| Case Report | ||
Open Vet J. 2023; 13(9): 1212-1218 Open Veterinary Journal, (2023), Vol. 13(9): 1212–1218 Case Report Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumor in a Warmblood mareSimone Della Tommasa*, Susanne Pauline Roth, Theresa Triebe, Walter Brehm, Katharina Luise Lohmann and Sabita Diana StöckleDepartment for Horses, University of Leipzig, Leipzig, Germany *Corresponding Author: Simone Della Tommasa. Department for Horses, University of Leipzig, Leipzig, Germany. Email: della.tommasa [at] vetmed.uni-leipzig.de Submitted: 13/07/2023 Accepted: 16/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
AbstractBackground: Granulosa cell tumors (GCTs) are the most common ovarian tumors in mares. The classical presentation of a GCT is a unilaterally enlarged ovary appearing as a multicystic honeycomb mass. In rare cases, GCTs cause hemoperitoneum as a result of the rapid growth of the tumor. The clinical diagnosis of GCT is usually based on history, rectal examination, ultrasonographic examination, and serum hormone analysis, and surgical removal of the affected ovary is the treatment of choice. The different surgical approaches are based on the dimension of the GCT. Case Description: A 7-year-old mare was referred to the department for horses due to suspicion of a large colon impaction. The mare presented with clinical signs of colic, fever, and signs of hypovolemic shock. Rectal and ultrasonographic examination showed hemoperitoneum and a honey-comb mass within the abdomen, and a GCT as the cause of an acute hemoperitoneum was diagnosed based on the serum level of anti-Müllerian hormone. After stabilization of the mare, the GCT was removed through a ventral midline incision. Because of the enormous dimensions of the GCT, intra-abdominal partial resection of the tumor using a tenotomy knife was necessary to exteriorize the ovarian pedicle. At 3 months follow-up, the mare was ridden for her intended use. Conclusion: This report provides an approach to an uncommon case of a very large and heavy GCT. Keywords: Granulosa cell tumor, Ovariectomy, Hemoperitoneum, Hemorrhage, Laparotomy. IntroductionGranulosa cell tumors (GCTs) are the most common ovarian tumors in mares (Meagher et al., 1977; Sundberg et al., 1977; McCue et al., 2006), representing >85% of reproductive neoplasms (McCue, 1998) and approximately 2.5% of all neoplasms in horses (Sundberg et al., 1977). All GCTs derive from the follicular granulosa cells (Sundberg et al., 1977; Kennedy et al., 1998), with a subset of granulosa theca cell tumors (GTCTs) also comprising a distinct theca-derived component (Kennedy et al., 1998) that can be demonstrated structurally by histopathology and functionally by a capacity for androgen secretion (Neto et al., 2010). The classical presentation of a GCT is a unilaterally enlarged ovary lacking a palpable ovulation fossa, and a small, inactive contralateral ovary. In addition, many GCTs (>83% of cases) appear as a multicystic (“honeycomb”) mass (Sherlock et al., 2016; Murase et al., 2018), while some (7% of cases) present as a solid ovarian mass (Sherlock et al., 2016) or even a single fluid-filled cyst (Hinrichs and Hunt, 1990; McCue, 1998; Renaudin et al., 2021). Affected mares may exhibit prolonged anoestrus, continuous or intermittent oestrus (nymphomania behavior), or aggressive, stallion-like behavior (Meagher et al., 1977; Hinrichs and Hunt, 1990; Neto et al., 2010). Without a histopathologically examined biopsy, there is no definitive diagnostic test for GTCT and GCTs in horses, and identification is usually based on history, rectal examination, ultrasonographic examination, and serum hormone analysis (Hinrichs and Hunt, 1990; Christman et al., 1999). Surgical removal of the affected ovary is the treatment of choice, (Meagher et al., 1977; Perino and Didier, 1985; McCue, 1998) as these tumors rarely metastasize (Bosu et al., 1982; Perino and Didier, 1985; Meagher et al., 1977). The surgical approach of choice for the removal of a GCT is a laparoscopic ovariectomy in the standing mare (Smith and Mair, 2008; Sinovich et al., 2022). Ovariectomy under general anesthesia is preferred in horses with large GCTs that cannot be removed via flank laparotomy. GCTs and GTCT have been reported to cause hemoperitoneum as a result of rupture before or during surgery (Alexander et al., 2004; Harper et al., 2010; Sherlock et al., 2016; Worsman et al., 2018). This rupture may be caused by the rapid growth of the tumor. Patient presentation of a hemorrhagic GCT is nonspecific and does not always present as a hemorrhagic shock (Hinrichs and Hunt, 1990; Dechant et al., 2006; Conwell et al., 2010; Crabtree, 2011; Renaudin et al., 2021). Due to the lack of specificity in clinical signs, it is difficult to arrive at a definitive diagnosis before surgery, but GCT should be considered as a differential diagnosis in the case of hemoperitoneum in mares (Hinrichs and