| Research Article | ||
Open Vet J. 2024; 14(2): 617-629 Open Veterinary Journal, (2024), Vol. 14(2): 617–629 Original Research Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactorChidozie C. Ugwu1,2, Mohd Hair-Bejo1,3*, Mat I. Nurulfiza3,4, Abdul R. Omar1,3 and Aini Ideris1,31Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Selangor, Malaysia 2Department of Animal Science and Technology, Federal University of Technology, Owerri, Imo State, Nigeria 3Institute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor, Malaysia 4Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia *Corresponding Author: Mohd Hair-Bejo. Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Selangor, Malaysia. Email: mdhair [at] upm.edu.my Submitted: 20/09/2023 Accepted: 15/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
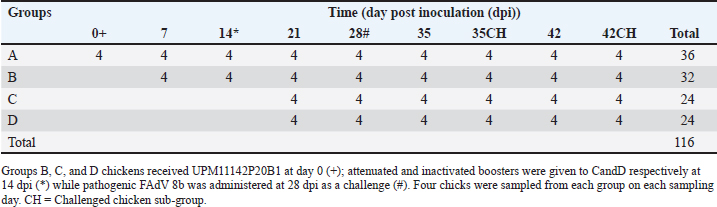
AbstractBackground: Fowl adenovirus (FAdV) 8b causes huge economic losses in the poultry industry worldwide. Attenuated FAdV 8b could be useful in preventing FAdV infections globally and scale-up obstacles could be solved by bioreactor technology. Aim: This study was carried out to attenuate the FAdV 8b isolate, propagate it in a bioreactor, molecularly characterize the passage isolates, and determine the immunogenicity, efficacy, and shedding of the virus of chickens. Methods: FAdV serotype 8b (UPM11142) isolate was passaged on chicken embryo liver (CEL) cells until attenuation and propagated in a bioreactor (UPM11142P20B1). Hexon and fiber genes of the isolates were sequenced and analyzed. UPM11142P20B1 was administered to 116-day-old broiler chickens divided into four groups, A (control), B (non-booster), C (booster with UPM11142P20B1), and D (booster with inactivated UPM11142P5B1). Eight chickens from each group were challenged. Body weight (BW) and liver weight (LW), liver: BW ratio (LBR), FAdV antibody titer, T lymphocyte sub-populations in the liver, spleen and thymus; and challenge virus load in the liver and shedding in cloaca were measured at weekly intervals. Results: The isolate caused typical cytopathic effects on CEL cells typical of FAdV. Novel molecular changes in the genes occurred which could be markers for FAdV 8b attenuation. BW, LW, and LBR were similar among groups throughout the trial but the uninoculated control-challenged group (UCC) had significantly higher LBR than the inoculated and challenged groups at 35 dpi. Non-booster group had higher FAdV antibodies at all time points than the uninoculated control group (UCG); and the challenged booster groups had higher titer at 35 dpi than UCC. T lymphocytes increased at different time-points in the liver of inoculated chickens, and in the spleen and thymus as well, and was higher in the organs of inoculated challenged groups than the UCC. There was a significantly higher challenge virus load in the liver and cloaca of UCC chickens than in the non-booster chickens. Conclusion: UPM11142P20B1 was safe, efficacious, significantly reduced shedding, and is recommended as a candidate vaccine in the prevention and control of FAdV 8b infections in broiler chickens. Keywords: Attenuation, Fowl adenovirus 8b, Humoral/cellular immunity, Vaccine, Virus shedding. IntroductionFowl adenovirus (FAdV) 8b is a group E Aviadenovirus in the family Adenoviridae (Islam et al. 2023). It causes inclusion body hepatitis (IBH) mainly in broilers that are 3–7 weeks old and causes mortality ranging from 2% to 30%; and is characterized by sudden death without clinical signs, enlarged, friable, and discolored liver with petechial hemorrhages; and hemorrhages in other organs (Norina et al., 2016; Revajova et al., 2017; Abd El-Ghany, 2023). Basophilic or eosinophilic intranuclear inclusion bodies are commonly detected in the liver and inflammatory mononuclear infiltration could be found in other organs (Revajova et al., 2017). Vertical transmission and brief pathogenesis associated with IBH are major concerns that can be overcome with live-attenuated vaccines. By mimicking natural infection, live-attenuated vaccines offer superior immunogenic competency and a better mode of administration (Isakova-Sivak and Rudenko, 2015). Attenuated vaccines are produced by weakening the virus to be incapable of disease causation but still retain its infectivity and immunogenicity. But the virus could be prone to reversion to virulence (Dai et al., 2019), and require