| Review Article | ||
Open Vet. J.. 2022; 12(2): 256-263 Open Veterinary Journal, (2022), Vol. 12(2): 256–263 Review Article Transcranial photobiomodulation (laser) therapy for cognitive impairment: A review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD)Curtis Wells Dewey1,2*, Matthew Warren Brunke1,3 and Kasie Sakovitch11Elemental Pet Vets, PLLC, Freeville, New York, USA 2Chi University, Reddick, Florida, USA 3Veterinary Surgical Centers Rehabilitation, Vienna, Virginia, USA *Corresponding Author: Curtis Wells Dewey. Elemental Pet Vets, PLLC, 1610 Dryden Road, Freeville, NY 13068, USA. Email: elementalpetvets [at] outlook.com Submitted: 28/02/2022 Accepted: 09/04/2022 Published: 17/04/2022 © 2022 Open Veterinary Journal
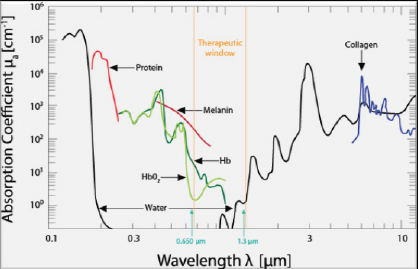
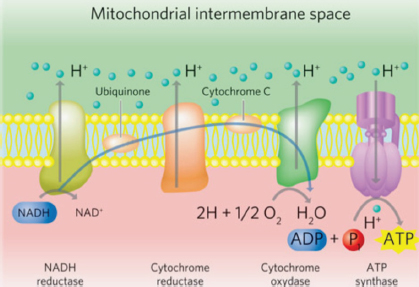
AbstractAlzheimer’s disease (AD) is a common degenerative brain disorder of aging people which shares many clinical and pathological features with canine cognitive dysfunction (CCD). CCD is considered a naturally occurring model of human AD. Transcranial photobiomodulation therapy (tPBMT), also known as transcranial laser therapy, entails delivering photons of near infrared to infrared light from the skin surface of the scalp to the underlying brain. Specific molecular cellular receptors, called chromophores, absorb this energy, and use it to initiate biological reactions with potential therapeutic benefit. Improvement in cognitive ability using tPBMT has been documented in rodent AD models and human clinical trials. The purposes of this review are to provide an overview of the suspected molecular mechanisms of action of tPBMT for the treatment of cognitive decline and to propose potential application of this treatment modality for dogs affected by CCD. Keywords: Canine, Cognitive, Dysfunction, Alzheimer’s, Photobiomodulation. IntroductionCanine cognitive dysfunction (CCD) is a naturally occurring degenerative brain disorder of aging dogs, analogous to human Alzheimer’s disease (AD) (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019). The pathophysiology of AD/CCD is multifactorial, including brain vascular compromise, neuronal mitochondrial dysfunction, deposition of toxic β-amyloid protein around neurons and blood vessels, oxidative brain damage, and inflammation. These interrelated processes lead to the progressive loss of dendrites, synapses, and neurons over time, with subsequent cognitive decline (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019). As with AD, CCD has no cure and there is a continual need for therapeutic options to mitigate and reverse the effects of the disease process. Photobiomodulation therapy (PBMT), also called laser therapy, is a popular therapeutic modality applied to numerous veterinary disorders. Delivery of photons of light energy in the near infrared to infrared wavelength range (approximately 600–1,200 nm) to tissues (Fig. 1) stimulates molecules called photoacceptors in cells; these molecules contain chromophores capable of absorbing specific wavelengths of light. Once stimulated, the chromophores convert the light energy into biological reactions with potentially beneficial effects. The primary photoacceptor in living tissues believed to be responsible for most PBMT-stimulated biological effects is cytochrome c oxidase (CCO). CCO is a polypeptide enzyme located in the inner mitochondrial membrane; it is an enzyme complex (complex IV) near the termination of the electron transport chain (oxidative phosphorylation), responsible for production of adenosine triphosphate (ATP), nitric oxide (NO), and several reactive oxygen (ROS) molecules (Fig. 2). In general, the positive effects of PBMT include decreased inflammation, decreased pain, and accentuated wound healing (Anders et al., 2017; Riegel and Godbold, 2017; Stephens, 2019). Transcranial photobiomodulation therapy (tPBMT) refers to the delivery of laser photons through the scalp and skull to reach the underlying brain tissue. Multiple molecular mechanisms of action for tPBMT for treatment of AD have been elucidated, with biological effects that include increased neuronal mitochondrial energy production (ATP synthesis), improved brain blood flow, reduction of brain β-amyloid load and deposition, attenuation of dendrite and neuronal loss, and reduced inflammatory and oxidative neuronal injury (Lapchak, 2012; Barrett and Gonzalez-Lima, 2013; Gonzalez-Lima et al., 2014; De la Torre, 2016, 2017, 2020; Hamblin, 2016, 2019; Saltmarche et al., 2017; Hennessy and Hamblin, 2017; Enengl et al., 2020). In addition to direct and indirect molecular mechanisms mediated by CCO stimulation, several non-CCO-mediated molecular mechanisms of action for tPBMF have been discovered with potential beneficial effects to cognitive function (Lapchak, 2012; Hennessy and Hamblin, 2017; Hamblin, 2019; Sommer, 2019; De la Torre et al., 2020; Enengl et al., 2020).
