size and highly congested. Keywords: Omphalocele, Herniorrhaphy, Congenital, Lamb, Embryology. IntroductionOmphalocele is an uncommon congenital defect in the ventral abdominal wall (Fazili et al., 2016; Raghavendran et al., 2020). The defect leads to an improper closing of the abdominal wall, resulting in herniating the umbilical sac (Raghavendran et al., 2020). The sac is filled with internal organs, such as intestines, and covered by an amniotic membrane (Christison-Lagay et al., 2011). The definite mechanism of developing the defect has not been achieved despite the numerous presented theories (Khan et al., 2019). During the early embryonic stage, if a deformity occurred in the ectodermal placodes which normally located at the umbilical ring (Khan et al., 2019); this would end with embryonic dysplasia (Hartwig et al., 1989; Russo et al., 1993). Such malformation was suggested to disrupt the body wall folding process and consequently failure of closing the abdominal wall properly and enlarging the umbilical ring (Noden and Lahunta, 1985; Hartwig et al., 1989; Watanabe et al., 2017; Raghavendran et al., 2020). Another assumption of arising omphalocele was attributed to the inability to return the herniated part to the abdominal cavity (McGeady et al., 2006; Christison-Lagay et al., 2011; Sagar et al., 2011). This was suggested to occur due to the developmental arrest during the presence of a part of the midgut in the umbilical sac (Gray and Skandalakis, 1972). In addition to the varied explanation of omphalocele pathogenesis, information about its etiology and prevalenceis significantly inadequate in domestic animals. Some authors, such as Raghavendran et al. (2020), did not consider omphalocele as a hereditary case. However, Roberts (1986) agreed with the inherited anomaly suggestion, which might be based on the likely associated factors such as inbreeding (Gutierrez et al., 1999). The prevalence of omphalocele, was generally believed to be rare in domestic animals (Baird, 1993), but there is no authentic information about its occurrence in lamb. Omphalocele was an urgent condition and required immediate treatment (Fazili et al., 2016; Sharma et al., 2018). Nevertheless, its prognosis is generally good (Fazili et al., 2014) unless the defect is severe especially if associate developmental anomalies accompany it in the heart, lungs, and other organs (Baird and MacDonald, 1981; Kamata et al., 2008). The current case report presents a full description of two newborn lambs with omphalocele, including the surgical intervention, postoperative management, and recommendations. Case DetailsTwo newborn male lambs, local breed (Libyan Barbary sheep), were presented at Al-Etgaan clinic, Tripoli, Libya on two separate occasions. The first and the second lambs were presented within 15 and 5 hours of their birth, respectively. The lambs were full-term and born normally (with a bit of difficulty for the second lamb) but without any assistance. In the first lamb, a large pendulous soft sac, 7.5 cm in diameter, was hanging from the ventral aspect of its abdomen. The membranous wall of the sac was thin (but not easy to cut) and hairless. It was somehow congested and contaminated with sand and dirt (Fig. 1). There were several intestinal loops, which appeared relatively normal, enclosed within the sac. The umbilical ring had well-defined boundaries with a diameter of 2 cm. The weight of the lamb was 3.7 kg, and its temperature and heart rate were generally within normal.