Hunt, 1990; Dechant et al., 2006; Crabtree, 2011; Renaudin et al., 2021). This case report describes a warmblood mare with acute intra-abdominal hemorrhage caused by a large and heavy (24 kg) GCT that was removed by a ventral midline laparotomy. Case DetailsA 7-year-old warmblood mare was referred to the Department for Horses, University of Leipzig, because of a suspected impaction of the ascending large colon. On initial examination, the mare showed tachycardia (60 beats/minute), tachypnea (40 breaths/minute), and fever (38.7°C). She had pale mucous membranes and a prolonged capillary refill time of more than 3 seconds. Borborygmi were decreased and she showed mild to moderate abdominal discomfort. A routine ultrasonographic examination of the abdomen revealed a large amount of echogenic, swirling free fluid suggestive of hemoperitoneum (Fig. 1). Free fluid was visible inguinal bilaterally and ventrally and hemoperitoneum was confirmed by abdominocentesis. The abdominal fluid was red, turbid, and contained mostly erythrocytes (5.73 T/l). Transrectal palpation revealed a firm structure in the ventral abdominal cavity, which extended across the abdomen from the right to the left side. This structure was initially suspected to be an impaction. Nasogastric intubation yielded no reflux. Hematologic examination showed profound anemia (PCV 14%, reference interval 27%–39%, RBC 2.79 T/l, reference interval 6.6–9.8 T/l, hemoglobin 2.9 mmol/l, reference interval 7.2–10.2 mmol/l), and leucocytosis (15 g/l, reference interval 4.4–12 g/l), which was characterized by neutrophilia (13.95 g/l, reference interval 1.6–8.7 g/l). The mare also showed hypoproteinaemia (total protein 54.7 g/l, reference interval 57.8–78.7 g/l) and azotemia (creatinine: 249 μmol/l, reference interval 83.7–156.4 μmol/l, urea 22.77 mmol/l, reference interval 2.51–7.34 mmol/l) that was suspected to be prerenal in origin. Plasma concentrations of cholesterol (3.5 mmol/l, reference interval 1.72–2.95 mmol/l) and triglycerides (4.82 mmol/l, reference interval 0.13–0.61 mmol/l), as well as the activities of creatine kinase (4,873 U/l, reference interval 146–354), lactate dehydrogenase (2,182 U/l, reference interval 223.9–536.3 U/l), and aspartate amino transferase (1,597, reference interval 213.2–626.7) were elevated. Minor electrolyte derangements were present. The mare was stabilized with crystalloid fluid therapy (Lactated Ringer’s solution) and received a blood transfusion. Since the initial blood donor showed clinical signs of colic after collection of 4 l of blood, a further 2 l of blood from a second blood donor was collected. After the transfusion of approximately 450 ml of blood, the mare developed a cardiac arrhythmia based on auscultation that resolved without additional treatment after pausing the transfusion. The arrhythmia had resolved by the time an ECG tracing was obtained, and the remainder of the transfusion was administered without further complications under ECG monitoring. The transfusion volume was limited to 6 l of blood as the owners declined transfusion of a larger volume due to financial constraints. The mare was cautiously treated with laxatives (enteral and parenteral fluids, macrogol 0.265 g/kg) based on the initial suspicion of colonic impaction. Because of a perceived increased risk of sepsis with hemoperitoneum, the mare received metaphylactic antibiotics (amoxicillin 10 mg/kg IV BID, Amoxicillin-Natrium®, CP-Pharma; gentamicin 6.6 mg/kg IV SID, Genta®, CP-Pharma). Additional treatments included flunixin meglumine (0.55 mg/kg IV BID, Flunidol®, CP-Pharma) as an analgesic and etamsylat (7 mg/kg IV TID, Hemosilate®, Ecuphar GmbH, Greifswald/Germany) to support hemostasis. During the following 3 days, the hemoperitoneum regressed and the mare no longer showed signs of abdominal discomfort. Ultrasonographically, a mass with a honeycomb structure was visible bilaterally in the inguinal region of the abdomen (Fig. 2). The firm structure that was palpable transrectally was unchanged, and transrectal ultrasonography revealed that it was the same mass as seen inguinally. Differential diagnoses included a GCT, GTCT, and hemangiosarcoma. Since the owner declined laparoscopy and biopsy, the blood concentration of anti-Mullerian hormone was determined and was highly suggestive of a GCT (22.96 ng/ml; reference interval for healthy mares <4 ng/ml, equivocal 4–7 ng/ml, suggestive of GCT >7 ng/ml). The mare was discharged from the hospital 14 days after the initial presentation since the owners initially declined further treatment due to a guarded prognosis and financial limitations. At the time of hospital discharge, the mare was clinically stable, and the anemia had improved (PCV 29%, reference interval 27%–39%, RBC 5.91 T/l, reference interval 6.6–9.8 T/l, hemoglobin 6.3 mmol/l, reference interval 7.2–10.2 mmol/l) and the amount of intra-abdominal free fluid had significantly decreased.
