| Original Article | ||
Open Vet J. 2023; 13(1): 108-113 Open Veterinary Journal, (2023), Vol. 13(1): 108–113 Original Research Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formulaAboubaker M. Garbaj1, Samira A. Farag1, Jihan A. Sherif1, Aml F. Lawila2, Hanan L. Eshamah1, Salah M. Azwai3, Fatim T. Gammoudi3, Hesham T. Naas1, Allaaeddin A. El Salabi4 and Ibrahim M. Eldaghayes3*1Department of Food Hygiene and Control, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Food and Drug Control Center, Tripoli, Libya 3Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 4Department of Environmental Health, Faculty of Public Health, University of Benghazi, Benghazi, Libya Submitted: 11/10/2022 Accepted: 25/12/2022 Published: 21/01/2023 *Corresponding Author: Ibrahim Eldaghayes. Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, P.O. Box 13662, Tripoli, Libya. Email: ibrahim.eldaghayes [at] vetmed.edu.ly © 2023 Open Veterinary Journal
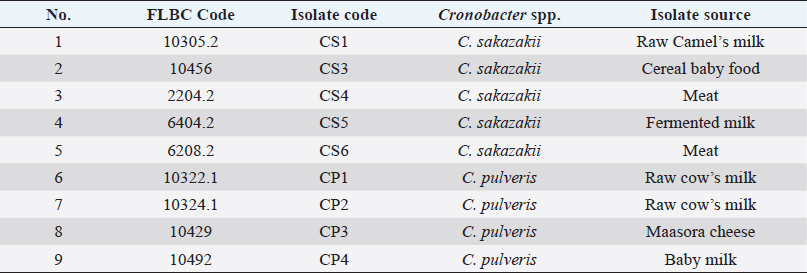
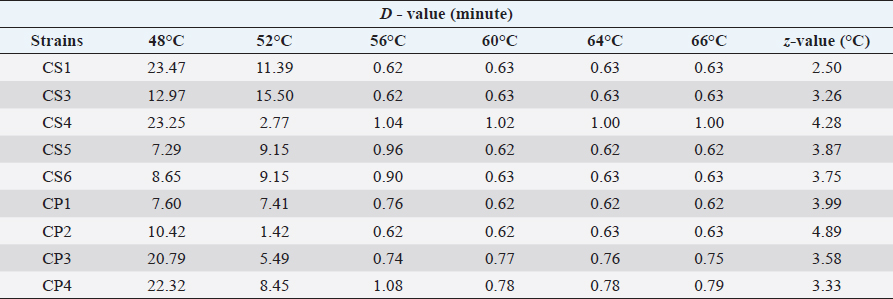
AbstractBackground: Cronobacter sspecies are the most significant foodborne pathogen in infant milk formula (IMF). These pathogens have been incriminated in severe forms of neonatal meningitis, sepsis, and necrotizing enterocolitis with a high mortality rate. Aim: This study was performed to elucidate the effect of heat stress on Cronobacter spp. (C. sakazakii and C. pulveris) in reconstituted IMF (RIMF). Methods: The reconstituted formula was inoculated with five C. sakazakii isolates and four C. pulveris isolates separately. The nine isolates of Cronobacter spp. were heated in RIMF at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C. The D- and z-values were determined by using linear regression analysis. Results: The D˗values of all isolates of C. sakazakii (CS1, CS3, CS4, CS5, and CS6) at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C were in the ranges 7.29–23.47, 2.77–15.50, 0.62–1.04, 0.62–1.02, 0.62–1.00, 0.62–1.00 minutes, respectively; while, the z˗values extended from 2.50°C to 4.28°C. The D˗ values of C. pulveris isolates (CP1, CP2, CP3, CP4) were in the ranges 7.60–22.32, 1.42–8.45, 0.62–1.08, 0.62–0.78, 0.62–0.78, 0.62–0.79 minutes at 48°C, 52°C, 56°C, 60°C, 64°C, 66°C, respectively and the calculated z-values ranged from 3.33°C to 4.89°C. Conclusion: This study may contribute to improving the understanding of the behavior of C. sakazakii and C. pulveris isolates in RIMF at various heat stress temperatures and may participate in the effective control of these pathogens in infant food production. Keywords: Cronobacter sakazakii, Cronobacter pulveris, Thermo-tolerance, D˗value, z˗value, Infant milk formula. IntroductionBreast milk is usually the most important source of nutrition for newborns and is essential for survival, immunological development, health, and early growth in life (Baker et al., 2021). Alternatively, non-breast-fed newborns can be fed on commercial powdered infant milk formula (IMF) that provides a nutritious alternative to breast milk (Costa et al., 2020). Infant formula is considered