Fig. 1. The therapeutic window for PBMT. Source: Riegel and Godbold (2017). Reprinted with permission.
Fig. 2. The mitochondrial electron transport chain. Source: Stephens (2019). Reprinted with permission. Mechanisms of actionMitochondrial effects of tPBMTNeuronal cellular energy failure due to mitochondrial dysfunction has been recognized as an early event in age-related cognitive decline. Both decreased cerebral blood flow and toxic effects of Aβ contribute to mitochondrial dysfunction (Landsberg et al., 2012; De la Torre, 2017, 2020; Da Luz Eltchechem et al., 2017; Chapagain et al., 2018; Dewey et al., 2019). Neuronal mitochondria are the primary source of the brain’s energy requirements via production of ATP. In addition to their role in neuronal energy production, functional mitochondria can mitigate the toxic effects of Aβ on neuronal cellular function. As mitochondrial function declines, the adverse effects of Aβ on cellular function (including that of the mitochondria) worsens (Gonzalez-Lima et al., 2014; Da Luz Eltchechem et al., 2017; De la Torre, 2017, 2020; Lu et al., 2017). Mitochondrial CCO has two subunits that contain copper (CuA and CuB) and these metal ions are responsible for reducing molecular oxygen to water, generating ATP in the process. These metallic components represent the chromophores of CCO and—when stimulated by appropriate wavelengths of light—cause accelerated electron transfer reactions of the cellular respiratory apparatus. The two absorption peaks for the stimulation of mitochondrial CCO are approximately 660 and 810 nm (Lapchak, 2012; Hamblin, 2016, 2019; Anders et al., 2017; Salehpour et al., 2017; Enengl et al., 2020). In addition to evidence that tPBMT at appropriate wavelengths stimulates neuronal mitochondrial ATP production via direct activation of CCO, there is also evidence that levels of CCO are amplified (Gonzalez-Lima et al., 2014; De la Torre, 2017, 2020; Salehpour et al., 2017; Hamblin, 2019). Additionally, tPBMT triggers a process called retrograde mitochondrial signaling, in which the stimulated mitochondria signal the cell nucleus to alter gene expression in order to augment mitochondrial function, as well as generate new mitochondria (biogenesis) (Hennessy and Hamblin, 2017; Hamblin, 2019). Another beneficial effect on mitochondrial function related to tPBMT has been demonstrated that is not CCO-dependent. Microscopic water layers adjacent to the inner mitochondrial membrane, referred to as interfacial water layers (IWL), become more viscous under conditions of oxidative stress (as occurs with AD/CCD). This viscosity impedes the function of ATP synthase, the final step in the oxidative respiratory chain. Application of PBMT at a wavelength of 670 nm has been shown to decrease IWL viscosity with subsequent improvement in mitochondrial ATP synthase function and cellular ATP production (Hamblin, 2019; Sommer, 2019). Effects of tPBMT on beta-amyloid (Aβ) loadAccumulation of the neurotoxic protein Aβ in brains of AD/CCD patients is well established. Formerly thought of as a primary aspect of the disease process, more recent evidence suggests that Aβ deposition is a consequence of other factors, such as vascular compromise and inflammation (Dewey et al., 2019; Hamblin, 2019). Furthermore, there is growing evidence that pathological alteration in the gastrointestinal microbial distribution (dysbiosis) is a major inciting factor for brain vascular damage and inflammation in AD/CCD; since Aβ is a known antimicrobial protein, its accumulation in AD/CCD may be a response to microbes and/or their toxins (Ambrosini et al., 2019; Dewey and Rishniw, 2021). Amyloid precursor protein (APP) is the source of Aβ, which is preferentially produced when β-secretase and γ-secretase activities predominate over α-secretase activity (as occurs with AD/CCD) (De Taboada et al., 2011; Hamblin, 2019). Aβ is known to trigger the production of reactive oxygen and nitrogen species, as well as several proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α), all of which can lead to