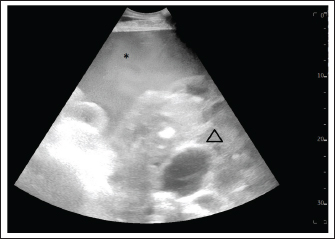
Fig. 1. Ultrasound examination of the abdomen on the first admission. The image was taken at the left inguinal region. Hemoperitoneum was suspected based on a large amount of echogenic and swirling free fluid (*) and was confirmed by abdominocentesis. The honeycomb structure (triangle) was visible in the entire ventral side of the abdomen, pushing against the spleen and stomach on the left side and reaching the kidney and liver on the right side. The honeycomb structure was visible up to the 9th intercostal space.
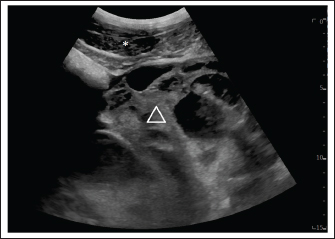
Fig. 2. Ultrasound examination of the abdomen on admission, one month after initial discharge. The image was acquired in the left inguinal region. The hemoperitoneum had completely resolved. The hemorrhage caused severe subcutaneous oedema (*). The honeycomb structure is visible (triangle) and has not increased in size. Without hemoperitoneum, it was easier to follow the shape of the GCT, excluding interaction with other organs. Four weeks following hospital discharge, the owners contacted the department for horses again and consented to surgical removal of the intraabdominal mass. Surgical treatmentThe mare was re-admitted for unilateral ovariectomy 4 weeks after hospital discharge. She appeared bright, alert, and responsive, and her vital parameters were within normal limits. On ultrasonographic examination, a minor amount of free abdominal fluid was evident. The honeycomb-structured mass was visible on both sides of the abdomen, extending from the kidneys to the ventral midline. Hematologic examination showed moderate anemia (PCV 20%, reference interval 27%–39%, RBC 4.98 T/l, reference interval 6.6–9.8 T/l, hemoglobin 4.9 mmol/l, reference interval 7.2–10.2 mmol/l, MCHC 24.5 mmol/l, reference interval 25.32–27.64 mmol/l). Exploratory laparotomy was considered the safest approach taking into consideration all the advantages and disadvantages. A two-step surgery and the use of a motorized morcellator were considered first; however, these approaches were deemed not feasible for different reasons. The size of the tumor was expected to preclude sufficient visibility after insertion of the optic into the abdomen, and it was anticipated that it would not be possible to move the GCT sufficiently using laparoscopic instruments to visualize the ovarian pedicle. Furthermore, the use of a motorized morcellator had not been described previously for use in large GCT. Preoperatively, the mare received amoxicillin (10 mg/kg IV BID, Amoxicillin-Natrium®, CP-Pharma), and gentamicin (6.6 mg/kg bwt iv SID, Genta®, CP-Pharma). Systemic analgesia was provided with levomethadonhydrochloride (0.1 mg/kg iv, L-Polamivet®, MSD Animal Health) and flunixin meglumine (1.1 mg/kg bwt SID iv, Flunidol®, CP-Pharma). The mare was premedicated with romifidine (0.08 mg/kg iv, Sedivet®, Boehringer Ingelheim) and was induced with rapid injection of Diazepam (0.06 mg/kg, Solupam®, Dechra) in combination with ketamine (2.2 mg/kg iv, Ursotamin®, DK-Pharma GmbH). After induction of anesthesia, the horse was intubated and positioned in dorsal recumbency. Anaesthesia was maintained with a mixture of isoflurane (MAC of ~1.3% cf) and oxygen (MAC of 5 l/minute) in combination with a constant rate infusion of ketamine (2.2 mg/kg/hour, Ursotamin®, DK-Pharma GmbH). Immediately after a 30 cm long ventral midline incision, the GCT was visible in the surgical field (Fig. 3). It extended across the abdomen from the right to the left side. On the right side, it was adjacent to the caecum and the ascending colon, and the small intestine was visible underneath the GCT. Cranially, the GCT extended to the