to be a microbiologically high-quality product, however, some microorganisms have been identified in neonatal infections associated with infant formula feeding (Garbaj et al., 2015). Moreover, powdered infant formula (PIF) manufacturing relies on the blending of many soluble substances and additives into dried base powder. This factor may be considered a critical health risk and serve as a main source of foodborne pathogens that transmit to neonates (Yemiş and Delaquis, 2020). Based on some epidemiological studies on the incidence of neonatal infections, Cronobacter spp., particularly C. sakazakii, is the most important foodborne pathogens in PIF (Al-Holy et al., 2010; Yemiş and Delaquis, 2020). Surveillance studies have identified Cronobacter spp. from different environments and foods such as milk powder, cereal formula, various dairy products, dried foods, meat products, spices, vegetables, rice, plants, etc. (Osaili et al., 2009). This ubiquitous microorganism, Gram-negative, non-spore-forming bacilli has been reported to cause septicemia, meningitis, sepsis, and necrotizing enterocolitis in newborns at low infection doses (Chauhan et al., 2020). In addition, C. sakazakii has the ability to withstand and tolerate sever stress conditions for instance high osmotic pressure and extreme dehydration (low water activity) compared to other Cronobacter spp. (Bai et al., 2019; Zhang et al., 2020). Reconstituted infant formula is known to provide an optimal environment for the growth of these pathogens, survive slight heat stress during preparation, and pose a significant potential risk to newborns (Xu et al., 2015). Moreover, some microbiological studies revealed that Cronobacter spp. has the ability to grow rapidly for long periods of time at room temperature in baby bottles (Al-Holy et al., 2009; Azwai et al., 2022). Therefore, World Health Organization (WHO)/FAO suggested that PIF should be reconstituted with water at a temperature of at least 70°C (Zhang et al., 2020). However, this target temperature may be difficult to adjust during home reconstitution of PIF or may not be suitable for direct feeding of neonates. Additionally, the PIF has different reconstituted instructions from different sources which may recommend lukewarm water with an alternative advised temperature (Yang et al., 2015). As a result, serious concerns have been raised about the safety of infant formula during the feeding of neonates worldwide. As C. sakazakii and C. pulveris (recently, Stephan et al. (2014), proposed reclassifying C. pulveris to Franconibacter pulveris) were previously detected in dairy and meat products sold in the Libyan market (Garbaj et al., 2017); therefore, C. sakazakii and pulveris needed to be evaluated for their resistance to heat stress which, is one of the most important factors affecting the behavior of these isolates in reconstituted IMF (RIMF). In contrast, from too many studies on the thermo-tolerance of Cronobacter spp., there is hardly any data available on variability and behavior of single isolate of C. sakazakii in rehydration PIF