| Original Article | ||
Open Vet J. 2023; 13(2): 225-232 Open Veterinary Journal, (2023), Vol. 13(2): 225–232 Original Research Molecular prevalence and genetic diversity of Toxoplasma gondii in free-range chicken in Northeastern LibyaHana A. Ali Awad1,2, Teguh Wahju Sardjono3*, Loeki Enggar Fitri3, Aulanni’am Aulanni’am4 and Monier A. Mohamed Sharif51Doctoral Program in Medical Science, Faculty of Medicine, Universitas Brawijaya Jalan Veteran Malang, Malang, Indonesia 2Department of Parasitology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al Bayda, Libya 3Department of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 4Department Chemistry, Biochemistry Laboratory, Faculty of Sciences, Universitas Brawijaya, Malang, Indonesia 5Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al Bayda, Libya *Corresponding Author: Teguh Wahju Sardjono. Department of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia. Email: teguhws [at] ub.ac.id Submitted: 06/12/2022 Accepted: 28/01/2023 Published: 19/02/2023 © 2023 Open Veterinary Journal
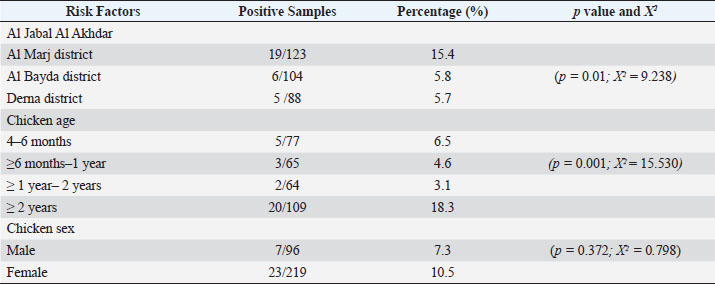
AbstractBackground: Toxoplasma gondii is one of the zoonotic protozoa parasites. It can prevalently infect humans and warm-blooded animals, causing human health problems and substantial economic losses to the livestock industry worldwide. Chicken is one of the potential sources of toxoplasmosis, but there is no report of the prevalence of toxoplasmosis and their genotypes in free-range chickens in Libya. Aim: This study aims to conduct a survey of molecular prevalence and identify the T. gondii genotype in free-range chickens and its association with the risk factors of age, gender, and region in Northeastern Libya. Methods: This study was conducted by examining a total of 315 free-range chicken organs (brain and heart) derived from three administrative districts in Northeastern Libya. The molecular prevalence was determined by PCR technique using B1 gene amplification. and the T. gondii genotype was determined by nested PCR-RFLP of GRA6 gene amplicon with restriction enzymes (MseI). Results: The overall molecular prevalence of T. gondii in free-range chicken in all three districts was 9.5% (30/315), and the highest (15.4%) was in the Al-Marj district (p=0.01; x 2=9.238). The highest prevalence of T. gondii by age was in chickens aged more than 2 years (p=0.001; x 2=15.530). The difference in T. gondii prevalence in male and female chickens was not significant (p =0.372; x 2=0.798). The predominant genotype I (93.3%) had identified at position 544 and 194 bp at the GRA6 marker, and only two positives were from genotype II (6.7%) at 700 and 100 bp fragments. Conclusion: The molecular prevalence of toxoplasmosis in free-range chicken in three districts in Northeastern Libya was 9.5%, and the highest rate was shown in the Al Marj district. Chicken by age more than 2 years had more risk to transmit toxoplasmosis in human. There was no different infection risk by consuming male or female free-range chicken. It is the first report to determine the predominant genotype, which was genotype I. Keywords: B1 gene, GRA6 gene, Genotyping, Free-range chicken, Toxoplasma gondii. IntroductionToxoplasma gondii is a zoonotic parasite ranked among the most important foodborne pathogens worldwide (Fabian et al., 2020). Food animals are reservoirs of T. gondii and one of the sources of parasite transmission to humans giving T. gondii its medical and veterinary importance (Tonouhewa et al., 2017). Humans get infected by consuming raw or uncooked meat containing tissue cysts, contaminated food or water with oocysts; or, from mother to fetus through vertical transmission in a primary infection (Hamilton et al., 2019; Salinas et al., 2021). More than a third of the world’s population has been infected with T. gondii which can have serious consequences for immunodeficient or immunocompromised people (Mao et al., 2021). First, genotyping studies have suggested the existence of T. gondii clonal populations with three main genotype lineages types I, II, and III, and rare recombinant strains (Howe and Sibley, 1995). Following that, the three genotypic variations of T. gondii were found to exist in Western Europe and America which differ in phenotype, including pathogenicity (Liu et al., 2015). Recently, more significant genetic variability has been shown using multilocus markers. The worldwide distribution of T. gondii genotypes is well-known in Western Europe and America, while there is only a little information on it in Asia and Africa. In Africa, lineages type II and type III, African lineages named Africa 1,2,3, and recently Africa 4 have been identified using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) or microsatellite (MS) markers (Lachkhem et al., 2021; Galal et al., 2022). Chicken is one of the most consumed meats in Libya, and free-range chicken meat is frequently