| Short Communication | ||
Open Vet. J.. 2021; 11(4): 598-602 Open Veterinary Journal, (2021), Vol. 11(4): 598–602 Short Communication Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horsesJosé da Costa Pimenta1*, Maria José Saavedra1,2,3, Gabriela Jorge da Silva4 and Mário Cotovio1,31Departamento de Ciências Veterinárias, ECAV, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal 2CITAB e Inov4Agro—Centro de Investigação e Tecnologias Agroambientais e Biológicas, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal 3CECAV e AL4AnimalS—Centro de Ciência Animal e Veterinária, Universidade de Trás-os- Montes e Alto Douro, Vila Real, Portugal 4Faculdade de Farmácia e Centro de Neurociências e Biologia Celular, Universidade de Coimbra, Portugal *Corresponding Author: José Pimenta. Departamento de Ciências Veterinárias, ECAV, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal. Email: josepimenta [at] utad.pt Submitted: 20/05/2021 Accepted: 07/10/2021 Published: 05/11/2021 © 2021 Open Veterinary Journal
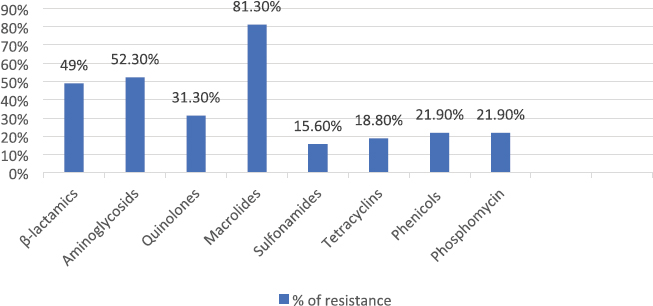
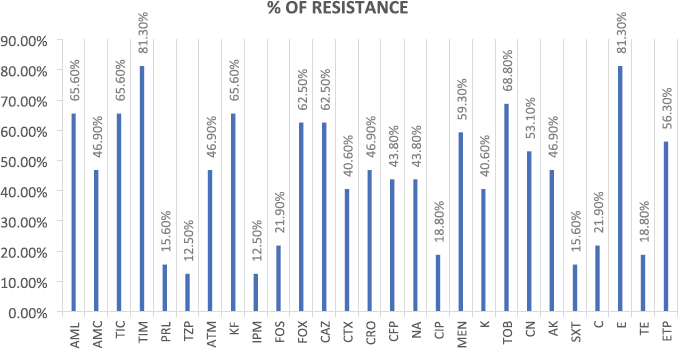
AbstractBackground: Many emergent pathogenic agents are cross-transmitted from animals to humans. Horses are considered as potential reservoirs of commensal, zoonotic, and multidrug-resistant bacteria. Equine bites could lead to infections caused by these agents, considering equine species as a public health concern. The more it is known about the equine oral microbiota the best secondary problems created by their commensal flora can be controlled. There are very few reports of Serratia rubidaea, a zoonotic and opportunistic bacterium, both in human and veterinary medicine. Aim: This study aimed to evaluate the Gram-negative microbiota of healthy equine oral cavities and their antimicrobial susceptibility. Methods: During equine routine oral procedures, eight healthy horses were selected for this study, after discarding any abnormal dental conditions. Samples were collected from the subgingival space and gingival margin from the tooth 406 and both the identification and antimicrobial susceptibility test of Gram-negative bacteria were performed. Results: This study reports the isolation of 32 Gram-negative agents, 27 of which were multidrug-resistant to the antimicrobial classes tested. High resistance rates were obtained to commonly used antimicrobial drugs, particularly macrolides and aminoglycosides as to carbapenems that are specific to human medicine. Two multi-drug resistance strains of S. rubidaea were found in the mouth of two healthy horses. Conclusion: Most Gram-negative isolates found in healthy horses were zoonotic and multi-drug resistant. This is a strong reason to consider the horse as an animal with a major place in the “One Health” concept. Equine clinicians should take precautions when working with horses’ mouths. Antimicrobial sensitivity tests should be taken into consideration when finding the appropriate antimicrobial therapy protocol. To the authors’ best knowledge, this is the first report about isolation of S. rubidaea from the mouth of the equine species. Keywords: Antimicrobial Resistance, Horse, Oral microbiota, Serratia. IntroductionAntimicrobial resistance is a global cause of concern due to the exponential development of multi drug resistance (MDR) to the antibiotic classes commonly used in clinical practice (Isgren, 2018; Nadeem et al., 2020). Horses are potential reservoirs of antimicrobial resistant bacteria, which can be cross transmitted with humans via human-horse contact, presenting this way a public health concern (Bourély et al., 2020). Furthermore, the transmission of resistance genes between human and animal isolates has already been shown (van Duijkeren et al., 2010; Dolejska et al., 2011). Therefore, it is important to identify and characterize equine zoonotic pathogens and their associated resistance phenotypes. However, multiple studies about the MDR of equine agents and their evolution will be necessary to estimate the potential public health problem that these animals can constitute (Bourély et al., 2020). Highly resistant bacterial isolates increase the risk of a delayed appropriate antimicrobial therapy increasing morbidity, mortality, and costs of treatment (Weese et al., 