constant molecular monitoring. Another drawback to the availability of live vaccines post-development is scale-up obstacles limiting large-volume production, but this could be overcome with the bioreactor technology. Again, the absence of cross-protection is an important epidemiologic feature of IBH that requires serotype-specific vaccine development. But the effectiveness of vaccines is dependent on their ability to induce enough T cells like CD8+ cytotoxic T cells (Thimme et al. 2003). Live-attenuated FAdV 4 with low titer and low antibody production still provided protection to chicks (Steer-Cope et al., 2019) which suggests the possibility of other protective avenues including cellular immunity worth exploring. Therefore, attenuated FAdV 8b could induce sufficient humoral and cell-mediated immunity and be useful in the prevention and control of IBH which has been rising worldwide. This study was carried out to attenuate FAdV 8b, propagate the isolate in microcarrier-adapted chicken embryo liver (CEL) cells in the bioreactor, and evaluate the pathogenicity, humoral, cellular, and blocking immunogenicity in broiler chickens. Materials and MethodsVirus, attenuation, and propagation in bioreactorFAdV 8b isolate named UPM11142 from a 2011 outbreak of IBH in a broiler farm in Malaysia was used. Daily mortality started from 7 days of age and ranged from 0.5% to 1% (Sohaimi et al., 2019). The isolate was filtered through a 0.45 μm syringe filter (Corning, USA) before use. Aseptically harvested liver from 15-day-old specific pathogen-free (SPF) embryonated chicken eggs (CEE) was used to prepare the CEL cells as previously (Soumyalekshmi et al., 2014). Confluent CEL cells in tissue culture flasks at passage 5 (UPM08136CELP5) were used to passage fifteen times to yield CELP6–CELP20; and monitored molecularly after every five passages. UPM11142CELP5 isolate was propagated in a stirred tank bioreactor as previously described (Ugwu et al., 2020). All glassware used was siliconized prior to sterilization. The stirred tank Bioreactor (Sartorius, Goettingen, Germany) was set up and Cytodex 1 microcarriers were prepared following the manufacturer’s recommendation. CEL cells adapted to Cytodex 1 microcarriers and FAdV 8b propagated in a stirred tank bioreactor as previously described while the infective dose (TCID50) was determined (Reeds and Muench, 1938). Polymerase chain reaction (PCR)HexA1/HexB1 primers targeting hexon gene and fbrF/fbrR primers designed from KU517714.1 reference strain targeting fiber gene were used for PCR as previously reported (Ugwu et al., 2020). Amplicons were sequenced (first base laboratories, Malaysia), analyzed and sequences were deposited in GenBank through Bankit software, and accession numbers were obtained. Inoculum and challenge virusThe FAdV 8b (UPM11142P20) was propagated one time in a stirred tank bioreactor using CEL cells adapted to Cytodex™ 1 microcarriers to produce UPM11142P20B1 (Ugwu et al, 2020) which was used as inoculum in the experimental animal trial. The UPM11142CELP5 isolates passaged 2x in SPF CEE to obtain UPM11142P5EP2 with a titer of 108 EID50/ml was filtered through 0.45 μm syringe filter and used as a challenge virus for the experimental animal trial. UPM11142P5B1isolate was inactivated with binary ethylene imine as previously described (Ugwu et al., 2022). Immunogenicity, safety, and efficacy of attenuated FAdV serotype 8b isolate UPM11142CEL20B1 on commercial broiler chickensOne hundred and sixteen day-old broiler chicks randomly divided into four main groups A, B, C, and D made up of 36, 32, 24, and 24 chickens respectively (Table 1). Feed and water were provided ad libitum, and clinical signs were monitored daily and recorded for 42 days. All chicks in groups B, C, and D were inoculated subcutaneously with 0.5 ml of attenuated FAdV isolate of UPM11142CEL20B1 (105.8 TCID50/ml) on day one of age (day 0 postinoculation (pi)) while chicks in group A were uninoculated. Chickens in group C received an attenuated booster while those in D group received an inactivated booster (108.3 TCID50/ml) at 14 day pi (dpi) through the subcutaneous route. On day 28 pi, eight chickens each from all the groups were challenged intramuscularly with 0.5 ml of UPM11142CEL5EP2 (108 TCID50/ml). Table 1. Study design for the experimental trial of the efficacy and immunogenicity of UPM11142P20B1 on commercial broiler chickens.