in countries such as Libya. Therefore, this study was performed to demonstrate the effect of heat stress on Cronobacter spp. (C. sakazakii and C. pulveris isolated from food of animal origin from Libya) in RIMF. Materials and MethodsBacterial strains used in this studyThe nine strains of Cronobacter spp. that have been used in this study (Table 1), were obtained from Foodborne Libyan Type Bacterial Collection (FLBC), these strains were isolated from raw milk, powdered (IMF), some dairy products, and meat from different localities in Libya and stored in cryobeads banking system at −80°C (Garbaj et al., 2017). A single bead was streaked with a wire loop under the aseptic condition on Nutrient agar (NA; CM0003, Oxoid, UK) and incubated at 37°C for 24 hours. Cells from single colonies of each strain were streaked on Tryptone Soya agar slants (TSA; Oxoid, UK), incubated at 37°C for 24 hours, and kept refrigerated until they were used. Inoculum preparationThe bacterial cultures were separately transferred from TSA slants to Brain Heart Infusion broth ((BHI; CM0225, Oxoid, UK). The strains were transferred individually into sterilized test tubes containing 10 ml BHI and incubated at 37°C for 24 hours. Within this stage, the bacteria are considered to be in the early stationary phase, which is known as the most resistant phase to antibiotics. Preparation of RIMFIMF was purchased from local supermarkets and pharmacies in Tripoli, Libya. The recommended amount of powder was added and reconstitution of IMF in sterile distilled water was carried out according to the manufacturer’s guideline (two scoops equal 8.6 g per 60 ml of warm water). Ten milliliters (ml) of RIMF were added into sterile test tubes and sterilized at 121°C for 15 minutes to get rid of the background microorganisms. Thermo-tolerance of Cronobacter spp.Thermo-tolerance of Cronobacter spp. was tested according to Al-Holy et al. (2009). The reconstituted milk was prepared and inoculated with Cronobacter spp., as follows: IMF was reconstituted according to the manufacturer’s instructions. About 10 ml samples of RIMF were sterilized at 121°C for 15 minutes. The isolate was transferred from TSA slants into (BHI) broth and incubated at 37°C for 24 hours. Ten-fold serial dilution of the BHI culture was performed in buffered peptone water solutions (RM 001˗500G HI media, India) by adding 1 ml from cultured BHI onto 99 ml of buffered peptone water dilution. A portion of 100 µl from each dilution was then aseptically plated (0.1 ml in duplicate) onto (TSA) (Oxoid CM 131) and incubated at 37°C for 24 hours (stationary phase). Table 1. Cronobacter strains used in this study.
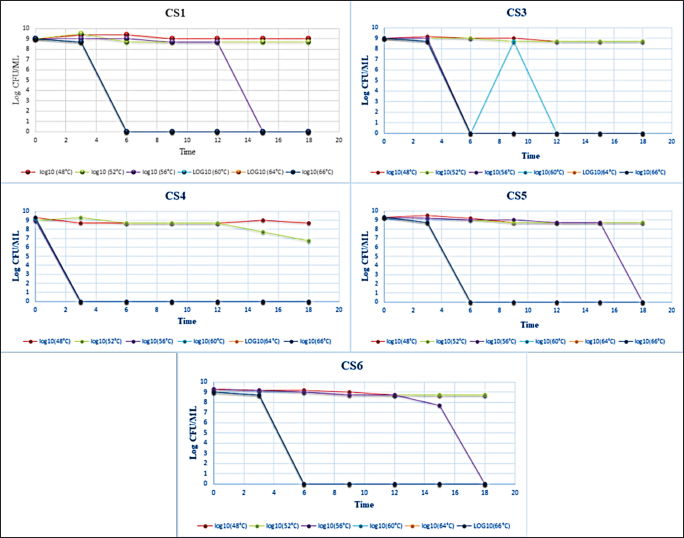
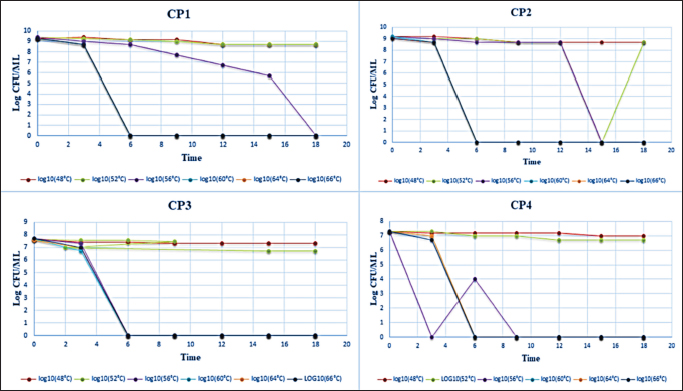
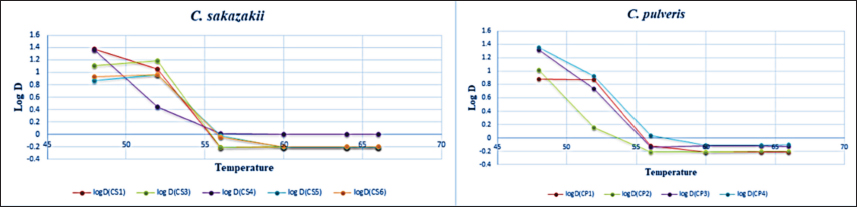