cellular damage. Aβ accumulation also leads to neuronal mitochondrial damage. Another detrimental action of Aβ is stimulating premature neuronal cell death (apoptosis) via various signaling pathways (De Taboada et al., 2011; Da Luz Eltchechem et al., 2017; Hamblin, 2019). Aβ can also cause neuronal damage via increasing formaldehyde (FA) levels in the brain. FA accumulates in AD brains due to decreased activity of formaldehyde dehydrogenase (FDH), the enzyme responsible for clearing FA. Aβ is thought to inactivate FDH and, in turn, elevated FA stimulates increased accumulation of Aβ (Huang et al., 2020). In vitro and in vivo studies have demonstrated the ability of PBMT to cause disaggregation of Aβ (Yagi et al., 2010; De Taboada et al., 2011; Da Luz Eltchechem et al., 2017; Yue et al., 2019; Zhang et al., 2019; Tao et al., 2021). There are several mechanisms via which tPBMT is believed to decrease brain Aβ load in AD/CCD. Such mechanisms include suppression of β-secretase activity and stimulation of enzymes responsible for degrading Aβ peptides (De Taboada et al., 2011; Zhang et al., 2019). Microglia have been shown to play a pivotal role in the generation and dissolution of Aβ in rodent AD models. There are two main types of activated microglia, designated as M1 and M2. M1 microglia are elevated in AD and are responsible for producing pro-inflammatory cytokines, disruption of the blood–brain barrier, neuronal damage, and ultimately cognitive decline. M2 microglia are considered neuroprotective, releasing anti-inflammatory cytokines, and enhancing neurotrophic factor release (Tang and Le, 2016). PBMT has been shown to shift the balance of microglia to the M2 phenotype in rodent models (Hamblin, 2019; Tao et al., 2021). In one study involving a mouse AD model, tPBMT application with 1,070 nm light led to decreased brain levels of Aβ and improvement of cognitive test scores. This study also showed a decrease in the M1 microglia population (particularly perivascularly), increased cerebral vascular density, and increased localization of microglia around brain Aβ depositions. These results suggest that tPBMT reduces brain amyloid load via modifying microglial activity, most likely via increased Aβ phagocytosis and improved blood supply (angiogenesis) (Tao et al., 2021). In another rodent study of AD, tPBMT at 630 nm wavelength decreased Aβ deposition and improved memory via activation of FDH, subsequently diminishing FA and Aβ accumulation (Huang et al., 2020). Another mechanism of tPBMT-mediated reduction of brain Aβ levels was elucidated in a mouse AD model, in which 632.8 nm wavelength light caused a shift of APP to a non-amyloidogenic pathway, reducing Aβ load and improving cognition and memory. In this study, it was found that tPBMT led to CCO-mediated activation of a sirtuin protein (SIRT-1), which downregulated activity of β-secretase (Zhang et al., 2019). Aβ-induced neuronal apoptosis is facilitated by the enzyme glycogen synthase kinase 3β (GSK3β). An important transcription factor that promotes cellular survival, β-catenin, is inactivated when phosphorylated by GSK3β. An in vitro cell culture study showed that PBMT exposure of cells to 632.8 nm wavelength light inhibited GSK3β activity with subsequent increase in β-catenin levels and inhibition of Aβ-induced apoptosis (Liang et al., 2012). tPBMT and brain-derived neurotrophic factor (BDNF)BDNF is a central nervous system (CNS) signaling peptide crucial to maintenance and preservation of synapses and neurons in the brain, as well as neurogenesis (Hu and Russek, 2008; Zuccato and Cattaneo, 2009; Hennessy and Hamblin, 2017; Hamblin, 2019). A primary site of neurogenesis is the hippocampus, which undergoes atrophy in AD/CCD (Halliday, 2017; Dewey et al., 2020). In addition to its regulatory role in the normal brain, BDNF has demonstrated neuroprotective effects against Aβ-mediated neurotoxicity (Arancibia et al., 2008). In both animal models of AD and brains of AD patients, a deficiency of BDNF and BDNF messenger RNA in key areas of the brain (e.g., hippocampus