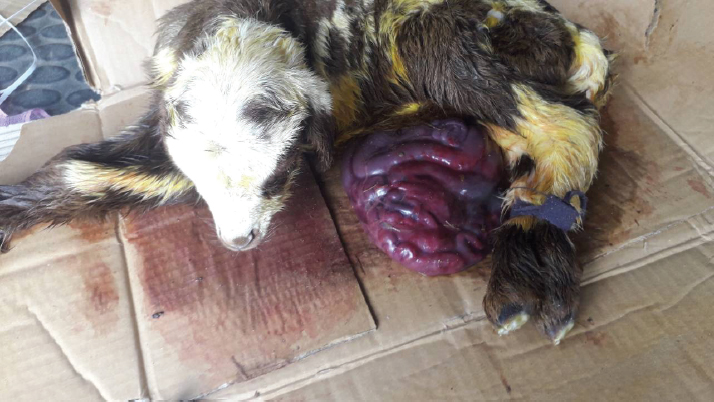
Fig. 1. A male newborn lamb (the first lamb) with omphalocele. The herniated sac congested and contained a number of intestinal lobes protruding from the umbilical ring. The animal was completely recovered and showed no complication postoperatively. The second lamb was unable to stand and was hypothermic (36.5°C). There was an increase in both heart and respiratory rates. The herniated sac was huge in relation to the body size (Fig. 2). It was hairless, disrupted, and very contaminated. The sac and its contents were significantly congested. Physical examination of the abdominal wall, including its muscles and skin, showed no abnormality in any animal. Based on the physical and the clinical examinations, the cases had enough evidence to be diagnosed as congenital omphalocele. The sac in each case was rinsed using warm normal saline and wrapped with moist gauze. After that, a gentle attempt was made to return the herniated portion to the abdominal cavity without cutting or opening the sac. As the contents were irreducible and the umbilical ring was proportionately small, the attempts to return the contents failed. Therefore, herniorrhaphy intervention was decided in the two cases. The lambs were positioned in dorsal recumbency. The area around the umbilicus was prepared. Povidin iodine (10%) was scrubbed in the surgical region for aseptic surgery. 4 ml of local anesthesia (Lidocaine 2%) was injected (infiltrated) around the umbilical area. The site of the surgical incision around the sac was scrubbed by alcohol and povidin iodine. An elliptical incision was made in the skin and the subcutaneous tissues without cutting the muscles layer, 1 cm from the umbilical ring. Few attempts were taken for reducing the contents. However, even with the incision, the reduction through the ring was still difficult and risky. Hence, the surgical incision was extended cranially for about 2 cm in the linea alba. Then, the intestinal loops were gently returned to their proper position in the abdominal cavity after cutting and eliminating the covering membranes of the sac. Other structures of the umbilical cord were cut and stitched. The surgical incision at the level of muscles and their tissues was closed with two layers of the simple continuous pattern using absorbable suture material (Polyglactin 910) No. 0 USP. The skin was also closed with the horizontal mattress suture pattern using non-absorbable suture material (silk) No. 0 USP. The wound was sprayed with oxytetracycline and methyl violet spray (derma aerosol). The lambs were received a broad spectrum antibiotic 0.5 ml of (Procaine benzylpenicillin 200 mg, Dihydrostreptomycin sulphate 200 mg) intramuscularly daily for 5 days postoperatively. The first lamb was monitored and kept with its dam in a clean restricted area. On the seventh postoperative day, the external stitches were removed, and the lamb was in a normal state with gaining weight. The second lamb was not presented after the date of operation for further treatments. Discussion24 hours postoperatively, the first lamb started walking actively, suckling, and defecating. After 2 weeks of the surgery, the animal showed no complications and was completely recovered. The second lamb was reported to have died shortly after the operation.
Fig. 2. A male newborn lamb (the second lamb) with omphalocele. The herniated sac was very large and highly congested. The case died shortly after the operation. The umbilicus is a typical region for congenital abdominal defects such as congenital umbilical hernia in which incomplete closure of the abdominal wall lead to protrud intestinal loops subcutaneously at the umbilical area (McGeady et al., 2006), gastroschisis where some intestinal loops were protruding via an abdominal wall defect right to the umbilicus (Christison-Lagay et al., 2011) and omphalocele. Based on the anatomical structure and feature of the protruded part, omphalocele or exomphalos (Raghavendran et al., 2020) was a defect characterized by the presence of a herniated sac hanging from an enlarged umbilical ring (McGeady et al., 2006). The sac was covered by a membrane