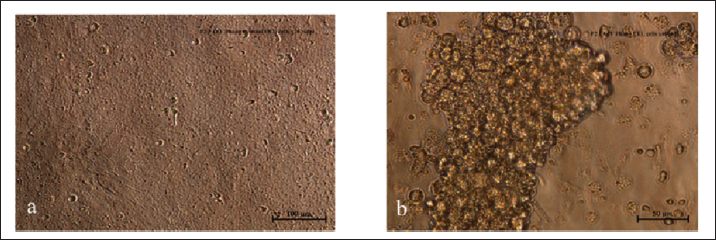
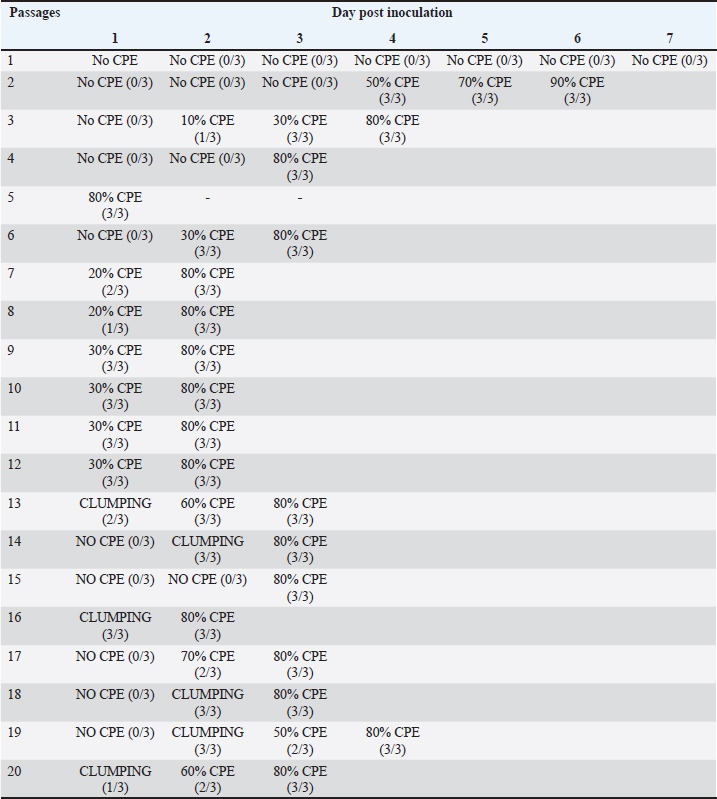
Sample collectionAs indicated in Table 1, eight chickens were sampled from each group on each sampling day. Clinical signs were observed and recorded each day. Body weight (BW), liver weight (LW), and gross lesions of each chicken were recorded on sampling days while the liver BW ratio was calculated. Samples of liver, spleen, and thymus from each chicken were collected for histopathological examination and cellular immunophenotyping. Samples of liver from all chickens were collected for qPCR detection of FAdV. Blood samples were collected, and serum was extracted from each chicken sampled for measurement of FAdV antibody titer. Liver samples and cloacal swabs were collected for evaluation of viral load and shedding, respectively. The swabs were suspended in 2 ml of DMEM media (without supplements) and stored at -20°C for further processing. Gross lesions and histopathological changesGross lesions of liver, spleen, and thymus from each chicken were observed and recorded prior to fixing in 10% buffered formalin for 48 hours (Zhao et al., 2016). They were processed, stained with Eosin and hematoxylin and were examined on slides under a simple light microscope (Leica DM LB2). The images were captured (Leica DFC295) and recorded. Enzyme-linked immunosorbent assay (ELISA) analysis of serum samplesBlood samples from each chicken were collected and serum harvested within 24 hours and stored at -20°C until use. ELISA test was carried out with each serum sample using ELISA kit (BioChek, UK) according to the Manufacturer’s recommendation and was read at 405 nm on ELISA reader (Dynatech MR7000, USA). Flow cytometric immunophenotypingEach organ sample from each chicken in each group was processed and stained with antibodies specific for CD3+, CD4+, and CD8+ and 10,000 live cells counted on BD FACS Calibur flow cytometer (Becton, Dickinson, and Company Biotec., San Diego, CA, USA), and analyzed as previously reported (Ugwu et al., 2022). Viral genome copy number of FAdV challenge virus in the liver and cloaca of challenged chickensFAdV hexon gene-specific primers and probe were used to generate standard curve and amplification plot and to conduct quantitative PCR on CFX96™ Real-Time PCR Detection System (Bio-Rad, USA) using DNA from each cloacal swab and liver sample according to a protocol previously reported (Ugwu et al., 2023). The CT values of each replicate was obtained and used to determine the mean CT of each sample; and calculate the FAdV genome copy number of each group. Data presentation and statistical analysisData generated were analyzed with a two-way repeated measures ANOVA on SPSS 25.0 for Windows (SPSS Inc., Chicago, IL) on a 5% probability level and results were presented as tables and figures. Ethical approvalThis study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Universiti Putra Malaysia Institutional Animal Care and Use Committee (IACUC) in a letter with ref number; UPM/IACUC/AUP-R086/2018 dated 8th March, 2019). ResultsAttenuation of FAdV isolate UPM11142 in CEL cellsNo CPE was observed at 1 dpi of P1, but 80% CPE which included clumping, rounding, shining and complete detachment of CEL cells from the monolayer was observed at 1 dpi of P5 (Fig. 1). At P19, 80% CPE was observed at 4 dpi and the delayed CPE continued to P20 (3 dpi) (Table 2).