and cerebral cortex) has been identified (Hu and Russek, 2008; Arancibia et al., 2008; Zuccato and Cattaneo, 2009). In rodent and primate AD models, delivery of BDNF to the brain via gene therapy or infusion of BDNF protein resulted in reversal of synaptic and neuronal loss and improvement in cognitive function (Nagahara et al., 2009). In an in vitro study, mouse hippocampal neurons damaged by Aβ were rescued by BDNF upregulation induced by exposure to PBMT at 632.8 nm wavelength. In this study, it was determined that PBMT exerted its effect via inducing a transcription factor called cyclic AMP response element binding protein (CREB); CREB stimulated increased transcription of BDNF mRNA and BDNF protein production via a kinase called extracellular signal regulated kinase (Meng et al., 2013). Anti-inflammatory and antioxidant effects of tPBMTNeuroinflammation and oxidative stress are prominent pathologic processes contributing to neuronal loss and cognitive decline in AD/CCD (Gonzalez-Lima et al., 2014; Hennessy and Hamblin, 2017; Chapagain et al., 2018; Dewey et al., 2019; Hamblin, 2019). There are numerous pro-inflammatory pathways responsible for these processes, some of which were previously described (e.g., Aβ-mediated inflammation, microglial-mediated inflammation, etc.). In one study involving human gingival fibroblast cells, PBMT at 635 nm was shown to inhibit both cyclo-oxygenase 2 and the NF-kB (nuclear factor kappa light chain enhancer of activated B cells) transcription pathway, leading to an anti-inflammatory effect (Lim et al., 2013). One mechanism of oxidative injury in AD is Aβ-induced activation of nicotinamide adenine dinucleotide phosphate oxidase in brain astrocytes and microglia, leading to superoxide anion production; these anions subsequently cause damage to adjacent neurons. In addition to this direct damage, ROS like superoxide anion leads to activation of cytosolic phospholipase A2 (cPLA2). cPLA2 activation leads to mitochondrial damage, with further production of damaging ROS molecules. In a study of cultured rat astrocytes exposed to Aβ, it was shown that stimulating the cells with PBMT at 632.8 nm light suppressed both superoxide anion production and activation of cPLA2 (Yang et al., 2010). Effects of tPBMT on brain blood flowDiminished cerebral blood flow is a key pathological feature of AD/CCD that begins early in the disease process (De la Torre, 2016, 2017, 2020; Chapagain et al., 2018; Dewey et al., 2019). PBMT has been shown to improve blood flow via stimulating NO production, as well as by promoting angiogenesis (Chen et al., 2008; Hennessy and Hamblin, 2017; Hamblin, 2019; De la Torre et al., 2020). NO is an important vasodilator and PBMT can increase its production by both mitochondrial stimulation and stimulating nitric oxide synthase (NOS), the enzyme necessary for NO production in tissues (Chen et al., 2008; De la Torre et al., 2020). Transcranial PBMT at 808 nm wavelength was shown in a mouse model to increase cerebral blood flow by 30%, presumably via stimulating NOS and subsequent NO production (Uozumi et al., 2010). Vascular endothelial growth factor (VEGF) is a peptide signaling molecule that has a central role in angiogenesis. Augmented VEGF expression and increased angiogenesis was demonstrated in a rat ischemic skin flap model in which the tissue was exposed to PBMT at wavelengths of 660 and 780 nm (Cury et al., 2013). tPBMT as a potential therapy for CCDTo the authors’ knowledge, there are no clinical reports regarding the use of tPBMT for the treatment of CCD. Although the ideal parameters and treatment frequency regarding tPBMT for AD/CCD are not well established, there are data from rodent AD studies and human clinical trials that can be used to formulate a tPBMT protocol for CCD (Barrett and Gonzalez-Lima, 2013; Naeser et al., 2014; Saltmarche et al., 2017; Salehpour et al., 2017; Chan et al., 2018). Important parameters to consider when devising a tPBMT protocol include wavelengths (nm), dose (Joules per square centimeter; J/cm2), power density (watts