2015). Multidrug resistance, pathogenic, and zoonotic potential are factors that contribute to a higher interest in some bacteria species (Bindu et al., 2015; Maddox et al. 2015). Given that nearly 75% of emergent pathogenic agents are anthropozoonotic, affecting environment, human and animal health, the “One Health” approach seems a good way to face this problem (McEwen and Collignon, 2018; Nichol and Magnus, 2018). Serratia spp. are Gram-negative bacteria that usually cause opportunistic infections (Karkey et al., 2018). Serratia rubidaea is an opportunistic and zoonotic bacterium, not very well described in both veterinary and human medicine. It is mostly found in the environment (water, soil, and vegetables) and reports about its isolation are rare. Infections are mostly related with clinically debilitated individuals or secondary to invasive procedure and prolonged broad-spectrum antibiotic administration (Litterio et al. 2012; Karkey et al., 2018). Although rare, nosocomial infections with S. rubidaea are reported, presenting tropism to blood, respiratory and urinary tracts, which confers them significance in human medicine (Sekhsokh et al., 2007; Gentille et al., 2014). There is also a report about the presence of this agent in a wound infection after a horse bite (Litterio et al., 2012). This study reports the isolation of 32 Gram-negative agents, 27 of which were multidrug-resistant to the antimicrobial classes tested. We also describe the rate of resistance to equine and human clinically used antimicrobial drugs, in pathogens isolated from healthy horses’ mouths. Two multi-drug resistant strains of S. rubidaea were identified in the mouth of two healthy and unrelated horses. Materials and MethodsAnimalsRoutine oral examinations were performed in two different equestrian centres, 1 with 15 horses and another 1 with 18. Eight healthy adult horses of different breeds and ages, without any kind of oral disease and deprived of systemic or topical antimicrobial therapy in the last 6 months were selected for this study. After a regular physical exam, an oral examination was performed to discard any type of gingivitis, periodontitis, or any other pathological condition of the teeth and mouth. The entire procedure was conducted in accordance with the European Animal Welfare Directives (Directive 98/58/CE and Decreto-lei 64/2000). Sample collectionSamples were collected as previously described by Gao et al. (2016). The mouth was washed with a sterile saline solution to remove food accumulated. Then, using a sterile curette and a swab, a sample was taken from the subgingival space and gingival margin of the tooth 406. Samples were placed in tubes with Stuart transport medium, kept at 4°C, and sent to the Medical Microbiology Laboratory—Antimicrobials, Biocides and Biofilms Unit, Department of Veterinary Sciences, UTAD. Sample processingSamples were cultured in tubes with Brain Hearth Infusion (BHI) liquid medium and incubated at 36°C, for 24 hours. After this period, tubes that presented a turbid medium were considered as having a positive bacterial growth and Gram-negative selective and differential growth mediums [Pseudomonas Aeromonas Selective Agar, Chromocult Coliform Agar, MacConkey and BHI] were used for the isolation process. Species identificationAfter subculture, the isolates were inoculated into the specific identification cards of the automated VITEK® 2 system using the standard protocol: Gram-negative bacilli (ID-GNB) (bioMérieux). Susceptibility testingAntimicrobial susceptibility test was performed in Muller-Hinton agar using the disc diffusion test. The zones of growth inhibition were evaluated according to the recommendations of Clinical and Laboratory Standards Institute (CLSI, 2020), after an incubation of 24 hours at 36°C. Twenty-seven antimicrobials (AM) of eight different classes that include β-lactams, aminoglycosides, quinolones, macrolides, sulfonamides, tetracyclines, amphenicols, and phosphonic acid derivates group were tested. For counting effects, the intermedium (I) was considered as resistant (R) because of the lower antibiotic susceptibility. Strains exhibiting resistance to 3 or more categories were considered MDR. Ethical approvalNo ethical approval was needed for this study. Results and