stomach and the caudal part of the lateral right lobe of the liver. Following the caudal surface of the GCT, the association of the GCT with the right ovary was ascertained. The right uterine horn and oviduct were palpable. Exteriorization of any part of the intestine was not possible due to the dimension and weight of the GCT. The ovarian pedicle and the uterus horn were also positioned underneath the GCT and could not be exteriorized. Due to the size of the mass, the only way to exteriorize the GCT and to ligate blood vessels was after its dissection into smaller segments. For the procedure, a tenotomy knife was used to transect the GCT in its length and approximately 1/3 of the mass was removed. Dissection of the GCT was performed blindly under manual control. The partial resection of the GCT was started in the ventral part of the GCT, which was visible after the ventral midline incision. Following the structure of the GCT, the dissection was continued dorsally, in the sagittal plane of the GCT. During the dissection, the bowel was protected under the hand of the surgeon performing the dissection, and an assistant provided support by removing the bowel from the incision line. The consistency of the GCT was firm and hard. The last centimeter of the dissected GCT, which was still connected to the main GCT, was bluntly separated by hand to avoid damage to the organs by the tenotomy knife. After partial ovariectomy, the GCT, the right ovarian pedicle, and the right uterine horn could be exteriorized (Fig. 4). As bleeding had occurred during dissection, blood vessels of the ovarian pedicle were sealed using a vessel-sealing device (Ligasure®, Covidien) before the remaining part of the GCT was removed (Fig. 5). The ovarian bleeding stopped as soon as the ovarian pedicle was sealed. After GCT removal, the abdomen was lavaged with 20 l sterile saline solution to remove fibrin clots remaining from the previous hemoperitoneum, and to prevent adhesion formation (Della Tommasa et al., 2022) The ventral midline was closed in four layers, using a simple continuous pattern for the peritoneum, a conventional continuous technique for the fascia, and simple continuous pattern for the subcutaneous and intra-cutaneous tissues. In the recovery stall, a belly bandage was applied. The mare recovered uneventfully. The removed GCT weighed 24.6 kg and had a diameter of 107 cm. Histopathology confirmed the diagnosis showing the presence of an extensively necrotic GCT with severe hemorrhages, multifocal fibrinous-purulent inflammation, and focal hematoma formation. Due to intraoperative bleeding, the mare developed severe anemia (PCV 13%) causing an increased heart rate (68 beats per minute). Therefore, a blood transfusion was performed. A different horse than the ones used for the first transfusion served as a blood donor. During the transfusion, the mare developed an adverse reaction showing fever and urticaria; therefore, the transfusion was stopped after 1.35 l and the mare received prednisolone-21-hydrogensuccinate as sodium salt (2 mg/kg, Prednisolut® 250 mg, mibe GmbH Arzneimittel, Brehna/Germany). As vital parameters normalized during the subsequent hour, transfusion of additional blood was not pursued. During hospitalization, the mare received antibiotics (amoxicillin 10 mg/kg IV BID, Amoxicillin-Natrium®, CP-Pharma, CP-Pharma; gentamicin 6.6 mg/kg IV SID, Genta®, CP-Pharma) as well as flunixin meglumine (0.55 mg/kg IV BID, Flunidol®, CP-Pharma) for 5 days after surgery. The laparotomy incision was well-adapted and showed no signs of surgical site infection. The PCV stabilized and increased to 24% before discharge from the hospital 8 days following surgery.