per square centimeter; W/cm2), and whether the light should be delivered as a continuous wave (CW) or pulsed wave (PW) (Stephens, 2019). Multiple wavelengths of light have been investigated as candidates for tPBMT, primarily those at or near the wavelengths of 660, 810, 980, and 1,064 nm. The two wavelengths thought to be most potentially beneficial, based on penetration depth into the brain, are approximately 660 and 810 nm (Tedford et al., 2015; Wang and Li, 2018). As mentioned previously, these two wavelengths match the two absorption peaks for mitochondrial CCO (Lapchak, 2012; Hamblin, 2016, 2019; Anders et al., 2017; Salehpour et al., 2017; Enengl et al., 2020). There is no consensus on the optimum dose of tPBMT delivery; however, an approximate dose for rodents of 10 J/cm2 and a range of 25–60 J/cm2 for people have been approximated based on various studies (Gonzalez-Lima and Barrett, 2014; Salehpour et al., 2017; Enengl et al., 2020). Higher power densities have the advantage of decreasing treatment time and improving penetration into tissues (Stephens, 2019). There is an increased risk of tissue heating with higher power density, but this rarely represents a clinical problem. There is some evidence that PW delivery of tPBMT is more effective than CW delivery (Hashmi et al., 2010; Enengl et al., 2020). One potential advantage of PW tPBMT delivery is the ability to use higher power densities (with greater penetration) while mitigating thermal effects (Hashmi et al., 2010). The ideal treatment frequency for tPBMT is yet another unknown. Based on some studies, treatment (at least initially) three times per week seems warranted (Naeser et al., 2014; Salehpour et al., 2017). These experimental studies were conducted for a finite time period (6 weeks). Long-term treatment protocols for tPBMT for cognitive impairment remain to be determined. In addition to technical considerations, there are several practical considerations to address when adapting tPBMT to dogs with CCD. Veterinary practices typically have high power medical lasers (class 3b or class 4) that require expertise and training to apply safely; lasers of these categories can treat patients very quickly, even if multiple regions require therapy (e.g., polyarthropathy). These lasers can cause thermal and retinal damage and are not suitable for home use (Bartels, 2017; Riegel and Godbold, 2017). Because of the proximity of the laser source to the eyes, the authors recommend protective eyewear for the patient when using higher power lasers (Fig. 3). It is unlikely that most pet owners will be able to transport their pets to a facility to receive tPBMT several times per week for an extended time period. Several wearable devices have been developed for treating people with cognitive decline. These devices deliver tPBMT via light-emitting diodes (LED) that contact the surface of the scalp; LED devices are very low power, compared to conventional medical lasers, so treatment sessions are fairly long (25–30 minutes) (Naeser et al., 2014; Hennessy and Hamblin, 2017; Saltmarche et al., 2017; Hamblin, 2019; Huang, et al., 2020). Similar devices have not been developed for dogs. Such devices would need to be available in several head sizes and may not be tolerated for the length of required treatment time in CCD patients. An alternative would be to have pet owners deliver tPBMT to their dogs at home with a small, hand-held laser unit of a lower power. Class 1 lasers do not pose the threat thermal or retinal damage and can be safely used at home by pet owners (Fig. 4). Although recommended tPBMT doses cannot be delivered as rapidly with class 1 lasers compared with higher powered devices, the authors estimate treatment times of 5–10 minutes, which may be practical for at home use. It is important to emphasize that such treatment times are estimates and that clinical trials are imperative to establish effective clinical protocols.
Fig. 3. Application of tPBMT to a dog using a class 4 laser. This class of laser should be used only by trained veterinary personnel.