which was composed of two layers, outer amnion and inner peritoneum (Christison-Lagay et al., 2011). In between them, there was Wharton’s jelly (Khan et al., 2019) which was a gelatinous connective tissue within the umbilical cord (Mitchell et al., 2003). The content of the sac showed some variations among the reported cases in different species. In agreement with the current findings, Fazili et al. (2014) reported loops of intestines only in the herniated sac of a lamb with omphalocele. However, in calves, the sac can have the intestines only (Fazili et al., 2016) or intestines with a portion of the liver (Baird, 2008; Raghavendran et al., 2020). Whereas in a goat kid, intestines, liver, and even spleen were all seen in the sac (Sharma et al., 2018). During the early embryonic development, the intestine elongated, and part of it herniated normally from the intra-embryonic coelom into a part of the extra-embryonic coelom (Fletcher and Weber, 2013), which was known as the umbilical sac (McGeady et al., 2006). In sheep, this physiological umbilical herniation was estimated to occur at around the third to the fourth week of the gestational period (McGeady et al., 2006). Afterward, the herniated intestinal loop returned into the intra-embryonic coelom or the abdominal cavity and the abdominal wall enclosed (Sharma et al., 2018). The congenital omphalocele occurred when the body folds disrupted and failed to close the abdominal wall during embryonic development (Noden and Lahunta, 1985; Watanabe et al., 2017; Raghavendran et al., 2020) and/or when the herniated loops failed to return into the abdominal cavity from the embryonic coelom (McGeady et al., 2006; Christison-Lagay et al., 2011; Sagar et al., 2011). The abdominal wall showed no abnormality in its morphological conformation in any of the presented lambs despite the unclosed and slightly wide umbilical ring. Perhaps the defect in these animals was more related to the inability to return back the intestine after being elongated. But without solid evidence, it might not be reasonable to exclude the effect of the improper closure of the abdominal wall. Unfortunately, proving the etiology of omphalocele seems to be faraway in the veterinary field, at least at the moment. Therefore, more scientific investigations are required to identify and clarify the pathogenesis of such an urgent defect in lambs. Omphalocele was an urgent condition that needed immediate surgical intervention for the newborns (Fazili et al., 2016; Sharma et al., 2018). Despite the prognosis being poor or good, the successful treatment depended on critical factors such as the status and size of the herniated part, the severity of the disrupted abdominal wall (Christison-Lagay et al., 2011; Sagar et al., 2011), the time of intervention after birth (Fazili et al., 2014), and the associated potential anomalies (Christison-Lagay et al., 2011). In both of the lambs, the herniated sac was soft and had only intestinal loops. Such content offered more flexibility during manipulation and reduction of the sac. If the liver with its gall bladder was displaced, there would be more difficulties, whether in reducing the sac or replacing this vital organ into its proper anatomical position. This is in addition to the risk of injuring the liver or its gall bladder during excising the sac (Christison-Lagay et al., 2011). Regarding the size of the herniated sac in relation to the available space in the abdominal cavity, the intra-abdominal pressure should be carefully considered during returning of the herniated contents and closure of the wall (Christison-Lagay et al., 2011). If this pressure was increased, then several complications might occur, such as acute hepatic congestion, renal failure, and intestinal infarction (Dunn and Fonkalsrud, 1997). In the first lamb, increasing the intra-abdominal pressure was excluded. The reasons for that were the relatively smaller size of the protruded sac and the normal abdominal conformation, which appeared capacious enough to contain the entire herniated portion without any complication. In contrast, the sac in the second case was proportionately huge in