Finally, the dilution that had a microbial load of 108 CFU/ml was used for the inoculation of the prepared RIMF. Heat treatment was conducted in a water bath at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C. The tubes were immersed completely in the heated water bath where the temperature was controlled and adjusted at the target temperature. Following each heat treatment, each tube was then removed from the water bath and immersed immediately into ice slush. At each heat treatment, 100 µl from the inoculated IMF was taken in duplicate at different time points: 0, 3, 6, 9, 12, 15, and 18 minutes. Quantification of the colonies was then performed using the overlay method (Al-Holy et al., 2008). The samples were spread-plated on TSA supplemented with 0.1% (w/v) of sodium pyruvate. The plates were then incubated for 2 hours at 37°C and a thin layer (8 ml) of violet red bile agar (HIMIDIA M 581, India) was overlaid onto TSA and the plates were re-incubated for an additional 22 hours at 37°C. Standard regression analysis was performed by log-linear models in Excel (Microsoft 2010) and the D-value was determined by taking the negative reciprocal of the slope. The z-value was calculated using linear regression of the log D-values at six temperatures: 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C. Statistical analysisData were presented as mean ± SD. The decimal reduction time (D-value), the time required at a certain temperature to reduce a specific microbial population, and the z-value (the number of degrees of temperature change necessary to change the D-value) were determined by using Microsoft Excel (log-linear models). ResultsThe microbial quantity of each strain showed relatively similar values, it ranged from 107 to 109 CFU/ ml. Table 2 shows decimal reduction times (D˗value and z˗value) of C. sakazakii and C. pulveris strains inoculated in RIMF. In this study, D˗values were calculated at six different temperatures. Results in Table 2 revealed that the D˗values of all strains of C. sakazakii (CS1, CS3, CS4, CS5, and CS6) at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C were in the ranges 7.29–23.47, 2.77–15.50, 0.62–1.04, 0.62–1.02, 0.62–1.00, 0.62–1.00 minutes, respectively, while, the z˗ values ranged from 2.50°C to 4.28°C. Results represented in Table 2 also revealed the D˗values of all strains of C. pulveris (CP1, CP2, CP3, CP4) were in the ranges 7.60–22.32, 1.42–8.45, 0.62–1.08, 0.62–0.78, 0.62–0.78, 0.62–0.79 minutes at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C, respectively, whereas, the z˗ values were ranged from 3.33°C to 4.89°C. Figures 1 and 2 illustrated the decimal reduction time curve (D˗values) of all C. sakazakii and C. pulveris isolates in RIMF at 48°C–66°C. While atypical thermal death time curve of the same isolates of C. sakazakii and C. pulveris in RIMF at 48°C –66°C was shown in Figure 3. DiscussionIMF product has been the focus of studies on the inactivation and inhibition of Cronobacter as the primary vehicle of contamination (Muytjens et al., 1983). The thermal and non-thermal electromagnetic radiation effects were thought to be involved in the mechanism of microbial killing (Najdovski et al., 1991). The thermal resistance of Cronobacter spp. is considered a vital parameter likely assisting infection of infant formula. Thus, the extent of thermo-tolerance between different