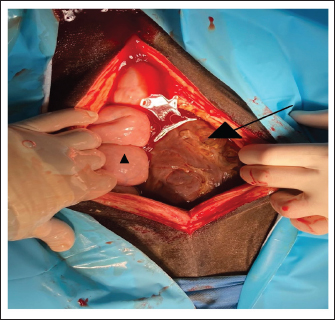
Fig. 3. Surgical view following ventral midline laparotomy with the horse in dorsal recumbency. Cranial is at the bottom of the image. The GCT is seen against the abdominal wall (arrow). A small segment of the small intestine was visible (triangle). The dimension of the tumor did not allow any exteriorization from the large or small intestine and any manipulation was possible. The mare was discharged with recommendations for an additional 5 weeks of stall confinement with only shorthand walks (5 minute daily, increasing by 5 minute each week). Follow-up information was obtained by e-mail 3 months after the surgery and showed that the mare was doing well and being ridden again. During the follow-up period, the mare did not show any abdominal discomfort, colic signs, or stallion-like behavior. The serum concentration of the ani-Muellerian hormone was not determined after the surgery.
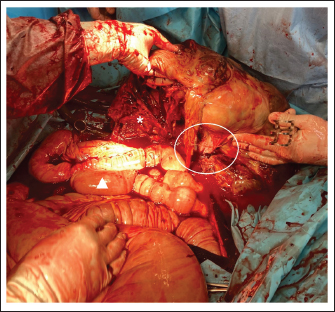
Fig. 4. Intraoperative image of the GCT. Cranial is at the bottom of the image. The ventral midline incision was widened to remove part of the resected GCT and to exteriorize the tumor still attached to the uterine horn. Part of the tumor (*) was resected with a tenotomy knife and the typical honeycomb-like appearance is visible. The right ovarian pedicle (circle) was exteriorized and resected with a sealed device. The bleeding stopped as soon as the ovarian pedicle was sealed. The intestine (triangle) was finally exteriorized after partial removal of the tumor and the abdominal cavity was inspected.
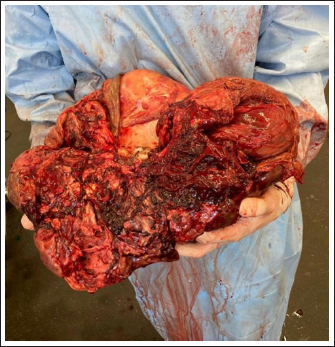
Fig. 5. The GCT was removed after a partial intra-abdominal resection. Part of the tumor that had been resected intra-abdominally is missing. The triangle indicates where the resection was performed. Due to the high blood supply to the tumor, bleeding is inevitable during intra-abdominal resection. The time between partial resection and sealing of the ovarian pedicle must be minimized to stop bleeding as soon as possible. DiscussionThe most commonly described causes of hemoperitoneum in horses include neoplasia (approximately 13% of cases), idiopathic hemorrhage (22%), uterine injury (22%), blood vessel rupture (20%), and splenic lesions (19%) (Sherlock et al., 2016). Trauma is thought to explain as many as 9% of cases (Worsman et al., 2018), while hemoperitoneum due to ovarian hematoma or GCT is less common. GCT was reported to cause hemoperitoneum as a result of rupture before or during surgery (Alexander et al., 2004; Harper et al., 2010; Sherlock et al., 2016; Worsman et al., 2018), and spontaneous rupture may occur due to rapid growth of the tumor (Worsman et al., 2018). In reported cases of hemoperitoneum caused by GCT, hemorrhage was severe and sometimes life-threatening due to systemic cardiovascular compromise. The treatment of choice for GCT is surgical removal (Alexander et al., 2004; Crabtree, 2011; Sherlock et al., 2016) and several surgical approaches for ovariectomy have been described. These include midline, flank, and paramedian laparotomy, colpotomy, and bilateral or unilateral laparoscopic approaches in the standing sedated horse (Smith and Mair, 2008; Gee et al., 2012; Ragle, 2012; Daniel et al., 2015; Colbath et al., 2017; Sinovich et al., 2022). Conservative treatment in some cases was followed by surgical removal at a later stage (Alexander et al., 2004) once medical stabilization was achieved. In the case reported here, the duration of tumor growth before clinical presentation was unknown; however, the clinical appearance at the time of first admission would support Worsman et al.'s (2018) theory that spontaneous rupture may occur due to rapid tumor growth. Usually, these tumors are due to increased excretion of specific reproductive hormones by the tumor, such as testosterone and inhibin, and in this particular case, the young age of the mare should also be considered. In fact, GCT can be found in mares of any age, but the average age of affected mares is 11 years. Juvenile GCTs are very rare and have presented often with hemoperitoneum in weanlings in other studies (Green et al., 1988; McKinnon and Barker, 2010). Why GCTs in young horses tend to grow so fast and produce hemorrhages is not entirely clear, but could be related