DiscussionAfter the isolation process, 32 Gram-negative isolates were identified, including Escherichia coli (n=14), Enterobacter cloacae complex (n=4), Klebsiella pneumoniae (n=3), S. rubidaea (n=2), Pseudomonas fluorescens (n=2), Enterobacter aerogenes (n=1), Kluyvera intermedia (n=1), Pantoea agglomerans (n=1), Pasteurella pneumotropica (n=1), Serratia plymuthica (n=1), Shigella sonnei (n=1), Sphingomonas paucimobilis (n=1). Twenty-seven isolates were MDR. The phenotypic resistance presented by antibiotics classes was higher to macrolides (81.3%) and aminoglycosides (52.3%) and lower to sulfonamides (15.6%) and tetracyclines (18.8%) (Fig. 1). Concerning percentage of resistance to each antimicrobial, Figure 2 illustrates that the overall results were elevated, with the higher percentage of resistance corresponding to erythromycin and ticarcillin-clavulanic acid, both with 81.3%, while piperacillin-tazobactam and imipenem have the lower percentage, both with 12.5%. Table 1 contemplates resistance rates of some isolates toward AM classes tested. Only bacterial species with at least two tested isolates are shown. All the agents show high resistance rates to macrolides and β-lactams while K. pneumoniae was the only with strong resistance to sulfonamides. Although some factors such as treatment with AM or hospitalization have been identified as risk factors for the development of resistance (Damborg et al., 2012), none of the horses included in this study had been submitted to any antimicrobial therapy in the previous six months. Furthermore, some horses had never had contact with an antimicrobial drug in their lives. As mentioned by Spijk et al. (2016), β-lactams are one of the most important AM categories in veterinary medicine; therefore, the high percentage of resistance makes antimicrobial therapy substantially less effective. Resistance to carbapenems, specially to ertapenem and meropenem is of critical concern to human medicine when facing a cross-transmitted agent infection.
Fig. 1. Percentage of resistance to antimicrobial classes tested. Upon our results, we recommend sulfonamides as the first choice of antimicrobial treatment (Fig. 1). Although there are many studies describing antimicrobial resistance in equine medicine, as far as authors know, none of them focus on the oral microbiota. Furthermore, bacterial resistance profiles change rapidly over time (Canton et al., 2008), remarking the importance of continuous surveillance. The more we know about equine oral microbiota as well the related patterns of resistance, the better we can implement an antimicrobial protocol for some diseases. Moreover, the potential for human to be exposed to resistant bacteria via close contact with horses is a problem that has received much attention (Maddox et al., 2012) Serratia rubidaea strains belong to samples collected from two horses from different stables. Their pattern of resistance demonstrates a phenotypic resistance to all the macrolides, tetracycline and meropenem, one of the carbapenems tested. One of the isolates presented resistance to all the aminoglycosides tested. Serratia rubidaea has already been isolated by Litterio et al. (2012) from an infected wound created by a horse bite in a 2-year-old boy. It is now possible to consider that the origin of this agent could be the horse’s mouth instead of the contamination with soil particles found in the wound. The possibility of wound infection by MDR S. rubidaea after a horse’s bite should be considered in human medicine, particularly in ridders or horse owners. Serratia rubidaea has also been reported as the cause of bacteraemia in a 15-year-old patient by Gentille et al. (2014), and in a 54-year-old patient by Sekhsokh et al. (2007). The Serratia rubidaea MDR profile should be considered in human medicine in cases of bacteraemia caused by this agent. The antimicrobial protocol used should be prudent and always based on microbiologic culture and in vitro antimicrobial susceptibility testing to make sure what is the best antibiotic for that strain. As far as this study goes, sulfonamides, amphenicols, and fosfomycin are good antimicrobial options. Table 1. Resistance rates toward AM classes tested. Only bacterial species with at least 2 tested isolates are shown.