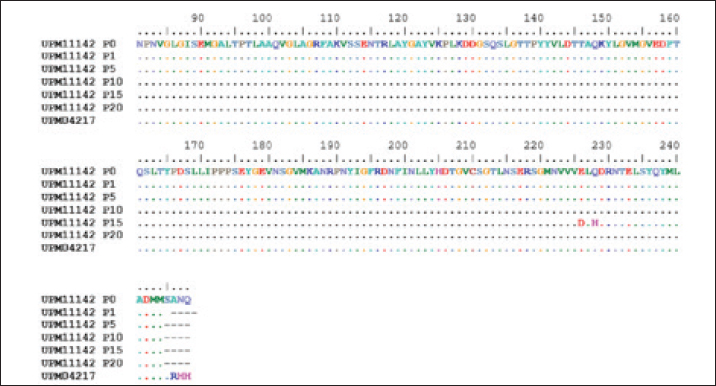
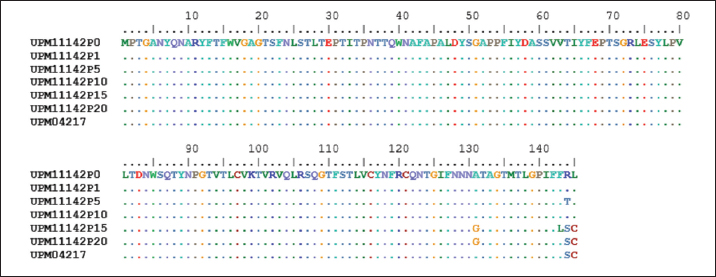
Fig. 1. Microscopic image of CEL cells infected FAdV 8b showing cytopathic effects. (a) Uninfected CEL cells (control); (b) UPM11142P5 showing clumping, shining and detachment of cells from monolayer (a, x10; b x20). Molecular analysis of hexon and fiber genesThe sequencing of hexon gene of the passage isolates produced 732 bp coding nucleotides which encoded 248 amino acid sequence. The blastn and blastp showed the isolate was 98%–100% and 99%–100% homologous with UPM04217 reference strain and other FAdV 8b isolates in GenBank respectively. Between UPM11142P0 and UPM11142P20 there was a G►31T change which resulted in V►11L (amino acid change) from passage 15 to 20. Other changes at passage 15 were, A►678T and G►684T resulted in E►226D and Q►228H amino acid coding (Figs. 2 and 4). Table 2. Percentage CPE of UPM11142 on CEL cells.
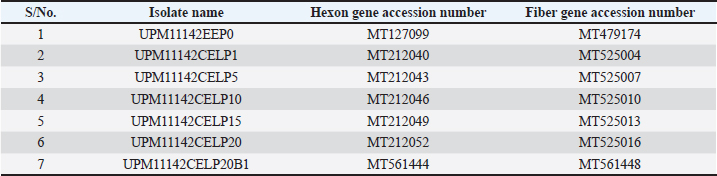
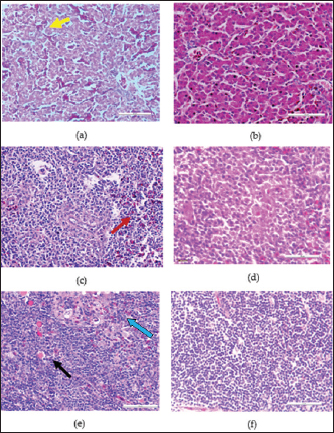
In the knob region of fiber gene, there was a synonymous change A►177G, and non-synonymous changes C►392G, G►393C, C►430A, T►432C, C►433T, T►434G and G►435C, all from passage 15 to 20 which resulted to changes in the amino acids from A►131G, R►144S and L►145C (Figs. 3 and 5). These sequences were deposited in the Genbank with accession numbers recorded (Table 3). Clinical signs, gross lesions, and histopathologic changesNo abnormal clinical signs, gross lesions, and histopathological changes were observed among the unchallenged group A chickens, and B–D groups (Fig. 6d–f). But challenged chickens in A group were depressed and anorexic within 2 days after the challenge. And also had pale, discolored, and enlarged liver, enlarged spleen, and thymus. Necrotic, congested liver with vacuoles (Fig. 6a), congested spleen with vacuoles and nuclear debris (Fig. 6b), and thymus with reduced thickness of the cortex, cell depletion in the medulla and signs of lymphoid depletion (Fig. 6c) were also observed.