to the stage of hormonal development in which weanlings are involved. It would be interesting to compare the levels of the different hormones at a young age to better understand the progression of the disease. The size of the GCT did not allow for a standing ovariectomy, which is routinely performed laparoscopically or hand-assisted (Ragle, 2012; Daniel et al., 2015). The two-step surgery and the use of a motorized morcellator were considered. The two-step surgery comprises the first step of detaching the ovarian pedicle in a standing laparoscopy, followed by removal by laparotomy in a second step. This method was not used here because it was expected that the size of the tumor would preclude sufficient visibility after insertion of the optic into the abdomen and that it would not be possible to move the GCT sufficiently using laparoscopic instruments to visualize the ovarian pedicle. The use of a motorized morcellator is described as a method of ovariectomy with good results (Lund et al., 2014); however, all of the treated mares underwent bilateral ovariectomy and none showed ovarian neoplasia (Lund et al., 2014). In addition, the dimensions of the ovaries of the treated mares were considered physiologic (Lund et al., 2014). Based on these assessments, it was decided to perform the ovariectomy under general anesthesia through a ventral midline incision. General anesthesia is always considered a high-risk option in critically ill horses; however, the mare appeared sufficiently stable based on clinical and hematological examination. Furthermore, diagnostic laparotomy allowed the exclusion of adhesions between the GCT and other organs by providing better visualization and evaluation of the ovarian pedicle (Conwell et al., 2010; Harper et al., 2010; Finding et al., 2011; Worsman et al., 2018). For safety reasons, a GCT should generally not be cut, but in this specific case, it was the only option. One of the main disadvantages of the described technique is that the GCT was dissected blindly in the abdomen. This is a high-risk technique because the bowel or other organs could be damaged. To avoid injury to the intestine or other organs, it was important to cut the GCT under manual control, staying in the middle of the tumor, and limiting transection to parts of the tumor that could be reached manually to protect the tenotomy knife. In addition, once the tumor was partially resected, bleeding was inevitable since the ovarian artery was still connected to the GCT. In fact, one of the main complications of this technique was the intra-operative hemorrhage following partial ovariectomy. Therefore, thetime between partial ovariectomy and sealing of the ovarian pedicle had to be minimized to prevent hypovolemic shock. The ligation of the ovarian pedicle is usually the first step in ovariectomy, but in this case, it was not possible because the pedicle lay under the GCT and could not be reached and ligated until partial resection of the tumor had been performed. All necessary instruments were ready to stop the bleeding once the ovarian pedicle could be reached, and preparations had been made for an immediate blood transfusion in case of massive bleeding. An additional limitation of the approach described here is the possibility of metastasis due to intra-abdominal splitting of the GCT. Aside from a key role in resolving possible adhesions, abdominal lavage with copious amounts of fluid during laparotomy was intended to remove tumor cells that may have been spread during resection and thereby limit the possibility of their diffusion and metastasis. Based on the previous literature, metastasis of GCT is rare (Bosu et al., 1982; Perino and Didier, 1985; Meagher et al., 1977); however, long-time follow-up will be necessary to assess this complication in the case presented here. In conclusion, this case report describes a strategy to solve a difficult presentation of a large GCT with a favorable outcome. The surgical approach included intra-abdominal splitting of the ovarian tumor to permit access to the ovarian pedicle and subsequent removal of the GCT via a midline laparotomy. Because of possible complications, primarily uncontrolled bleeding, and the unclear risk of metastasis of the GCT following intra-abdominal transection, this approach should only be used in specific and complicated cases, where conventional surgical techniques cannot be applied. AcknowledgmentNone. Conflict of interestThe authors declare that there is no conflict of interest Author contributionsConception and design of the case report SDT, SDS, and KLL; WB, SPR, and SDT performed the surgical treatment; KLL, SDS, and TJT performed the preoperative and postoperative treatment of the mare. All authors participated in the preparation of the draft manuscript. All authors reviewed the results and approved the final version of the manuscript. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Data availabilityThe authors confirm that the data supporting the findings of this study are available within the article. ReferencesAlexander, G.R., Tweedie, M.A., Lescun, T.B. and McKinnon, A.O. 2004. Haemoperitoneum secondary to granulosa cell tumour in two mares. Aust. Vet. J. 82(8), 481–484. Bosu, W.T., Van Camp, S.C., Miller, R.B. and Owen, R.R. 1982. Ovarian disorders: clinical and morphological observations in 30 mares. Can. Vet. J. 23(1), 6–14. Christman, S.A., Bailey, M.T., Wheaton, J.E., Troedsson, M.H., Ababneh, M.M. and Santschi, E.M. 1999. Dimeric inhibin concentrations in mares with granulosa-theca cell tumors. Am. J. Vet. Res. 60(11), 1407–1410. Colbath, A.C., Hackett, E.S., Lesser, C.S. and Hendrickson, D.A. 2017. Left paralumbar laparoscopic bilateral ovariectomy in mares. Vet. Surg. 46(4), 574–579. Conwell, R.C., Hillyer, M.H., Mair, T.S., Pirie, R.S. and Clegg, P.D. 2010. Haemoperitoneum in horses: a retrospective review of 54 cases. Vet. Rec. 167(14), 514–518. Crabtree, J. 2011. Review of seven cases of granulosa cell tumour of the equine ovary. Vet. Rec. 169(10), 251. Daniel, A.J., Easley, J.T., Story, M.R., Hendrickson, D.A. and Hackett, E.S. 2015. Standing hand-assisted laparoscopic removal of large granulosa cell tumours in horses using a specimen retrieval bag and morcellator. Equine Vet. Edu. 27, 505–509. Dechant, J.E., Nieto, J.E. and Le Jeune, S.S. 2006. Hemoperitoneum in horses: 67 cases (1989-2004). J. Am. Vet. Med. Assoc. 229(2), 253–258. Della Tommasa, S., Scharner, D., Brehm, W. and Troillet, A. 2022. Disorders of the sustentaculum tali and the medial trochlear ridge of the talus in horses: novel findings and surgical management of five cases. Vet. Rec. Case Rep. 10(3), 1–8. Finding, E.J.T., Eliashar, E., Johns, I.C. and Dunkel, B. 2011. Autologous blood transfusion following an allogenic transfusion reaction in a case of acute anaemia due to intra-abdominal bleeding. Equine Vet. Edu. 23(7), 339–342. Gee, E.K., Dicken, M., Archer, R.M., Herdan, C.L., Pauwels, F.E. and Drayton, B.M. 2012. Granulosa theca cell tumour in a pregnant mare: concentrations of inhibin and testosterone in serum before and after surgery. N. Z. Vet. J. 60(2), 160–163. Green, S.L., Specht, T.E., Dowling, S.C., Nixon, A.J., Wilson, J.H. and Carrick, J.B. 1988. Hemoperitoneum caused by rupture of a juvenile granulosa cell tumor in an equine neonate. J. Am. Vet. Med. Assoc. 193, 1417–1419. Harper, J., Stewart, A.J., Kuhnt, L., Waguespack, R.W., Holland, M. and Downs, C. 2010. Ultrasonographic appearance and abdominal haemorrhage associated with a juvenile granulosa cell tumour in a foal. Equine Vet. Edu. 22, 115–120. Hinrichs, K. and Hunt, P.R. 1990. Ultrasound as an aid to diagnosis of granulosa cell tumour in the mare. Equine Vet. J. 22, 99–103. Kennedy, P.C., Cullen, J.M., Edwards, J.F., Goldschmidt, M.H., Larsen, S. and Munson, L. 1998. Histological classification of the tumours of the genital system. In World Health Organization international histological classification of tumours of domestic animals. Ed., Kennedy PC. Washington, DC: Armed Forces Institute of Pathology, pp: 24–28. Lund, C.M., Ragle, C.A., Lutter, J.D. and Farnsworth, K.D. 2014. Use of a motorized morcellator for elective bilateral laparoscopic ovariectomy in standing equids: 30 cases (2007-2013). J. Am. Vet. Med. Assoc. 244(10), 1191–1197. McKinnon, A.O. and Barker, K.J. 2010. Granulosa theca cell tumours. Equine Vet. Edu. 22(3), 121–24. McCue, P.M. 1998. Neoplasia of the female reproductive tract. Vet. Clin. North Am. Equine Pract. 14, 505–515. McCue, P.M., Roser, J.F., Munro, C.J., Liu, I.K. and Lasley, B.L. 2006. Granulosa cell tumours of the equine ovary. Vet. Clin. North Am. Equine Pract. 