Cronobacter strains was evaluated by determining the thermal resistance rate by calculating the D-value and z-value of each strain. According to our findings, significant differences in thermal resistance were observed between the isolates (Table 2). It has been reported that the D48 value ranged from 7.29 minutes for CS5 to 23.47 minutes for CS1, and the D52 value ranged from 2.77 minutes for CS4 to 15.50 minutes for CS3. This is in agreement with some studies that reported this pathogen had a different and wide range of heat resistance (Edelson-Mammel and Buchanan, 2004; Al-Holy et al., 2009). Moreover, D60 ranged from 0.62 minutes for CS5 to 1.02 minutes for CS4 in this current study, which is in agreement with (Walsh et al., 2011) who found that D60 ranged from 0.10 to 0.73 minutes. However, D60 of Cronobacter isolates was 5.74 minutes which indicates a high heat resistance (Awadallah et al., 2018). This may be due to the examined Cronobacter strains being isolated from animal feces. These results revealed that it is relatively difficult to compare D-values among researchers. This could be attributed to the fact that the bacterial thermo-tolerance can generally be influenced by several factors including physiological conditions, the growing temperature of the inoculum, and heating menstruum (fat, solids, and sugar concentration) besides bacterial recovery method (Nazarowec-White and Farber, 1997). Moreover, these variations in D˗values may be due to differences in the environmental condition of the inspected isolates (Chauhan et al., 2020). The z˗value was also calculated in this study, it ranged from 2.50°C to 4.28°C, which was lower than previously reported for C. sakazakii. Both studies by Edelson-Mammel and Buchanan (2004) and Iversen et al. (2004) found that the z˗values were ranging from 5.6°C to 5.8°C. Nevertheless, Al-Holy et al. (2009) reported much higher z-values, 3.76°C and 10.11°C. Furthermore, the highest z˗ value (17.42°C) was reported for C. sakazakii by (Awadallah et al., 2018). With regard to C. pulveris, the D˗value and z˗value have not been reported to date. As shown in Table 2, the z˗value ranged from 3.33°Cto 4.89°C which was relatively similar to C. sakazakii results. In comparison, D48 ranged from 7.60 to 22.32 minutes while D52 ranged from 1.42 to 8.45 minutes which were lower than that reported for the same D-values of C. sakazakii. On the other hand, the findings of D56, D60, D64, and D66 values for C. pulveris strains were correspondingly equivalent to that reported for C. sakazakii. All of these results were in agreement with the WHO which recommends using hot water at more than 70°C to prepare infant formula to reduce the risk of viable Cronobacter cells (Al-Nabulsi et al., 2009). Table 2. D- and z-values of tested Cronobacter strains.
Fig. 1. Thermal inactivation of C. sakazakii strains (CS1, CS3, CS4, CS5 and CS6) at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C in RIMF.
Fig. 2. Thermal inactivation of C. pulveris strains (CP1, CP2, CP3 and CP4) at 48°C, 52°C, 56°C, 60°C, 64°C, and 66°C in RIMF.