Fig. 2. Comparison of aligned amino acid sequences of hexon gene of UPM11142 and UPM04217 reference strain showing changes between original and passage isolates.
Fig. 3. Comparison of aligned amino acid sequences of fiber gene of UPM11142 and UPM04217 reference strain showing changes between original and passage isolates.
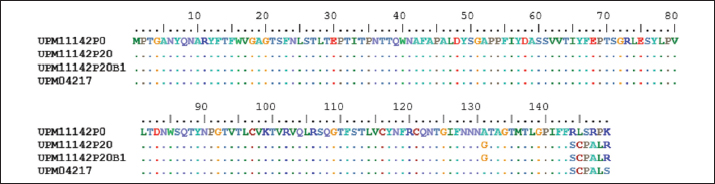
Fig. 4. Comparison of the aligned amino acid sequences of hexon gene of the attenuated UPM11142 isolate showing no changes between UPM11142P20 and UPM11142P20B1.
Fig. 5. Comparison of the aligned amino acid sequences of fiber gene of the attenuated UPM11142 isolate showing no changes between UPM11142P20 and UPM11142P20B1. Table 3. List of passage isolates of UPM11142 and the accession numbers of the hexon and fiber genes in Genbank.
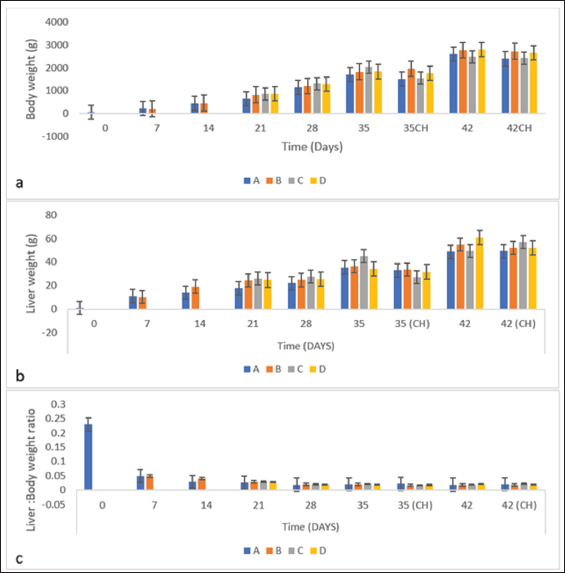
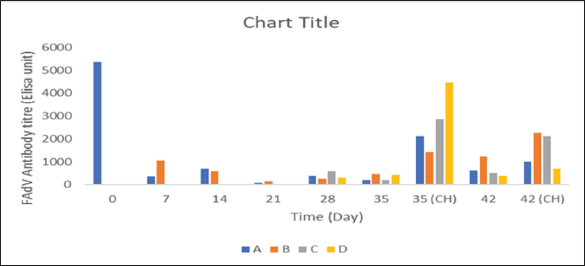
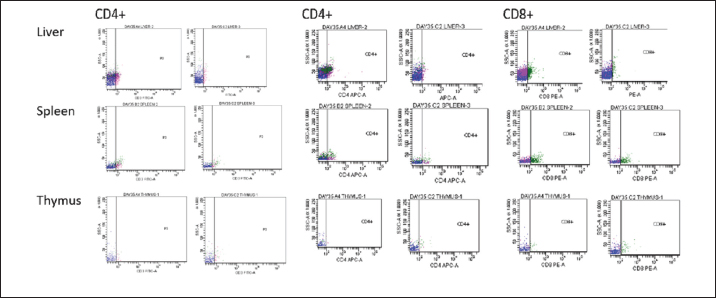
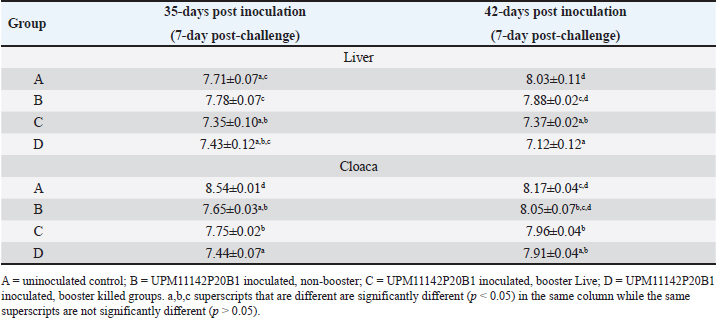
BW, LW, and liver: BW ratioThe uninoculated control chickens in the A group recorded significantly (p < 0.05) lower BW than the inoculated chickens in groups B, C, and D at 21 dpi but similar at other timepoints. The BW of group A was significantly lower (p < 0.05) than challenged control chickens (Fig. 7a). The LW of the chickens in the inoculated groups was significantly higher (p < 0.05) than that of the uninoculated group at 14 and 21 dpi but remained statistically similar throughout the rest of the trial (Fig. 7b). The liver—BW ratio of the challenged control chickens in group A was significantly higher (p < 0.05) at 35 dpi than that of the inoculated challenged chickens suggesting protection of the inoculated chickens from the challenge virus (Fig. 7c). FAdV antibody titerHigh maternal antibodies were recorded among chickens in the trial, but it declined significantly throughout the trial (Fig. 8). The non-booster group A recorded a significantly higher (p < 0.05) titer at 42 dpi than all the groups. The challenged chickens in the booster with the inactivated group at 35 dpi recorded much higher titer than other groups. Cellular immunophenotyping of organs of chicken inoculated with attenuated FAdV 8bThere were significantly higher CD3+ T lymphocytes in the liver of chickens in the inoculated groups at 21 dpi and higher (p > 0.05) at 14, 28, 35, and 42 dpi than chickens in the uninoculated control A group; higher (p > 0.05) in the spleen at 14, 21, 35, and 42 and in the thymus at 7, 14, 21, 28, and 42 dpi. They were also higher in the three organs among the inoculated challenged groups at 35 and 42 dpi and were significantly higher in the thymus at 35 dpi than the uninoculated challenged control group as illustrated in the dot plots (Fig. 9).
Fig. 6. HandE images of liver, spleen and thymus of challenged and unchallenged chickens inoculated with the attenuated UPM11142P20B1: (a)–(c) liver, spleen and thymus of uninoculated challenged control chickens, respectively; showing necrosis, congestion and vacuolation of the hepatocytes (black arrows); cellular vacuolation and necrosis in the spleen (black arrow) and lymphoid cells depletion in the thymus (black and white arrows), respectively; (d)–(f) inoculated challenged liver, spleen and thymus, respectively showing normal conformity at 35 dpi. HE, 40x. The CD4+ T lymphocytes were significantly higher at 14 dpi and higher (p > 0.05) at 21, 28, and 42 dpi in the liver; were significantly higher (p < 0.05) in the spleen at 7, 14, 21, and higher (p > 0.05) at 28 and 42 dpi and significantly