22, 799–817. Meagher, D.M., Wheat, J.D., Hughes, J.P., Stabenfeldt, G.H. and Harris, B.A. 1977. Granulosa cell tumours in mares—a review of 78 cases. Proc. Am. Assoc. Equine Pract. 23, 133–143. Murase, H., Ball, B.A., Tangyuenyong, S., Watanabe, G., Sato, F. and Hada, T. 2018. Serum anti-Müllerian hormone concentrations in mares with granulosa cell tumours versus other ovarian abnormalities. J. Equine Vet. Sci. 60, 6–10. Neto, A.C., Ball, B.A., Browne, P. and Conley, A.J. 2010. Cellular localization of androgen synthesis in equine granulosa-theca cell tumours: immunohistochemical expression of 17alpha-hydroxylase/17,20-lyase cytochrome P450. Theriogenology 74, 393–401. Perino, L.J. and Didier, P.J. 1985. Equine granulosa cell tumours. Equine Pract. 7, 13–17. Ragle, C.A. 2012. Advances in equine laparoscopy. Hoboken, NJ: John Wiley & Sons. Renaudin, C.D., Kelleman, A.A. and Keel, K. 2021. Equine granulosa cell tumours among other ovarian conditions: diagnostic challenges. Equine Vet. J. 53, 60–70. Sherlock, C.E., Lott-Ellis, K., Bergren, A., Withers, J.M., Fews, D. and Mair, T.S. 2016. Granulosa cell tumours in the mare: a review of 52 cases. Equine Vet. Edu. 28, 75–82. Sinovich, M., Archer, D.C., Kane-Smyth, J., Scarabelli, S., Ritchie, A. and Kelly, P.G. 2022. Haemoperitoneum associated with bilateral granulosa cell tumours in a pregnant mare treated by standing ovariectomy. Equine Vet Educ. 34(12), e526–e532. Smith, L.J. and Mair, T.S. 2008. Unilateral and bilateral laparoscopic ovariectomy of mares by electrocautery. Vet. Rec. 163, 297–300. Sundberg, J.P., Burnstein, T., Page, E.H., Kirkham, W.W. and Robinson, F.R. 1977. Neoplasms of equidae. J. Am. Vet. Med. Assoc. 170, 150–152. Worsman, F.C.F., Barakzai, S.Z., de Bont, M.P., Turner, S. and RubioMartınez, L.M. 2018. Treatment of haemoperitoneum secondary to ruptured granulosa cell tumours in two mares. Equine Vet. Edu. 32(2), 71–77. | ||
| How to Cite this Article |
| Pubmed Style Tommasa SD, Roth SP, Triebe T, Brehm W, Lohmann KL, Stockle SD. Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Vet J. 2023; 13(9): 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 Web Style Tommasa SD, Roth SP, Triebe T, Brehm W, Lohmann KL, Stockle SD. Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. https://www.openveterinaryjournal.com/?mno=158979 [Access: May 29, 2024]. doi:10.5455/OVJ.2023.v13.i9.17 AMA (American Medical Association) Style Tommasa SD, Roth SP, Triebe T, Brehm W, Lohmann KL, Stockle SD. Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Vet J. 2023; 13(9): 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 Vancouver/ICMJE Style Tommasa SD, Roth SP, Triebe T, Brehm W, Lohmann KL, Stockle SD. Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Vet J. (2023), [cited May 29, 2024]; 13(9): 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 Harvard Style Tommasa, S. D., Roth, . S. P., Triebe, . T., Brehm, . W., Lohmann, . K. L. & Stockle, . S. D. (2023) Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Vet J, 13 (9), 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 Turabian Style Tommasa, Simone Della, Susanne Pauline Roth, Theresa Triebe, Walter Brehm, Katharina Luise Lohmann, and Sabita Diana Stockle. 2023. Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Veterinary Journal, 13 (9), 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 Chicago Style Tommasa, Simone Della, Susanne Pauline Roth, Theresa Triebe, Walter Brehm, Katharina Luise Lohmann, and Sabita Diana Stockle. "Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare." Open Veterinary Journal 13 (2023), 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 MLA (The Modern Language Association) Style Tommasa, Simone Della, Susanne Pauline Roth, Theresa Triebe, Walter Brehm, Katharina Luise Lohmann, and Sabita Diana Stockle. "Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare." Open Veterinary Journal 13.9 (2023), 1212-1218. Print. doi:10.5455/OVJ.2023.v13.i9.17 APA (American Psychological Association) Style Tommasa, S. D., Roth, . S. P., Triebe, . T., Brehm, . W., Lohmann, . K. L. & Stockle, . S. D. (2023) Successful intra-abdominal resection of a 24 kg ovarian granulosa cell tumour in a Warmblood mare. Open Veterinary Journal, 13 (9), 1212-1218. doi:10.5455/OVJ.2023.v13.i9.17 |