consumed, as it is supposed to be healthier than that of caged chickens. However, consuming raw or undercooked chicken meat may expose an individual to human toxoplasmosis (Vieira et al., 2018). As per their feeding habit, free-range chickens have been used widely in ascertaining the environmental contamination with T. gondii oocyst; and identifying the genetic variation of T. gondii worldwide (Hamilton et al., 2019), but there is no information of the prevalence of T. gondii infection and their genotypes in free-range chickens in Libya. This research aimed to assess the molecular prevalence and identify the T. gondii genotype in free-range chicken and its association with the risk factors of age, gender, and region in Northeastern Libya. Materials and MethodsStudy period and locationThis cross-sectional study was undertaken in the Al Jabal Al Akhdar region of Northeast Libya at different farms in three districts. It lasted from March 2021 to February 2022. Sample collsectionA total of 315 samples were taken and grouped according to the origin of the materials. Those were three administrative districts in the Al Jabal Al Akhdar region (Al Marj, Al Bayda, and Derna districts). All purchased free-range chicken were kept by farmer. Based on information from veterinarian, chickens were grouped by gender into males and females and were categorized into four groups by age of chicken (Table 1). The chickens were slaughtered under sterile conditions, and tissue samples (heart and brain) were obtained. Samples were stored at −20°C before being transported in cold boxes to the laboratory of Animal Health Research in the Ministry of Agriculture in Egypt. Sample preparation and DNA extractionAbout one gram of each chicken tissue (heart and brain) was homogenized individually by tissue lyser (Qiagen, Hilden, Germany), in sterile Phosphate Buffered Saline (PBS) with two glass beads (5 mm). About 180 μl of ATL buffer and 20 μl of proteinase K were added to 200 μl homogenate into a 1.5 ml microcentrifuge tube, then incubated at 56°C till tissue lysis. DNA was extracted using a commercial kit (QIAamp DNA Mini kit, Animal Tissue protocol, catalog number 51304) following the manufacturer’s instructions. The extracts were stored at −20°C for further use. Molecular detection of B1 gene by PCREmerald Amp GT PCR Master Mix (Takara, code number. RR310A) kit to amplify the T. gondii B1 gene, was performed using the specific primers described by Lin et al. (2000). Briefly, the final volume was 25 µl of PCR reaction as follows: 5 µl DNA template, 12.5 µl of Emerald Amp GT PCR, 5.5 µl of PCR grade water (dH2O), 1 µl of each primer (20 pmol). The cycling conditions were standardized as primary denaturation of strands at 95°C for 5 minutes, followed by 35 cycles of amplification (at 94°C for 30 seconds, at 60°C for 30 seconds, at 72°C for 30 seconds), and the final extension at 72°C for 7 minutes. Genotyping by nested PCRThe nested PCR assay was performed to amplify the GRA6 gene. Briefly, the specific primers pair were described in the previous study (Armand et al., 2017). The first round of a 25 µl PCR reaction includes (20 pmol) forward primer (1 μl), (20 pmol) reverse primer (1 μl), (5 μl) of extracted DNA, Emerald Amp GTPCR master mix (12.5 μl), PCR grade water (5.5 μl). The cycling conditions were set up for 95°C for 5 minutes, followed by 35 cycles of 94°C for 30 seconds, 55°C for 40 seconds, and 72°C at 50 seconds, with a final extension at 72°C for 10 minutes. In the second round performed, the first amplified GRA6 product was cycled 35 times by the primary denaturation of strands at 95°C for 5 minutes, denaturation at 94°C for 30 seconds, followed by annealing at 58°C for 40 seconds, extension at 72°C for 45 seconds), and the final extension step at 72°C for 10 minutes. The success of amplicons was electrophoresed on 1.5% agarose gel and visualized under UV. Nested PCR-RFLPA nested PCR-RFLP assay was performed to digest the 750 bp nested PCR amplified GRA6 product using 1.5 U of MseI enzyme for genotyping. To prepare the RFLP reactions of 7 μl of PCR product was mixed with 1 μl of restriction enzyme and 1 μl of enzyme buffer, and the total volume was adjusted to 15 μl by adding distilled water. The preparation was then incubated at 37°C for 20 minutes. The digested products were separated by electrophoresis on 1.5% agarose gel at 60 minutes in ethidium bromide-stained and visualized on a trans-illuminator under UV light. Statistical analysisAll statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0. (IBM Corp., Armonk, NY). A chi-square test was used to determine whether there were significant differences between disease occurrence and independence. Variable (area, sex, and chicken age), statistical significance was defined as a p-value ≤0.05. Table 1. Risk Factor and its Association with the Molecular Prevalence of T. gondii in Positive Chickens.