Fig. 2. Percentage of resistance of each antimicrobial. (AML10): amoxicillin; (AMC30): amoxicillin/clavulanate; (TIC75): ticarcillin; (TIM85): ticarcillin-clavulanic acid; (PRL100): piperacillin; (TZP110): piperacillin-tazobactam; (ATM30): aztreonam; (KF30): cephalothin; (IMP10): imipenem; (FOS50): Fosfomycin; (FOX30): cefoxitin; (CAZ30): ceftazidime; (CTX30): cefotaxime; (CRO30): ceftriaxone; (CFP30): cefoperazone; (NA30): nalidixic acid; (CIP5): ciprofloxacin; (MEM10): meropenem; (K30): kanamycin; (TOB10): tobramycin; (CN10): gentamicin; (AK30): amikacin; (SXT25): trimethoprim-sulfamethoxazole; (C30): chloramphenicol; (E15): erythromycin; (TE30): tetracycline; (ETP10): ertapenem. Although rare, these infections can occur, and the etiologic agent could be cross-transmitted from the horse’s oral cavity with the particular concern of the massive MDR of these strains. Serratia rubidaea could make part of equine oral microbiome probably because of its presence in water, vegetables and soil. All individuals that work with horses, mostly veterinarians and dentists, which are in close contact with the horse’s mouth, should take these results into account and assure good clinical practices like the use of gloves during an oral examination. As mentioned by Spijk et al. (2016), these kind of studies aid practitioners in making an evidence based antimicrobial choice when prompt therapy is needed. The fact that all these zoonotic and MDR agents were found in healthy horses from two distinct equestrian centers, that were not submitted to antimicrobial treatment in the previous 6 months, is a strong reason to consider the horse as an animal with a major place in the “One Health” concept. Given the high number of MDR isolates (n=27), this study praises the importance of the antimicrobial sensitivity tests to adjust an antimicrobial protocol preventing the development of resistances. To the best of authors’ knowledge, this is the first report of the isolation of S. rubidaea from the mouth of healthy horses, and with resistance to antibiotics clinically relevant in human medicine which are used only in hospital settings (e.g., meropenem). AcknowledgementsThis work is supported by National Funds by FCT—Portuguese Foundation for Science and Technology, under the projects UIDB/04033/2020 and UIDP/04033/2020 (CITAB-Center for the Research and Technology of Agro-Environmental and Biological Sciences) and Associate Laboratory Inov4Agro-Institute for Innovation, Capacity Building and Sustainability of Agri-food Production. The research unit CECAV- Animal and Veterinary Research Centre and Associate Laboratory for Animal and Veterinary Science - AL4AnimalS received funding from the FCT, reference of the project UIDB/CVT/0772/2020. Conflict of interestThe authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors’ contributionMJS and MC contributed conception and design of the study; JP and MC collected the samples; JP, MJS, GS and MC performed all the laboratory assays; JP wrote the first draft of the manuscript; JP, MJS, GS and MC critically revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. ReferencesBindu, B.S., Mishra, D.P. and Narayan, B. 2015. Inhibition of virulence of Staphylococcus aureus—a food borne pathogen—by squalene, a functional lipid. J. Funct. Foods 18, 224–234. Bourély, C., Cazeau, G., Jarrige, N., Haenni, M., Gay, E. and Leblond, A. 2020. Antimicrobial resistance in bacteria isolated from diseased horses in France. Equine Vet. J. 52, 112–119. Canton, R., Novais, A., Valverde, A., Machado, E., Peixe, L., Baquero, F. and Coque, T. 2008. Prevalence and spread of extended- spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14, 144–153. Clinical and Laboratory Standards Institute (CLSI). 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. Wayne, PA: CLSI. Damborg, P., Marskar, P., Baptiste, K. and Guardabassi, L. 2012. Faecal shedding of CTX-M-producing Escherichia coli in horses receiving broad spectrum antimicrobial prophylaxis after hospital admission. Vet. Microbiol. 154, 298–304. Dolejska, M., Duskova, E., Rybarikova, J., Janoszowska, D., Roubalova, E., Dibdakova, K., Maceckova, G., Kohoutova, L., Literak, I., Smola, J. and Cizek, A. 2011. Plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli isolates from an equine clinic and a horseback riding centre. J. Antimicrob. Chemother. 66, 757–764. Gao, W., Chan, Y., You, M., Lacap-Bugler, D., Leung, W. and Watt, R. 2016. In-depth snapshot of the equine subgingival microbiome. Microb. Pathog. 