higher (p < 0.05) in the at 14 dpi and higher (p > 0.05) 7, 21, and 28 dpi in the thymus among the inoculated groups than in the uninoculated control group. CD4+ cells were also higher in the liver among the inoculated challenged groups at 35 and 42 dpi and were significantly higher in the thymus at 42 dpi than the uninoculated challenged control group. The CD8+ T lymphocytes were significantly higher at 21 dpi and higher (p > 0.05) at 14, 28, and 42 dpi in the liver; they were significantly higher (p < 0.05) in the spleen at 14 and 21 dpi and in the thymus at 21 dpi among the inoculated groups than in the uninoculated control group. The inoculated challenged groups had significantly higher CD8+ cells in the thymus at 42 dpi and higher (p > 0.05) in the liver and spleen at 35 and 42 dpi than the uninoculated challenged control group. Viral load in the liver and shedding in the cloaca of challenged chickensThe FAdV challenge virus load in the liver of the A group was significantly higher than that of the challenged booster groups at 35 and 42 dpi (Table 4). which indicates significantly high virus clearance in the liver of inoculated chickens. The challenge virus copy number in the cloaca of chickens in the A group was significantly higher than that of the B, C, and D groups in the two time points which is an indication of highly reduced FAdV Challenge virus shedding among the inoclated chickens. This shows that the attenuated FAdV 8b, UPM11142P20B1 induced blocking immunity which is a critical need for control and eradication of the virus in the environment. DiscussionSerial passage of viruses in susceptible host cells is expected to produce attenuation leading to low pathogenic and might maintain its immunogenicity (Hair-Bejo, 2010). Serial passage of UPM11142 isolate on CEL cells produced typical cytopathic effects which were similar to FAdV 4 on CEL cells (Soumyalekshmi et al., 2014). However, there was a delay in the exhibition of cytopathic effects at passages 19 and 20 which is an indication of possible attenuation. CPE within 2 days translating to 6 days as observed could indicate attenuation but such presumption cannot alone be confirmatory. But the passages caused novel molecular changes in the hypervariable L1 loop and knob regions of the hexon gene and the fiber gene respectively and were presumed attenuated. Interestingly, the attenuated isolate did not show any changes when passaged once in a stirred tank bioreactor which is an indication of the stability of the isolate in the bioreactor. This breakthrough will increase virus yield, improve availability, and reduce cost. Again, this bioreactor propagated, attenuated UPM11142P20B1 was efficacious when inoculated into day-old broiler chickens where they induced humoral and cell-mediated immunity, significantly reduced viral load in the liver and shedding in the cloaca. There was a valine to leucine mutation that occurred in UPM11142P20 which could be a marker for attenuation. It is one of the most common polymorphisms in transmembrane protein which could be explained easily because it requires only one nucleotide change to occur (Molnár et al., 2016). However, since both residues are nonpolar and hydrophobic amino acids with slight structural differences, the effect of the change could be minimal. But V►11L change in this study is similar to V►458L mutation that occurred in Hemagglutinin gene of Influenza virus which led to decreased pathogenicity after six passages in MDCK (Madin-Darby canine kidney) cells (Zarubaev et al., 2018) and