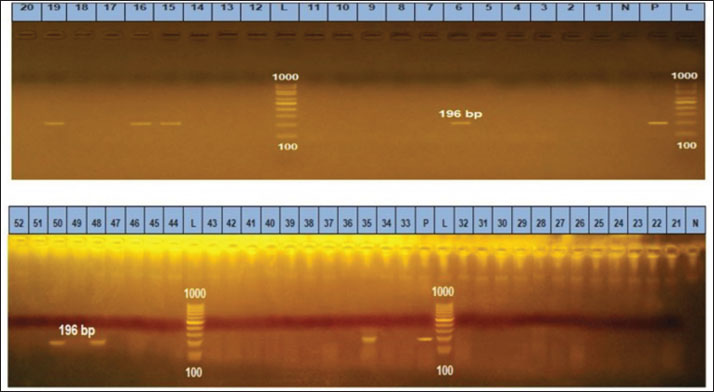
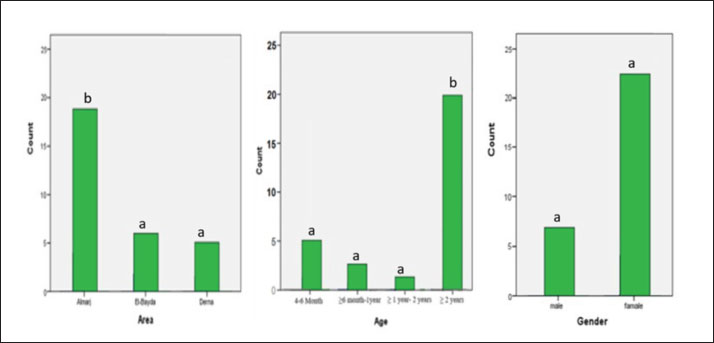
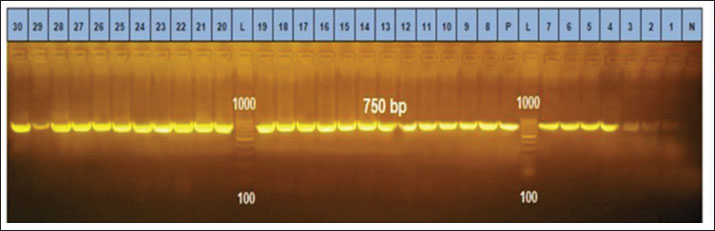
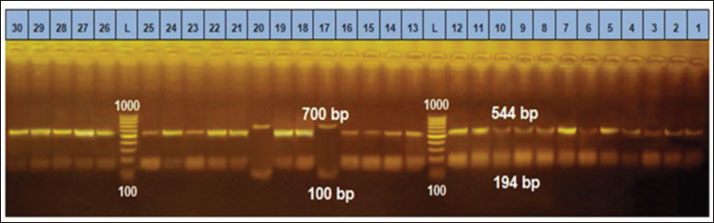
Ethical approvalThe free-range chicken sampling started after obtaining approval from the Libyan National Committee for Biosafety and Bioethics (approval number: SH/3/2021). ResultsMolecular prevalence of T. gondii and the associated risk factorsThe overall molecular prevalence of T. gondii in the 315 free-range chicken brain and heart tissues was 9.5% (30/315). The PCR amplification products of the B1 gene were the predicted amplicon size of 196 bp as in (Fig. 1). The highest prevalence was revealed of T. gondii in the chicken tissue obtained from the Al Marj district 15.4% (19/123), followed by Al Bayda district at 5.8% (6/104) and then Derna district at 5.7% (5/88), the prevalence rate among districts was a significantly different (p=0.01; x 2=9.238). The prevalence rate among age groups was significantly different (p =0.001; x 2=15.530), The highest prevalence rate was 18.3% (20/109) for the chickens aged (≥2 years;) followed by those in the age group 4–6 months at 6.5% (5/77). Chickens aged ≥6 months to 1 year and ≥1 year to 2 years, had prevalence rates of 4.6% (3/65), and 3.1% (2/64), respectively. As per the sex, the prevalence was higher in the female group at 10.5% (23/219), compared to the male group at 7.3% (7/96). However, it was not significant (p =0.372; x 2=0.798) (Table 1, Fig. 2). Genetic characterizationNested-PCR resultTo determine the genetic characterization of isolates, all positive B1 gene PCR products samples (30) were subjected to GRA6 nested-PCR amplification. As a result, all samples were detected positive by visualizing the 750 bp fragment on the agarose gel (Fig. 3). Nested PCR-RFLP resultsAll 30 positive samples of the second nested-PCR GRA6 were genotyped (100%) using the RFLP method and restriction enzymes (MseI). According to the PCR-RFLP, there are two genotypes among all positive samples; 28 samples were from genotype I (93.3%), which showed 544–bp and 194–bp fragments and only two samples were positive from genotype II (6.7%). This sample was detected at 700-bp and 100-bp fragments (Fig. 4). DiscussionAscertaining the scope of genetic diversity of T. gondii in Libya is important in assessing its potential impact on public health. However, there was no information about the genotypes of T. gondii in domestic animals, including chickens, in Libya. Therefore, this is the first study to use PCR assay and PCR-RFLP to diagnose and identify T. gondii strains, which report their molecular prevalence in chickens in