94, 76–89. Gentille D., Pérez M. and Centelles M. 2014. Bacteremia by a Serratia rubidaea with an atypical quinolones resistance phenotype. Rev. Chilena Infectol. 31, 351–352. Isgren, C. 2018. Antimicrobial resistance in horses. Vet. Rec. 183, 316–318. Karkey, A., Joshi, N., Chalise, S., Joshi, S., Shrestha, S., Nguyen, T., Dongol, S., Basnyat, B., Baker, S. and Boinett, C. 2018. Outbreaks of Serratia marcescens and Serratia rubidaea bacteremia in a central Kathmandu hospital following the 2015 earthquakes. Trans. R. Soc. Trop. Med. Hyg. 112, 467–472. Litterio, M., Arazi, S., Hernández, C. and Lopardo, H. 2012. Isolation of Serratia rubidaea from a mixed infection after a horse bite. Rev. Argent. Microbiol. 44, 272–274. Maddox, T., Clegg, P., Diggle, P., Wedley, A., Dawson, S., Pinchbeck, G. and Williams, N. 2012. Cross-sectional study of antimicrobial-resistant bacteria in horses. Part 1: Prevalence of antimicrobial resistant Escherichia coli and methicillin-resistant Staphylococcus aureus. Equine Vet. J. 44, 289–296. Maddox, T., Clegg, P., Williams, N. and Pinchbeck, G. 2015. Antimicrobial resistance in bacteria from horses: epidemiology of antimicrobial resistance. Equine Vet. J. 47, 756–765. McEwen, S. and Collignon, P. 2018. Antimicrobial Resistance: a “One Health” Perspective. Microbiol. Spectr. 6, 1–26. Nadeem, S., Gohar, U., Tahir, S., Mukhtar, H., Pornpukdeewattana, S., Nukthamna, P., Ali, A., Bavisetty, S. and Massa, S. 2020. Antimicrobial resistance: more than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 46, 578–599. Nichol, A. and Magnus, D. 2018. The “One Health” Approach to Zoonotic Emerging Infectious Diseases. Am. J. Bioeth. 18, 1–2. Sekhsokh, Y., Arsalane, L., Ouenass, M., Doublali, T., Bajjou, T. and Amine, I. 2007. Bactériémie à Serratia rubidaea [Serratia rubidaea bacteremia]. Med. Mal. Infect. 37, 287–289. Spijk, J., Schmitt, S., Fürst, A. and Schoster, A. 2016. A retrospective analysis of antimicrobial resistance in bacterial pathogens in an equine hospital (2012–2015). Schweiz. Arch. Tierheilkd. 158, 433–442. van Duijkeren, E., Moleman, M., Oldruitenborgh-Oosterbaan, M.M., Multem, J., Troelstra, A., Fluit, A.C., Wamel, W., Houwers, D., Neeling, A. and Wagenaar, J. 2010. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: an investigation of several outbreaks. Vet. Microbiol. 141, 96–102. Weese, J., Giguere, S., Guardabassi, L., Morley, P., Papich, M., Ricciuto, D. and Sykes, J. 2015. ACVIM Consensus Statement on Therapeutic Antimicrobial Use in Animals and Antimicrobial Resistance. J. Vet. Intern. Med. 29, 487–498. | ||
| How to Cite this Article |
| Pubmed Style Pimenta J, Saavedra MJ, Silva G, Cotovio M. Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Vet. J.. 2021; 11(4): 598-602. doi:10.5455/OVJ.2021.v11.i4.9 Web Style Pimenta J, Saavedra MJ, Silva G, Cotovio M. Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. https://www.openveterinaryjournal.com/?mno=82710 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.9 AMA (American Medical Association) Style Pimenta J, Saavedra MJ, Silva G, Cotovio M. Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Vet. J.. 2021; 11(4): 598-602. doi:10.5455/OVJ.2021.v11.i4.9 Vancouver/ICMJE Style Pimenta J, Saavedra MJ, Silva G, Cotovio M. Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 598-602. doi:10.5455/OVJ.2021.v11.i4.9 Harvard Style Pimenta, J., Saavedra, . M. J., Silva, . G. & Cotovio, . M. (2021) Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Vet. J., 11 (4), 598-602. doi:10.5455/OVJ.2021.v11.i4.9 Turabian Style Pimenta, Jose, Maria Jose Saavedra, Gabriela Silva, and Mario Cotovio. 2021. Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Veterinary Journal, 11 (4), 598-602. doi:10.5455/OVJ.2021.v11.i4.9 Chicago Style Pimenta, Jose, Maria Jose Saavedra, Gabriela Silva, and Mario Cotovio. "Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses." Open Veterinary Journal 11 (2021), 598-602. doi:10.5455/OVJ.2021.v11.i4.9 MLA (The Modern Language Association) Style Pimenta, Jose, Maria Jose Saavedra, Gabriela Silva, and Mario Cotovio. "Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses." Open Veterinary Journal 11.4 (2021), 598-602. Print. doi:10.5455/OVJ.2021.v11.i4.9 APA (American Psychological Association) Style Pimenta, J., Saavedra, . M. J., Silva, . G. & Cotovio, . M. (2021) Multidrug-resistant Serratia rubidaea strains in the oral microbiota of healthy horses. Open Veterinary Journal, 11 (4), 598-602. doi:10.5455/OVJ.2021.v11.i4.9 |