is proposed as the marker for delayed CPE in CEL cells infected with FAdV 8b. Non-synonymous base changes have been reported to lead to functional changes in proteins (Veno et al., 2019) and this could lead to attenuation of viruses. One of the main single nucleotide polymorphisms is the non-synonymous single nucleotide polymorphism which resulted in single amino acid polymorphism that could alter protein stability, charge, solubility, structure, or function and lead to changes in their pathogenic potentials (Naveed et al., 2016). An A►V change in the VP1 protein was linked to attenuation in IBDV (Pikuła et al., 2018). It is only probable that the base changes and the resultant changes in the amino acid residues in the hexon gene of UPM11142P20 could cause changes in their stability or functions. The L1 loop region is where most changes leading to the least homology of FAdV are domiciled and changes therein are markers for infectivity in FAdV (Sohaimi et al., 2018).
Fig. 7. (a) BW, (b) LW, and (c) body to liver-weight ratio of broiler chickens inoculated with attenuated UPM11142P20B1 FAdV strain. A=Uninoculated control, B=Inoculated non-booster, C=inoculated+attenuated booster, D=inoculated+inactivated booster, CH=challenged chickens. In the fiber gene, there were changes that included a base change A►177G in the shaft region. The role of the shaft in the infectivity or pathogenicity of FAdV is still not clear although Wu et al. (2003) even as the length of shaft could play a role in the ability of knob to interact with cell receptors to achieve endocytosis. However, since the change had no effect on the amino acid coding in that region, its effect on the stability or function of the fiber protein may not be significant. Arginine to serine and leucine to cysteine substitutions resulting from five nucleotide changes also occurred. Arginine is a charged amino acid and its substitution with serine which is a polar residue is likely to affect the conformity of the fiber protein. A similar mutation was reported in Herpes simplex virus, where arginine substitution with serine in the herpes DNA polymerase gene produced a variant that was resistant to phosphonoacetic acid and acyclovir antiviral drugs and hypersensitive to aphidicolin (Gibbs et al., 1988). On the other hand, leucine change to cysteine could affect the viability of fiber knob interaction with CEL cell receptors because although both are hydrophobes, leucine is nonpolar while cysteine is a polar amino acid. However, this kind of substitution for intracellular protein usually should produce neutral effects since cysteine usually offers stability effects when found in the core of proteins (Betts and Russell, 2003). Interestingly, all these changes occurred in the knob region in which changes therein is known to affect the pathogenicity of FAdV (Pallister et al., 1996). Fiber was suggested to be the first to experience molecular changes due to its location and the first to interact with the environment (Sohaimi et al., 2018). It is proposed that these changes in hexon and fiber genes are markers for attenuation of the FAdV 8b strain passaged in CEL cells. Incidentally, none of these changes have been reported before in FAdV 8b which makes them novel findings.
Fig. 8. FAdV antibody titer of broiler chickens inoculated with attenuated UPM11142P20B1 FAdV strain A=Uninoculated control, B=Inoculated non-booster, C=UPM08136P5B1 inoculated+attenuated booster, D=inoculated+inactivated booster, CH=challenged chickens. FAdV=Fowl adenovirus.