Libya. Most of the studies used serological methods to investigate the high prevalence of this parasite in different domestic hosts around Libya. However, these methods have low sensitivity because of low antibody levels (Mose et al., 2016). Although some previous reports that used different serological methods for diagnosis, displayed a high prevalence of T. gondii antibodies (Al-mabruk et al., 2013; Fadiel et al., 2021). The difference in the prevalence as per the area of origin of the chicken may be related to the number of chickens examined, and the contaminated environment. The higher prevalence of chicken-positive toxoplasmosis in the Al Marj district than in the Al Bayda and Derna districts could be due to the differences in agricultural activity, and livestock keeping, which is more common in the Al Marj district. As it is known, cats play a key role in spreading T. gondii oocysts in the environment, so we assume that the number of cats in the Al Marj district may be more than in other areas. Thus, this could explain the high prevalence of infection in free-range chicken in that region compared to other regions. Al Marj district’s surrounding environment is a rural area, as in the case of Brazil, where a high prevalence between 46.0% and 50% was free-range chickens in rural areas, indicating widespread contamination of the rural environment of that country with T. gondii oocysts (Sá et al., 2017; Tonouhewa et al., 2017).
Fig. 1. Gel electrophoresis of PCR amplification products of B1 in the brain and heart (L): DNA size marker 100–1,000 bp., N: Negative control; P: Positive control. Lanes 6, 15, 16, 19, 35, 48, 50 are positive samples (196 bp).
Fig. 2. Percentage of positive samples based on the area, chicken ages, and sex of chicken. Different notation means significant different (p-value ≤ 0.05, chi-square test). There is a significant association between the prevalence of T. gondii in different chickens ages. A significantly higher prevalence was observed in older chickens (more than 2 years) than in younger chickens. A high prevalence of toxoplasmosis in older chickens (≥2 years) was also reported in Kenya (Mose et al., 2016), and Pakistan (Khan et al., 2020). This could be attributed to older chickens having more outdoor access, considered a risk factor, and therefore being more predisposed to T. gondii infection. Being outdoors will expose them to soil contaminated with T. gondii oocysts.
Fig. 3. Gel electrophoresis after secondary PCR amplification product with GRA6 primers. N: Negative control; p: Positive control. The positive band is at 750 bp. Ladder weight: DNA size marker (L):100–1,000 bp.