Fig. 3. Thermal death time curve of strains of C. sakazakii (left) and C. pulveris (right) in RIMF ConclusionIn conclusion, IMF has been recognized as the major vehicle of transmission of C. sakazakii especially when prepared under unhygienic conditions. C. sakazakii was previously isolated from infant products sold in the Libyan market. The thermal resistance of Cronobacter spp. is considered a vital parameter assisting infection of infant formula. In this work, the extent of thermos-tolerance amongst dissimilar Cronobacter isolates was evaluated by calculating D-value and z-value for each isolate. The results of this study indicate that a significant disparity in thermal resistance between C. sakazakii isolates is noted. Some C. sakazakii isolates can grow and proliferate at cooling temperatures with time. With regard to C. pulveris, the z˗ value was relatively similar to C. sakazakii results. It is likely that the usage of hot water at more than 70°C is essential to prepare infant formula as WHO recommends. To date, only a few studies considered the growth variation in heat resistance amongst C. sakazakii strains in infant products. Moreover, this is the first study in Libya investigating the thermos-tolerance of Cronobacter species in RIMF. This study may contribute to reducing the risk of C. sakazakii survival during the preparation of infant food. Authors’ contributionsA.M.G, S.M.A, and S.A.F. planned and designed the study. S.M.A, S.A.F, J.A.S, A.F.L, and F.T.G carried out the laboratory work. All authors A.M.G, S.A.F, S.M.A, J.A.S, A.F.L, H.L.E, F.T.G, H.T.N, A.A.E.S. and I.M.D contributed equally to the analysis and presentation of data in addition to the preparation and revision of the manuscript. All authors have read and approved the final manuscript. Competing interestsThe authors declare that they have no competing interests. ReferencesAl-Holy, M.A., Lin, M., Al-Qadiri, H.M. and Rasco, B.A. 2008. A comparative study between overlay method and selective-differential media for recovery of stressed enterobacter sakazakii cells from infant formula. Food Microbiol. 25(1), 22–28. Al-Holy, M.A., Lin, M., Abu-Ghoush, M.M., Al-Qadiri, H. and Rasco, B.A. 2009. Thermal resistance, survival and inactivation of enterobacter sakazakii (Cronobacter spp.) in powdered and reconstituted infant formula. J. Food Saf. 29(2), 287–301. Al-Holy, M.A., Castro, L.F. and Al-Qadiri, H. 2010. Inactivation of cronobacter spp.(enterobacter sakazakii) in infant formula using lactic acid, copper sulfate and monolaurin. Lett. Appl. Microbiol. 50(3), 246–251. Al-Nabulsi, A.A., Osaili, T.M., Shaker, R.R., Olaimat, A.N., Ayyash, M.M. and Holley, R.A. 2009. Survival of Cronobacter species in reconstituted herbal infant teas and their sensitivity to bovine lactoferrin. J. Food Sci. 74(9), 479–484. Awadallah, M.A., Ahmed, H.A., Merwad, A.M., Abou Elez, R.M. and Saleh, K.M. 2018. Molecular characterization of cronobacter sakazakii in egypt, survival and thermoresistance at different temperatures: a potential public health risk. Vector Borne Zoo. Dis. 18(2), 101–107. Azwai, S.M., Farag, S.A., Garbaj, A.M., Sherif, J.A., Lawila, A.F., Eshamah, H.L., Gammoudi, F.T., Naas, H.T. and Eldaghayes, I.M. 2022. Survival and Viability of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula at various storage temperatures. EC Microbiol. 18(11), 20-29. Bai, Y., Yu, H., Du, G., Fei, S. and Shi, C. 2019. Survival and environmental stress resistance of cronobacter sakazakii exposed to vacuum or air packaging and stored at different temperatures. Front. Microbiol. 