Fig. 4. Application of tPBMT to a dog using a class 1 laser (photo courtesy of Iker Asteinza DVM). ConclusionTranscranial photobiomodulation therapy is a promising new treatment modality for patients with cognitive decline. It has shown efficacy in rodent AD models and human clinical trials and may be adaptable to CCD. Beneficial mechanisms of action for tPBMT for AD/CCD include increased energy (ATP) production via mitochondrial stimulation, reducing brain beta-amyloid (Aβ) load and adverse effects of this toxic protein, stimulating brain levels of BDNF, mitigating neuroinflammation and oxidative brain damage, and improving brain blood flow. Optimum dosing parameters and treatment frequency for tPBMT in CCD patients are unknown but data from rodent AD studies and human clinical trials can be used to approximate a logical protocol. Evaluating tPBMT in dogs afflicted with CCD will likely require at home therapy with an easily portable tPBMT delivery system that can deliver the necessary dose of light energy in a short time period. Future clinical studies evaluating tPBMT as a treatment modality for CCD will be necessary to devise short- and long-term protocols, as well as to evaluate the safety and efficacy of such protocols. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAmbrosini, Y.M., Borcherding, D., Kanthasamy, A., Kim, H.J., Willette, A.A., Jergens, A., Allenspach, K. and Mochel, J.P. 2019. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front. Aging Neurosci. 11, 1–14. doi: 10.3389/fnagi.2019.00130. Anders, J.J., Ketz, A.K. and Wu, X. 2017. Basic principles of photobiomodulation and its effects at the cellular, tissue, and system levels. In Laser therapy in veterinary medicine: photobiomodulation. Eds., Riegel, R.J. and Godbold, J.C. Ames, IA: Wiley, pp: 36–51. Arancibia, S., Silhol, M., Mouliere, M.J., Hollinger, I., Maurice, T. and Tapia-Arancibia, L. 2008. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 31, 316–326. Barrett, D.W. and Gonzalez-Lima, F. 2013. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neurosci. 230, 13–23. Bartels, K.E. 2017. Therapy laser safety. In Laser therapy in veterinary medicine: photobiomodulation. Eds., Riegel, R.J. and Godbold, J.C. Ames, IA: Wiley, pp: 29–35. Chan, A.S., Lee, T.K., Yeung, M.K. and Hamblin, M.R. 2019. Photobiomodulation improves the frontal cognitive function of older adults. Int. J. Geriatr. Psychiatry. 34, 369–377. Chapagain, D., Range, F., Huber, L. and Viranyi, Z. 2018. Cognitive aging in dogs. Gerontology 64(2), 165–171. doi: 10.1159/000481621. Chen, C., Hung, H. and Hsu, S. 2008. Low energy laser irradiation increases endothelial cell proliferation, migration and eNOS gene expression possibly via P13K signal pathway. Lasers Surg. Med. 40, 46–54. Cury, V., Moretti, A.I.S., Assis, L., Bossini, P., de Souza Crusca, J., Neto, C.B., Fangel, R., de Souza, H.P., Hamblin, M.R. and Parizotto, N.A. 2013. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1α and MMP-2. J. Photochem. Photobiol. 125, 164–170. Da Luz Eltchechem, C., Salgado, A.S.I., Zangaro, R.A., da Silva Pereira, M.C., Kerppers, I.I., da Silva, L.A. and Parreira, R. B. 2017. Transcranial LED therapy on amyloid-β toxin 25-35 in the hippocampal region of rats. Lasers Med. Sci. 32, 749–756. De la Torre, J.C. 2016. Cerebral perfusion enhancing interventions: a new strategy for the prevention of Alzheimer dementia. Brain Pathol. 26, 618–631. De la Torre, J.C. 2017. Treating cognitive impairment with transcranial low level laser therapy. J. Photochem. Photobiol. 168, 149–155. De la Torre, J.C., del Olmo, A. and Valles, S. 2020. Can mild cognitive impairment be stabilized by showering brain mitochondria with laser photons? Neuropharmacology 171, 107841. doi: 10.1016/j.neuropharm.2019.107841. De Taboada, L., Yu, J., El-Amouri, S., Gattoni-Celli, S., Richieri, S., McCarthy, T., Streeter, J. and Kindy, M.S. 2011. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J. Alzheimers Dis. 23, 521–535. Dewey, C.W., Davies, E.S., Xie, H. and Wakshlag, J.J. 2019. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. Small Anim. Pract. 49, 477–499. Dewey, C.W., Rishniw, M., Johnson, P.J., Platt, S., Robinson, K., Sackman, J. and O’Donnell, M.O. 2020. Canine cognitive dysfunction patients have reduced total hippocampal volume compared with aging control dogs: a comparative magnetic resonance imaging study. Open Vet. J. 10, 438–442. Dewey, C.W. and Rishniw, M. 2021. Periodontal disease is associated with cognitive dysfunction in aging dogs: a blinded prospective comparison of visual periodontal and cognitive questionnaire scores. Open Vet. J. 11, 210–216. Enengl, J., Hamblin, M.R. and Dungel, P. 2020. Photobiomodulation for Alzheimer’s disease: translating basic research to clinical application. J. Alzheimers Dis. 75, 1073–1082. Gonzalez-Lima, F., Barksdale, B.R. and Rojas, J.C. 2014. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem. Pharm. 88, 584–593. Gonzalez-Lima, F. and Barrett, D.W. 2014. Augmentation of cognitive brain functions with transcranial lasers. Front. Syst. Neurosci. 8, 36. DOI: 10.3389/fnsys.2014.00036. Halliday, G. 2017. Pathology and hippocampal atrophy in Alzheimer’s disease. Lancet Neurol. 16, 862–864. Hennessy, M. and Hamblin, M.R. 2017. Photobiomodulation and the brain: a new paradigm. J. Opt. 19(1), 013003. DOI: 10.1088/2040-8986/19/1/013003. Hamblin, M.R. 2016. Shining light on the head: photobiomodulation for brain disorders. BBA Clin. 6, 113–124. Hamblin, M.R. 2019. Photobiomodulation for Alzheimer’s disease: has the light dawned? Photonics. 6(3), 77. doi: 10.3390/photonics6030077. Hashmi, J.T., Huang, Y., Sharma, S.K., Kurup, D.B., De Taboada, L.D., Caroll, J.D. and Hamblin, M.R. 2010. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 42, 450–466. Hu, Y. and Russek, S.J. 2008. BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J. Neurochem. 105, 1–17. Huang, N., Yao, D., Jiang, W., Wei, C., Li, M., Li, W., Mu, H., Gao, M., Ma, Z., Lyu, J. and Tong, Z. 2020. Safety and efficacy of 630 nm red light on cognitive function in older adults with mild to moderate Alzheimer’s disease: protocol for a randomized controlled study. Front. Aging Neurosci. 12, 143. doi: 10.3389/fnagi.2020.00143. Landsberg, G.M., Nichol, J. and Araujo, J.A. 2012. Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet. Clin. Small Anim. 42, 749–768. Lapchak, P.A. 2012. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert. Rev. Med. Devices 9, 71–83. Liang, J., Liu, L. and Xing, D. 2012. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Rad. Biol. Med. 53, 1459–1467. Lim, W., Kim, J., Kim, S., Karna, S., Won, J., Jeon, S.M., Kim, S.Y., Choi, Y., Choi, H. and Kim, O. 2013. Modulation of lipopolysaccharide-induced NF-kB signaling pathway by 635 nm irradiation via heat shock protein 27 in human gingival fibroblast cells. Photochem. Photobiol. 89, 199–207. Lu, Y., Wang, R., Dong, Y., Tucker, D., Zhao, N., Ahmed, M.E., Zhu, L., Liu, T.C.Y., Cohen, R.M. and Zhang, Q. 2017. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol. Aging 49, 165–182. Meng, C., He, Z. and Xing, D. 2013. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J. Neurosci. 33, 13505–13517. Naeser, M.A., Zafonte, R., Krengel, M.H., Martin, P.I., Frazier, J., Hamblin, M.R., Knight, J.A., Meehan, W.P. and Baker, E.H. 2014. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma. 31, 1008–1017. Nagahara, A.H., Merrill, D.A., Coppola, G., Tsukada, S., Schroeder, B.E., Shaked, G.M., Wang, L., Blesch, A., Kim, A., Conner, J.M., Rockenstein, E., Chao, M.V., Koo, E.H., Geschwind, D., Masliah, E., Chiba, A.A. and Tuszynski, M.H. 2009. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nature Med. 15, 331–337. Riegel, R.J. and Godbold, J.C. 2017. Fundamental information. In Laser therapy in veterinary medicine: photobiomodulation. Eds., Reigel, R.J. and Godbold, J.C. Ames, IA: Wiley, pp: 9–18. Salehpour, F., Ahmadian, N., Rasta, S.H., Farhoudi, M., Karimi, P. and Sadigh-Eteghad, S. 2017. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiol. Aging 58, 140–150. Saltmarche, A.E., Naeser, M.A., Ho, K.F., Hamblin, M.R. and Lim, L. 2017. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed. Laser Surg. 35, 432–441. Sommer, A.P. 2019. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: the principles of low-level light therapy. Ann. Transl. Med. 7, S13–S20. Stephens, B.J. 2019. Light is just the catalyst. In Veterinary laser therapy in small animal practice. Eds., Redondo, M.S. and Stephens, B.J. Sheffield, UK: 5M Publishing, pp: 3–9. Tang, Y. and Le, W. 2016. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. Tao, L., Liu, Q., Zhang, F., Fu, Y., Zhu, X., Weng, X., Han, H., Huang, Y., Suo, Y., Chen, L., Gao, X. and Wei, X. 2021. Microglia modulation with 1070-nm light attenuates Aβ burden and cognitive impairment in Alzheimer’s disease mouse model. Light: Science & Applications. doi:10.1038/s41377-021-00617-3. Tedford, C.E., DeLapp, S., Jacues, S. and Anders, J. 2015. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brains. Lasers Surg. Med. 47, 312–322. Uozumi, Y., Nawashiro, H., Sato, S., Kawauchi, S., Shima, K. and Kikuchi, M. 2010. Targeted increase in cerebral blood flow by transcranial near-infrared irradiation. Lasers Surg. Med. 42, 566–576. Wang, P. and Li, T. 2018. Which wavelength is optimal for transcranial low-level laser stimulation? J. Biophotonics. 12(2), e201800173. doi: 10.1002/jbio.201800173. Yang, X., Askarova, S., Sheng, W., Chen, J.K., Sun, A.Y., Sun, G.Y., Yao, G. and Lee, C.M. 2010. Low energy light (632.8 nm) suppresses amyloid-β peptide-induced oxidative and inflammatory responses in astrocytes. Neurosci. 171, 859–868. Yue, X., Mei, Y., Zhang, Y., Tong, Z., Cui, D., Yang, J., Wang, A., Wang, R., Fei, X., Ai, L., Di, Y., Luo, H., Li, H., Luo, W., Lu, Y., Li, R., Duan, C., Gao, G., Yang, H., Sun, B., He, R., Song, W., Han, H. and Tong, Z. 2019. New insight into Alzheimer’s disease: light reverses Aβ-obstructed interstitial fluid flow and ameliorates memory decline in APP/PS1 mice. Alzheimers Dement. 5, 671–684. Zhang, Z., Shen, Qi., Wu, X., Zhang, D. and Xing, Da. 2019. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell. 19(1), e13054. doi: 10.1111/acel.13054. Zuccato, C. and Cattaneo, E. 2009. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 5, 311–322. | ||
| How to Cite this Article |
| Pubmed Style Dewey CW, Brunke MW, Sakovitch K. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J.. 2022; 12(2): 256-263. doi:10.5455/OVJ.2022.v12.i2.14 Web Style Dewey CW, Brunke MW, Sakovitch K. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). https://www.openveterinaryjournal.com/?mno=93802 [Access: November 23, 2025]. doi:10.5455/OVJ.2022.v12.i2.14 AMA (American Medical Association) Style Dewey CW, Brunke MW, Sakovitch K. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J.. 2022; 12(2): 256-263. doi:10.5455/OVJ.2022.v12.i2.14 Vancouver/ICMJE Style Dewey CW, Brunke MW, Sakovitch K. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J.. (2022), [cited November 23, 2025]; 12(2): 256-263. doi:10.5455/OVJ.2022.v12.i2.14 Harvard Style Dewey, C. W., Brunke, . M. W. & Sakovitch, . K. (2022) Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J., 12 (2), 256-263. doi:10.5455/OVJ.2022.v12.i2.14 Turabian Style Dewey, Curtis Wells, Matthew Warren Brunke, and Kasie Sakovitch. 2022. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Veterinary Journal, 12 (2), 256-263. doi:10.5455/OVJ.2022.v12.i2.14 Chicago Style Dewey, Curtis Wells, Matthew Warren Brunke, and Kasie Sakovitch. "Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD)." Open Veterinary Journal 12 (2022), 256-263. doi:10.5455/OVJ.2022.v12.i2.14 MLA (The Modern Language Association) Style Dewey, Curtis Wells, Matthew Warren Brunke, and Kasie Sakovitch. "Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD)." Open Veterinary Journal 12.2 (2022), 256-263. Print. doi:10.5455/OVJ.2022.v12.i2.14 APA (American Psychological Association) Style Dewey, C. W., Brunke, . M. W. & Sakovitch, . K. (2022) Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Veterinary Journal, 12 (2), 256-263. doi:10.5455/OVJ.2022.v12.i2.14 |