Fig. 4. PCR-RFLP analysis of GRA6 gene coding region with restriction enzymes (MseI). Lanes: L DNA size markers. (Between 1,000bp and 100 bp), Lanes: 17, and 20 digested PCR products of GRA6 for Type II & lanes: 1–16, 18, 19 and lanes: 21–30 digested PCR products of GRA6 gene for Type I. Although the sex risk factor in our study was not significant, the prevalence of female infection compared to males was high, which resembles the results of a previous study done in China on ducks (Lv et al., 2021); these results also match what was mentioned by Mose et al. (2016). The brain and heart tissues were used to detect DNA because both organs are included in the most common predilection sites for T. gondii (Lüder and Rahman, 2017). Unfortunately, in this study, a low prevalence of 9.5% was indicated in chicken brains and hearts, which may explain the false-negative results due to the small amount of chicken tissue taken for DNA extraction, whereas, only one gram of host tissue was used. A study conducted in Pakistan, using chicken heart targeting the B1 T. gondii gene, gave the same prevalence of 9.5% (Khan et al., 2020). However, our finding expressed a higher prevalence compared with the previous study in China that reported, the prevalence of T. gondii in chicken was only 8.17% (Zou et al., 2017). In contrast, previous study using PCR to detect the T. gondii B1 gene on three Caribbean islands showed a higher prevalence (24.4%, 17.1%, and 17.1%) in the free-range chicken (Hamilton et al., 2019). Another previous study in northern Iran reported a high prevalence of the B1 gene of T. gondii that reached 40% in free-range chicken heart samples (Abbaszadeh et al., 2022). Our result also expressed a lower prevalence compared to the Tunisian study performed by PCR target B1 which showed a much higher prevalence of 43.3% (Zrelli, 2022). According to our search in the literature, the highest prevalence of T. gondii in livestock and poultry animals was found in Asia in 2014 with 89.8% (Hajimohammadi et al., 2022). Molecular diagnostics of toxoplasmosis is generally based on the detection of a specific DNA sequence, using various assays and protocols, mostly from highly conserved regions such as the B1 gene, internal transcribed spacer–1 and 18S rDNA gene sequences (Ivovic et al., 2012). It is important to note, however, that molecular diagnostics, as a constantly evolving modern methodology, is not standardized even among the world’s leading laboratories. The differences are significant and numerous, and they span all aspects of the methodology, including target genes for parasite detection and markers for genotyping, equipment manufacturers, and protocols (Ivovic et al., 2012). In our study, the most common genotype using PCR-RFLP of GRA6 gene is the T. gondii type I. This study is consistent with the previous study in Iran, which genotype type I was reported as the most prevalent genotype based on the SAG2 gene using RFLP method (Mahami-Oskouei et al., 2017). Similar study showed T. gondii type I was predominant in Iran based on the GRA6 and SAG2 markers (Armand et al., 2017). A study in China revealed a high prevalence of T. gondii in chicken hearts from farmers’ markets, On the 77 positive samples, nested PCR-RFLP genotyping was performed using the T. gondii SAG3 and GRA6 gene loci. SAG3 genotyping revealed a mixed infection rate of 89.6% for type I and type I/II strains, while GRA6 genotyping revealed a type I strain with infection rate of 98.7% (Wang et al., 2020). In Libya, Several studies carried out on humans have shown a high prevalence of human toxoplasmosis (Gamal, 2015; Gashout et al., 2016). One study was undertaken in the area close to our study area in the economic capital city in Northeastern Libya (Benghazi). This particular study determined the genotype for ocular toxoplasmosis which showed (25%) of type I based on a single locus PCR-RFLP analysis based upon the SAG2 gene (Ali et al., 2018). The above study’s findings appear to be consistent with ours in this study, the predominance of the clonal type I lineage of T. gondii. Therefore, our findings may spur exploring the risk of human infection with T. gondii through consuming undercooked infected chicken meat. In contrast to our findings, the first report on