10, 303–309. Baker, P., Santos, T., Neves, P.A., Machado, P., Smith, J., Piwoz, E., Barros, A.J., Victora, C.G. and McCoy, D. 2021. First-food systems transformations and the ultra-processing of infant and young child diets: the determinants, dynamics and consequences of the global rise in commercial milk formula consumption. Mater. Child. Nutr. 17(2), 13097–13114. Chauhan, R., Bansal, S., Azmi, W. and Goel, G. 2020. Increased thermal tolerance in cronobacter sakazakii strains in reconstituted milk powder due to cross protection by physiological stresses. J. Food Saf. 40(4), 12810–12818. Costa, P.V., Vasconcellos, L., da Silva, I.C., de Mello Medeiros, V., Forsythe, S.J. and Brandão, M.L.L. 2020. Multi-locus sequence typing and antimicrobial susceptibility profile of cronobacter sakazakii and cronobacter malonaticus isolated from corn-based farinaceous foods commercialized in Brazil. Food Res. Int. 129, 108805–108811. Edelson-Mammel, S.G. and Buchanan, R.L. 2004. Thermal inactivation of enterobacter sakazakii in rehydrated infant formula. J. Food Prot. 67(1), 60–63. Garbaj, A., Abolghait, S., Lawila, A., Azwai, S., Naas, H., Moawad, A., Gammoudi, F., Barbieri, I., Abureema, S. and Eldaghayes, I. 2017. Moleculer identification, prevalence and antimicrobial susceptibility profile of Cronobacter spp. cultivated on a chromogenic medium in Libya. J. Mol. Microbiol. 1(1), 1–9. Garbaj, A.M., Ftayem, T., Eshamah, H.L., Abureema, S.F. and Said, K. 2015 Bacteriological quality of infant milk formula in Tripoli city, Libya. Libyan J. Vet. Med. Sci. 1(1), 11–15. Iversen, C., Lane, M. and Forsythe, S. 2004. The growth profile, thermotolerance and biofilm formation of enterobacter sakazakii grown in infant formula milk. Lett. Appl. Microbiol. 38(5), 378–382. Muytjens, H.L., Zanen, H., Sonderkamp, H.J., Kollée, L.A., Wachsmuth, I.K. and Farmer, J. 1983. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J. Clin. Microbiol. 18(1), 115–120. Najdovski, L., Dragaš, A.Z. and Kotnik, V. 1991. The killing activity of microwaves on some non-sporogenic and sporogenic medically important bacterial strains. J. Hosp. Infect. 19(4), 239–247. Nazarowec-White, M. and Farber, J. 1997. Thermal resistance of enterobacter sakazakii in reconstituted dried-infant formula. Lett. Appl. Microbiol. 24(1), 9–13. Osaili, T.M., Shaker, R.R., Ayyash, M.M., Al-Nabulsi, A.A. and Forsythe, S.J. 2009. Survival and growth of Cronobacter species (Enterobacter sakazakii) in wheat-based infant follow-on formulas. Lett. Appl. Microbiol. 48(4), 408–412. Stephan, R., Grim, C.J., Gopinath, G.R., Mammel, M.K., Sathyamoorthy, V., Trach, L.H., Chase, H.R., Fanning, S. and Tall, B.D. 2014. Re-examination of the taxonomic status of Enterobacter helveticus, Enterobacter pulveris and Enterobacter turicensis as members of the genus Cronobacter and their reclassification in the genera Franconibacter gen. nov. and Siccibacter gen. nov. as Franconibacter helveticus comb. nov., Franconibacter pulveris comb. nov. and Siccibacter turicensis comb. nov., respectively. Int. J. System. Evol. Microbiol. 64, 3402–3410. Walsh, D., Molloy, C., Iversen, C., Carroll, J., Cagney, C., Fanning, S. and Duffy, G. 2011. Survival characteristics of environmental and clinically derived strains of Cronobacter sakazakii in infant milk formula (IMF) and ingredients. J. Appl. Microbiol. 110(3), 697–703. Xu, Y.Z., Métris, A., Stasinopoulos, D., Forsythe, S. and Sutherland, J. 2015. Effect of heat shock and recovery temperature on variability of single cell lag time of Cronobacter turicensis. Food Microbiol. 45, 195–204. Yang, H.