relation to the abdomen. The intestinal loops showed a high degree of contamination, congestion, and most likely ischemic necrosis. The complications of the large proportionate size and the severe congestion of the sac seem to be the reasons for the lamb not surviving. Omphalocele was not considered a hereditary case (Thieme, 1992; Davis et al., 2008; Raghavendran et al., 2020). Since it was assumed as a result of a defect in folding the abdominal wall, there were some developmental abnormalities that commonly accompanied the case, such as cardiac anomalies (Baird and MacDonald, 1981) and pulmonary hypoplasia (Kamata et al., 2008), as well as chromosomal abnormalities (Brantberg et al., 2005). On the other hand, some authors assumed the omphalocele was an inherited defect (Roberts, 1986) in which some factors such as inbreeding seemed to have an important role in its occurrence (Gutierrez et al., 1999). Obviously, there was no definite agreement among researchers about the actual etiology or even the mechanism of the resultant omphalocele as mentioned above. There is no doubt that the congenital defects caused many livestock losses and consequently affected the owner’s finance (Raghavendran et al., 2020). However, the available data about the representative prevalence of omphalocele in domestic animals was very scant (Baird, 1993). Moreover, while this defect was reported to be accompanied by other anomalies in humans (Baird and MacDonald, 1981; Kamata et al., 2008), the veterinary publications lacked any comprehensive descriptions of the potential associated congenital defects (Baird, 2008). Perhaps this significant shortage in information was because of unreported cases or even the inadequate description of the cases in the field (Raghavendran et al., 2020). Rather than the few available studies on the prevalence of omphalocele in domestic animals such as on calves (Mee, 1994) and cats (Robinson, 1990), there is no information about the prevalence of the defect in lambs in the literature. ConclusionIt can be concluded that the prognosis of omphalocele in lambsis dependent ona number of considerations, including the instant attendance of the ill newborn,the level of contamination and integrity of the sac, and the surgical intervention time. In addition, the severity of any associated anomalies, if present, would increase the life threats. The fundamental scientific information about omphalocele in the veterinary field is very scant; hence, further studies and investigations are required. ReferencesBaird, A.N. 2008. Umbilical surgery in calves. Vet. Clin. North Am. Food Anim. Pract. 24(3), 467–477. Baird, A.N. 1993. Omphalocele in two calves. J. Am. Vet. Med. Assoc. 202, 1481–1482. Baird, P.A. and MacDonald, E.C. 1981. An epidemiologic study of congenital malformations of the anterior abdominal wall in more than half a million consecutive live births. Am. J. Hum. Genet. 33, 470–478. Brantberg, A., Blaas, H.G., Haugen, S.E. and Eik-Nes, S.H. 2005. Characteristics and outcome of 90 cases of fetal omphalocele. Ultrasound Obstet. Gynecol. 26, 527–537. Christison-Lagay, E.R., Kelleher, C.M. and Langer, J.C. 2011. Neonatal abdominal wall defects. Semi. Fetal Neonat. Med. 16, 164–172. Davis, N.M., Kurpios, N.A., Sun, X., Gros,J., Martin, J.F. and Tabin, C.J. 2008. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev. Cell 15, 134–145. Dunn, J.C. and Fonkalsrud, E.W. 1997. Improved survival of infants with omphalocele. Am. J. Surg. 173, 284–287. Fazili, M.R., Bhattacharya, H.K., Dar, S.H. and Athar, H. 2014. Congenital omphalocele and its surgical Management in lamb. Egypt. J. Sheep Goats Sci. 9(1), 77–80. Fazili, M.R., Dar, S.H., Bhattacharya, H.K. and Rehman, M. 2016. Congenital omphalocele in four calves, their surgical management and outcome. Indian J. Anim. Res. 50(5), 816–819. Fletcher, T.F. and Weber, A.F. 2013. Veterinary developmental anatomy. Veterinary embryology—class notes. Available from http://vanat.cvm.umn.edu/WebSitesEmbryo.html Gray, S.W. and Skandalakis, J.E. 1972. Embryology for surgeons: the embryological basis for the treatment of congenital defects. Philadelphia, PA: Saunders. Gutierrez, C., Rodriguez, J.L., Sagrera,M.C.,Corbera, J.A. and Montoya, J.A. 1999. Two cases of schistosomusreflexus and two of omphalsocele in the canarian goat. J. Appl. Anim. Res. 15, 93–96. Hartwig, N.G., Vermeij-Keers, C.H., De Vries, H.E., Kagie, M. and Kragt, H. 1989. Limb body wall malformation complex: an embryologic etiology? Hum. Pathol. 