the chicken population of India indicated T. gondii lineage as type III based on PCR-RFLP of the GRA6 gene (Biradar et al., 2014). A recent survey by Gorgani-Firouzjaee et al. (2022) using PCR-RFLP of SAG2 and GRA6 genes demonstrated the alleles of clonal type III in all isolates. These different results are likely because of various genotypes of T. gondii in different geographic regions. This may explain the high diversity of T. gondii lineages in these regions. The GRA6 gene is widely used as an appropriate marker for T. gondii. It can identify and distinguish its three and some atypical genotypes with a single PCR reaction, followed by an endonuclease (Msel) digestion (Danehchin et al., 2016). In this study, the PCR-RFLP assay on the products of the second steps of nested-PCR of GRA6 genes had been used to identify the T. gondii genotypes (Gorgani-Firouzjaee et al., 2022). The use of single molecular markers may represent a problem due to a large extent diversity may be lost or genotypically different parasites may not be distinguished efficiently (Fernández-Escobar et al., 2022). Therefore, this is the main limitation of this research for reliable genotype classification. To date, there is no commonly used set of markers to genotype T. gondii strains (Su et al., 2006). However, only loci GRA6 and SAG3 had enough high-quality sequences to perform a robust phylogenetic analysis (Fernández-Escobar et al., 2022). Most T. gondii isolates from human and animal sources in Northern America and Europe were grouped into one of three clonal lineages by multilocus enzymes electrophoresis, PCR-RFLP and MS typing. The rapid development of multilocus-sequencing methods as well as the description of a diverse panel of new PCR-RFLP and MS markers resulted in firm observations on the predominance of three clonal/archetypal types or lineages (Su et al., 2006). Unfortunately, traditional T. gondii typing methodologies have significant limitations because only very specific and restricted sites within a large T. gondii genome are assessed. Whole-genome sequencing data analysis has emerged as the most suitable approach for a thorough analysis of the genetic diversity in T. gondii (Fernández-Escobar et al., 2022). ConclusionThis study determined the T. gondii DNA for the first time and genotyped the T. gondii strain in free-range chickens in Libya, with type I as the most predominant genotype. Because of its potential to impact public health, the relatively high prevalence of infected chickens especially in Al Marj District, and those of chickens more than 2 years old, further studies need to be done. Studies on other domestic animals are also important to determine their genetic diversity to gain more knowledge of its control and prevention. AcknowledgmentsThe authors gratefully acknowledge the kind support and cooperation of the Universitas Brawijaya and the Libyan Embassy for facilitating the financial assistance to perform this research. Authors’ contributionsAll authors contributed to making the completion of this manuscript possible. Conceptualization: H.A.A., T.W.S., L.E.F., M.A.S., and A.A. Collecting samples and conducting the research trials: H.A.A., data curation: H.A.A., T.W.S., L.E.F., and M.A.S.; writing original draft preparation: H.A.A, supervised the research; T.W.S., L.E.F., M.A.S., and A.A., supervised the field research: M.A.S. Finishing the manuscript: H.A.A., T.W.S., L.E.F., M.A.S., A.A. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbbaszadeh, S., Teimouri, A., Mahmoudi, M.R., Roushan, Z.A., Hajipour, N., Majidi-Shad, B. and Sharifdini, M. 2021. Molecular detection of Toxoplasma gondii in chicken hearts from markets and retail stores in Northern Iran. Food Waterborne Parasitol. 27, e00166. Al-mabruk, A.A., ALKHunfas, S.R., El-Buni, A., Annajar, B. 2013. Seroprevalence of Toxoplasma gondii antibodies in sheep from Libya. Int. J. Adv. Res. 1(9), 1–6. Ali, R.H., Elkadi, M.A., Mokhtar, A., Kassem, H. and Mansur, F.A.F. 2018. Ocular toxoplasmosis in Benghazi, Libya: evidence of type I strain. IOSR J. Dent. Med. Sci. 17(12), 28–32. Armand, B., Solhjoo, K., Kordshooli, M.S., Davami, M.H., Pourahmad, M. and Orfaee, V. 2017. Toxoplasma gondii type I, predominant genotype isolated from sheep in South of Iran. Vet. World 10(4), 386–392. Biradar, S.S., Saravanan, B.C., Tewari, A.K., Sreekumar, C., Sankar, M. and Sudhakar, N.R. 2014. Genetic characterization of Toxoplasma gondii isolates from chickens in India by GRA6 gene sequence analysis. Acta Parasitol. 