-Y., Kim, S.-K., Choi, S.-Y., You, D.-H., Lee, S.-C., Bang, W.-S. and Yuk, H.-G. 2015. Effect of acid, desiccation and heat stresses on the viability of Cronobacter sakazakii during rehydration of powdered infant formula and in simulated gastric fluid. Food Control. 50, 336–341. Yemiş, G.P. and Delaquis, P. 2020. Natural compounds with antibacterial activity against cronobacter spp. In powdered infant formula: a review. Front. Nutr. 7, 595964–595978. Zhang, S., Xiong, J., Lou, W., Ning, Z., Zhang, D. and Yang, J. 2020. Inhibition of cronobacter sakazakii in reconstituted infant formula using triglycerol monolaurate and its effect on the sensory properties of infant formula. Int. J. Food Microbiol. 320, 1–36. | ||
| How to Cite this Article |
| Pubmed Style Garbaj AM, Farag SA, Sherif JA, Lawila AF, Eshamah HL, Azwai SM, Gammoudi FT, Naas HT, El-Salabi AA, Eldaghayes IM. Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Vet J. 2023; 13(1): 108-113. doi:10.5455/OVJ.2023.v13.i1.11 Web Style Garbaj AM, Farag SA, Sherif JA, Lawila AF, Eshamah HL, Azwai SM, Gammoudi FT, Naas HT, El-Salabi AA, Eldaghayes IM. Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. https://www.openveterinaryjournal.com/?mno=138198 [Access: April 30, 2024]. doi:10.5455/OVJ.2023.v13.i1.11 AMA (American Medical Association) Style Garbaj AM, Farag SA, Sherif JA, Lawila AF, Eshamah HL, Azwai SM, Gammoudi FT, Naas HT, El-Salabi AA, Eldaghayes IM. Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Vet J. 2023; 13(1): 108-113. doi:10.5455/OVJ.2023.v13.i1.11 Vancouver/ICMJE Style Garbaj AM, Farag SA, Sherif JA, Lawila AF, Eshamah HL, Azwai SM, Gammoudi FT, Naas HT, El-Salabi AA, Eldaghayes IM. Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Vet J. (2023), [cited April 30, 2024]; 13(1): 108-113. doi:10.5455/OVJ.2023.v13.i1.11 Harvard Style Garbaj, A. M., Farag, . S. A., Sherif, . J. A., Lawila, . A. F., Eshamah, . H. L., Azwai, . S. M., Gammoudi, . F. T., Naas, . H. T., El-Salabi, . A. A. & Eldaghayes, . I. M. (2023) Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Vet J, 13 (1), 108-113. doi:10.5455/OVJ.2023.v13.i1.11 Turabian Style Garbaj, Aboubaker M., Samira A. Farag, Jihan A. Sherif, Aml F. Lawila, Hanan L. Eshamah, Salah M. Azwai, Fatim T. Gammoudi, Hesham T. Naas, Allaaeddin A. El-Salabi, and Ibrahim M. Eldaghayes. 2023. Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Veterinary Journal, 13 (1), 108-113. doi:10.5455/OVJ.2023.v13.i1.11 Chicago Style Garbaj, Aboubaker M., Samira A. Farag, Jihan A. Sherif, Aml F. Lawila, Hanan L. Eshamah, Salah M. Azwai, Fatim T. Gammoudi, Hesham T. Naas, Allaaeddin A. El-Salabi, and Ibrahim M. Eldaghayes. "Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula." Open Veterinary Journal 13 (2023), 108-113. doi:10.5455/OVJ.2023.v13.i1.11 MLA (The Modern Language Association) Style Garbaj, Aboubaker M., Samira A. Farag, Jihan A. Sherif, Aml F. Lawila, Hanan L. Eshamah, Salah M. Azwai, Fatim T. Gammoudi, Hesham T. Naas, Allaaeddin A. El-Salabi, and Ibrahim M. Eldaghayes. "Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula." Open Veterinary Journal 13.1 (2023), 108-113. Print. doi:10.5455/OVJ.2023.v13.i1.11 APA (American Psychological Association) Style Garbaj, A. M., Farag, . S. A., Sherif, . J. A., Lawila, . A. F., Eshamah, . H. L., Azwai, . S. M., Gammoudi, . F. T., Naas, . H. T., El-Salabi, . A. A. & Eldaghayes, . I. M. (2023) Thermal tolerance of Cronobacter sakazakii and Cronobacter pulveris in reconstituted infant milk formula. Open Veterinary Journal, 13 (1), 108-113. doi:10.5455/OVJ.2023.v13.i1.11 |