20(11), 1071–1077. Kamata, S.N., Usui, N., Sawai, T., Nose, K. and Fukuzawa, M. 2008. Prenatal detection of pulmonary hypoplasia in giant omphalocele. Pediatr.Surg. Int. 24, 107–111. Khan, F.A., Hashmi, A. and Islam, S. 2019. Insights into embryology and development of omphalocele. Semi. Pediat. Surg. 28(2), 80–83. McGeady, T.A., Quinn, P.J., Fitz Patrick, E.S. and Ryan, M.T. 2006. Veterinaryembryology. Blackwell Publishing Ltd., pp: 205–224. Mee, J.F. 1994. Omphalocele in aborted and full-term dairy calves: a case series. Theriogenology 42, 1125–1131. Mitchell, K.E., Weiss, M.L., Mitchell, B.M., Martin, P., Davis, D., Morales, L., Helwig, B., Beerenstrauch, M., Abou-Easa, K., Hildreth, T. and Troyer, D. 2003. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 21, 50–60. Noden, D.M. and De Lahunta, A. 1985. Digestive system. In The Embryology of domestic animals. Baltimore, Maryland: Williams and Wilkins, pp: 292–311. Raghavendran, V.B., Rajasokkappan, S., Rajan, T. and Prabhu, R. 2020. Congenital exomphalos and its surgical correction in Holstein Friesian calf. J. Entomol. Zool. Stud. 8(5), 350–351. Roberts, S.J. 1986. Veterinary obstetric and genital diseases, 3rd ed. Woodstock, NY, pp: 78, 283, 335–336. Robinson, R. 1990. Genetics for dog breeders. Oxford, UK: Pergamon. Russo, R., D’Armiento, M., Angrisani, P. and Vecchione, R. 1993. Limb body wall complex: a critical review and a nosological proposal. Am. J. Med. Genet. 47(6), 893–900. Sagar, V.P., Vadde, K.S., Sai Krishna, S.K. and Venkateswarlu, S. 2011. An omphalocele in a buffalo calf: a case report. Buffalo Bull. 30(1), 10–11. Sharma, P., Kumar, S., Yadav, V.K. and Verma, S.P. 2018. Still birth with omphalocele (Congenital umbilical hernia) in fetus of goat. J. Entomol. Zool. Stud. 6(2), 2222–2224. Thieme, G. 1992. Developmental malformations of the fetal ventral body wall. Ultrasound Q. 10, 225–265. Watanabe, S., Suzuki, T., Hara, F., Yasui, T., Uga, N. and Naoe, A. 2017. Omphaloceleand gastroschisis in newborns: over 16 years of experience from a single clinic. J. Neonat. Surg. 6, 27. | ||
| How to Cite this Article |
| Pubmed Style Abdelhadi JM, Oheida A. Herniorrhaphy in two newborn lambs with omphalocele. Open Vet. J.. 2021; 11(4): 667-671. doi:10.5455/OVJ.2021.v11.i4.17 Web Style Abdelhadi JM, Oheida A. Herniorrhaphy in two newborn lambs with omphalocele. https://www.openveterinaryjournal.com/?mno=82495 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.17 AMA (American Medical Association) Style Abdelhadi JM, Oheida A. Herniorrhaphy in two newborn lambs with omphalocele. Open Vet. J.. 2021; 11(4): 667-671. doi:10.5455/OVJ.2021.v11.i4.17 Vancouver/ICMJE Style Abdelhadi JM, Oheida A. Herniorrhaphy in two newborn lambs with omphalocele. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 667-671. doi:10.5455/OVJ.2021.v11.i4.17 Harvard Style Abdelhadi, J. M. & Oheida, . A. (2021) Herniorrhaphy in two newborn lambs with omphalocele. Open Vet. J., 11 (4), 667-671. doi:10.5455/OVJ.2021.v11.i4.17 Turabian Style Abdelhadi, Jalal Mohamed, and Aiman Oheida. 2021. Herniorrhaphy in two newborn lambs with omphalocele. Open Veterinary Journal, 11 (4), 667-671. doi:10.5455/OVJ.2021.v11.i4.17 Chicago Style Abdelhadi, Jalal Mohamed, and Aiman Oheida. "Herniorrhaphy in two newborn lambs with omphalocele." Open Veterinary Journal 11 (2021), 667-671. doi:10.5455/OVJ.2021.v11.i4.17 MLA (The Modern Language Association) Style Abdelhadi, Jalal Mohamed, and Aiman Oheida. "Herniorrhaphy in two newborn lambs with omphalocele." Open Veterinary Journal 11.4 (2021), 667-671. Print. doi:10.5455/OVJ.2021.v11.i4.17 APA (American Psychological Association) Style Abdelhadi, J. M. & Oheida, . A. (2021) Herniorrhaphy in two newborn lambs with omphalocele. Open Veterinary Journal, 11 (4), 667-671. doi:10.5455/OVJ.2021.v11.i4.17 |