59(4), 666–674. Danehchin, L., Razmi, G. and Naghibi, A. 2016. Isolation and genotyping of Toxoplasma gondii strains in ovine aborted fetuses in Khorasan Razavi Province, Iran. Korean J. Parasitol. 54(1), 15–20. Fabian, B. T., Hedar, F., Koethe, M., Bangoura, B., Maksimov, P., Conraths, F.J., Villena, I., Aubert, D., Seeber, F. and Schares, G. 2020. Fluorescent bead-based serological detection of Toxoplasma gondii infection in chickens. Parasites Vectors. 13(1), 1–11. Fadiel, M.M., Hailazakis, N.E. and Abdulwahid, A.A. 2021. Seroprevalence and risk factors of Toxoplasma gondii in sheep and goats in Benghazi city East of Libya. 1. Libyan J. Sci. Technol. 13(2), 121–126. Fernández-Escobar, M., Schares, G., Maksimov, P., Joeres, M., Ortega-Mora, L.M. and Calero-Bernal, R. 2022. Toxoplasma gondii genotyping: a closer look into Europe. Front. Cell. Infect. Microbiol. 12, 842595. Galal, L., Ariey, F., Gouilh, M.A., Dardé, M.L., Hamidović, A., Letourneur, F., Prugnolle, F. and Mercier, A. 2022. A unique Toxoplasma gondii haplotype accompanied the global expansion of cats. Nat. Commun. 13, 5778. Gamal, M.A.B. 2015. Seroprevalence study of IgG antibodies to toxoplasma, and risk factors for toxoplasma infestation among pregnant women in Alkhoms state, Libya. 1(1), 15–19. Gashout, A., Amro, A., Erhuma, M., Al-Dwibe, H., Elmaihub, E., Babba, H., Nattah, N. and Abudher, A. 2016. Molecular diagnosis of Toxoplasma gondii infection in Libya. BMC Infec. Dis. 16, 157. Gorgani-Firouzjaee, T., Kalantari, N. and Ghaffari, S. 2022. Molecular identification and genotyping of Toxoplasma gondii isolated from sheep and cattle in northern Iran. Vet. Res. Forum 13(3), 371–378. Hajimohammadi, B., Ahmadian, S., Firoozi, Z., Askari, M., Mohammadi, M., Eslami, G., Askari, V., Loni, E., Barzegar-Bafrouei, R. and Boozhmehrani, M.J. 2022. A Meta-analysis of the prevalence of toxoplasmosis in livestock and poultry worldwide. EcoHealth 19(1), 55–74. Hamilton, C.M., Thomas, R.R.R., Oliveira, C.O.S., Innes, I.V.E.A. and Kelly, F.K.P.J. 2019. Prevalence and genetic diversity of Toxoplasma gondii in free - ranging chickens from the Caribbean. Acta Parasitol. 64(4), 738–744. Howe, D.K. and Sibley, L.D. 1995. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 172(6), 1561–1566. Ivovic, V., Vujanic, M., Zivkovic, T., Klun, I. and Djurkovic-Djakovic, O. 2012. Molecular detection and genotyping of Toxoplasma gondii from clinical samples. Toxoplasmosis Recent Adv. Available via https://www.intechopen.com/chapters/38945 Khan, M.B., Id, S.K., Rafiq, K. and Khan, S.N. 2020. Molecular identification of Toxoplasma gondii in domesticated and broiler chickens (Gallus domesticus ) that possibly augment the pool of human toxoplasmosis. PLoS One 15(4), e0232026. Lachkhem, A., Galal, L., Lahmar, I., Passebosc, K., Riahi, H., Plault, N., Dardé, M.L., Mercier, A. and Babba, H. 2021. First isolation and genotyping of Toxoplasma gondii strains from domestic animals in Tunisia. Sci. Rep. 2021(11), 9328. Lin, M.H., Che, T.C., Kuo, T.T., Tseng, C.C. and Tseng, C.P. 2000. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 38(11), 4121–4125. Liu, Q., Wang, Z.D., Huang, S.Y. and Zhu, X.Q. 2015. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 8, 292. Lüder, C.G.K. and Rahman, T. 2017. Impact of the host on Toxoplasma stage differentiation. Microbial Cell. 4(7), 203–211. LV, Q., Zheng, H., Yang, W. and Liu, G. 2021. Molecular Detection of Toxoplasma gondii and Neospora caninum in Domestic Ducks in Hunan Province China. Front. Vet. Sci. 8, 649603. Mahami-Oskouei, M., Moradi, M., Fallah, E., Hamidi, F. and Asl Rahnamaye Akbari, N. 2017. Molecular detection and genotyping of Toxoplasma gondii in chicken, beef, and lamb meat consumed in northwestern Iran. Iran. J. Parasitol. 12(1), 38–45. Mao, F., Yang, Y., Chen, Y., Zhang, Q., Ding, X., Ni, B., Xu, X., Jin, X. and Dai, Y. 2021. Seroprevalence and risk factors of Toxoplasma gondii infection among high-risk populations in Jiangsu Province, Eastern China. front. cell. Infect. Microbiol. 11, 783654. Mose, J.M., Kagira, J.M., Karanja, S.M., Ngotho, M., Kamau, D.M., Njuguna, A.N. and Maina, N.W. 2016. Detection of natural Toxoplasma gondii infection in chicken in Thika region of Kenya using nested polymerase chain reaction. Biomed. Res. Int. 2016, 7589278. Sá, S.G.de., Ribeiro-Andrade, M., Silva, L.T.R., Neto, O.L.S., Lima, D.C.V., Pedrosa1, C.deM., Bezerra, M.J.G. and Mota, R.A. 2017. Risk factors associated with Toxoplasma gondii infection in free-range chickens in the semiarid region of Brazil. Rev. Bras. Parasitol. Vet. 26(2), 221–225. Salinas, M.J.G., Campos, C.E., Peris, M.P.P. and Kassab, N.H. 2021. Prevalence of Toxoplasma gondii in retail fresh meat products from free-range chickens in Spain. J. Vet. Res. 65, 457–461. Su, C., Zhang, X. and Dubey, J.P. 2006. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol. 36(7), 841–848. Tonouhewa, A.B.N., Akpo, Y., Sessou, P., Adoligbe, C., Yessinou, E., Hounmanou, Y.G., Assogba, M.N., Youssao, I. and Farougou, S. 2017. Toxoplasma gondii infection in meat animals from Africa: systematic review and meta-analysis of sero-epidemiological studies. Vet. world 10(2),194–208. Vieira, F.E.G., Sasse, J.P., Minutti, A.F., Miura, A.C., de Barros, L.D., Cardim, S.T., Martins, T.A., de Seixas, M., Yamamura, M.I., Su, C. and Garcia, J.L. 2018. Toxoplasma gondii: prevalence and characterization of new genotypes in free-range chickens from south Brazil. Parasitol. Res. 117, 681–688. Wang, R., Zhao, N., Zhang, H., Wang, F., Li, H., Liu, Y., Zhao, X. and Zhang, X. 2020. Prevalence of Toxoplasma gondii infections in chicken hearts from farmers’ markets and supermarkets in the Tai’an Region of China. J. Food Prot. 83(2), 338–341. Zou, Y., Nie, L.B., Zhang, N.Z., Zou, F.C., Zhu, X.Q. and Cong, W. 2017. First genetic characterization of Toxoplasma gondii infection in poultry meat intended for human consumption in eastern China. Infec. Genet. Evol. 55, 172–174. Zrelli, S., Amairia S., Jebali, M. and Gharbi, M. 2022. Molecular detection of Toxoplasma gondii in tunisian free-range chicken meat and their offal. Parasitol. Res. 121, 3561–3567. | ||
| How to Cite this Article |
| Pubmed Style Awad HAA, Sardjono TW, Fitri LE, AA, Sharif MAM. Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Vet J. 2023; 13(2): 225-232. doi:10.5455/OVJ.2023.v13.i2.11 Web Style Awad HAA, Sardjono TW, Fitri LE, AA, Sharif MAM. Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. https://www.openveterinaryjournal.com/?mno=133000 [Access: April 28, 2024]. doi:10.5455/OVJ.2023.v13.i2.11 AMA (American Medical Association) Style Awad HAA, Sardjono TW, Fitri LE, AA, Sharif MAM. Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Vet J. 2023; 13(2): 225-232. doi:10.5455/OVJ.2023.v13.i2.11 Vancouver/ICMJE Style Awad HAA, Sardjono TW, Fitri LE, AA, Sharif MAM. Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Vet J. (2023), [cited April 28, 2024]; 13(2): 225-232. doi:10.5455/OVJ.2023.v13.i2.11 Harvard Style Awad, H. A. A., Sardjono, . T. W., Fitri, . L. E., , . A. A. & Sharif, . M. A. M. (2023) Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Vet J, 13 (2), 225-232. doi:10.5455/OVJ.2023.v13.i2.11 Turabian Style Awad, Hana A. Ali, Teguh Wahju Sardjono, Loeki Enggar Fitri, Aulanniam Aulanniam, and Monier A. Mohamed Sharif. 2023. Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Veterinary Journal, 13 (2), 225-232. doi:10.5455/OVJ.2023.v13.i2.11 Chicago Style Awad, Hana A. Ali, Teguh Wahju Sardjono, Loeki Enggar Fitri, Aulanniam Aulanniam, and Monier A. Mohamed Sharif. "Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya." Open Veterinary Journal 13 (2023), 225-232. doi:10.5455/OVJ.2023.v13.i2.11 MLA (The Modern Language Association) Style Awad, Hana A. Ali, Teguh Wahju Sardjono, Loeki Enggar Fitri, Aulanniam Aulanniam, and Monier A. Mohamed Sharif. "Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya." Open Veterinary Journal 13.2 (2023), 225-232. Print. doi:10.5455/OVJ.2023.v13.i2.11 APA (American Psychological Association) Style Awad, H. A. A., Sardjono, . T. W., Fitri, . L. E., , . A. A. & Sharif, . M. A. M. (2023) Molecular prevalence and genetic diversity of Toxoplasma gondii in free range chicken in Northeastern Libya. Open Veterinary Journal, 13 (2), 225-232. doi:10.5455/OVJ.2023.v13.i2.11 |