Fig. 9. Flow cytometric dot plots of CD3+, CD4+ and CD8+ in the liver spleen and thymus of challenged chickens at 35 dpi (7-day post-challenge). Since the uninoculated non-challenged chickens in this trial had similar or lower BW it means that the attenuated FAdV 8b is safe and well tolerated by the chickens in the inoculated groups. The uninoculated non-challenged chickens had a higher liver-to-BW ratio and since the enlargement of the liver is one of the most striking gross lesions associated with FAdV infections in chickens (Redondo et al., 2018), the attenuated FAdV 8b helped limit the effects of pathogenic FAdV challenge on the inoculated groups. There were clinical signs, gross lesions, and histopathological changes in the liver, spleen, and thymus of the uninoculated control, challenged chickens which were not observed among the inoculated challenged chicken groups showing that the attenuated FAdV 8b protected the inoculated chickens against pathogenic FAdV 8b challenge. Table 4. Copy number of FAdV challenge virus in the liver and cloaca of chickens inoculated with attenuated FAdV strain (UPM11142P20B1) and challenged.
There was an inducement of FAdV antibodies in the chickens inoculated with the attenuated FAdV 8b in this trial which was low in the beginning probably due to the observed high maternally derived antibodies (MDA). FAdV inducement of passive immunity had been reported (Mittal et al., 2014). However, this MDA declined till 21 dpi coinciding with the rise in antibody titer among chickens inoculated with UPM11142P20B1. MDA offers protection to offspring early in life but prevents early vaccine-induced immunity (Yosipovich et al., 2015), especially with live vaccines that need infection to induce protective immunity. Mechanisms of suppression of immune response by MDA include inhibition of B and T helper cells and of antigen processing, and activation of T suppressor cells (Crhanova et al., 2011); through physical epitope blocking, a process referred to as Epitope masking (Kim et al., 2011). Acquired immune suppression by MDA has been reported in Newcastle disease virus (Rauw et al., 2009). UPM11142P20B1 induced antibodies which was significantly higher among the B group than that of the uninoculated control chickens at 35 dpi and the A group at 42 dpi and at other time points after the decline of MDA. However, it was observed that the challenged D group with the highest antibody titer at 42 dpi had a significantly higher FAdV 8b load in the liver. This suggests a lack of virus neutralization. This challenged D group chickens also had significantly higher liver-to-BW ratio and histopathologic changes in the liver. There seems to be a relationship established between histopathologic changes in the liver, antibody titer and virus load in the liver in this trial which favors that the attenuated FAV 8b strain provided protection against FAdV 8b challenge. But it is obvious that cellular immunity could have contributed to the protective efficacy of the attenuated UPM11142P20B1 on chickens in this trial. The attenuated UPM11142P20B1 induced cellular immune response evidenced by increased CD3+, CD4+, and CD8+ T lymphocytes sub-population in the liver, spleen, and thymus of the broiler chickens. The inducement of cellular immune response is correlated to efficacy of viral vaccines (Thimme et al., 2003); and because live attenuated vaccines mimic natural infections and should induce cellular immunity (Rosendahl Huber et al., 2014). In this trial CD3+, CD4+, and CD8+ were induced up to 42 dpi. This is similar to the report of higher CD3 T cells for 25 dpi reported among chickens experimentally infected with FAdV 8 (Wang et al., 2012). There is a paucity of information regarding the cellular immune response of chickens to attenuated FAdV 8b which means that this report could form a baseline information on cell-mediated immune response of chickens to FAdV 8b vaccines thereby highlighting its novelty. The CMI response was more observed in the liver which is understandable as the liver is the target organ of infection. The lymphocytes were also induced in the spleen and thymus as well. The spleen is very important in the immune response to viral infections being the interface between peripheral blood and pathogens, and an increase in lymphocytes in the spleen among the inoculated groups, especially when challenged, suggests the development of memory cells which is important in the development of long-term protection against FAdV 8b in chickens (Wu et al., 2018). On the other hand, the thymus is where T-cell mature and CMI develop (Wang et al., 2012). There was a 83% and 86% decrease of CD8+ cells in the liver and thymus among the uninoculated challenged control chickens compared to 45% decrease in the thymus of chickens in the A group which suggests immune suppression through lymphoid depletion and apoptosis of lymphocytes. This is in line with the report of a decrease in CD4+ and CD8+ cells in the thymus of chickens infected with FAdV 4 (Steer-Cope et al., 2019). It would then mean that attenuated FAdV 8b would have protected the inoculated chickens from immunosuppression by limiting apoptosis of lymphocytes and lymphoid depletion. The FAdV challenge virus was shed in the cloaca for 14 days post-challenge in this trial. This is similar to FAdV 9 shedding for 14 days (Deng et al., 2013) and FAdV 4 for 28 days (Grgić et al., 2013) previously reported. The significantly higher (p < 0.05) shedding of FAdV challenge virus among the challenged D group than the A groups means UPM11142P20B1 induced blocking immunity which is remarkable putting into perspective the importance of horizontal transmission in FAdV infections. It is recommended that the ability of vaccines to prevent the shedding of viruses in the environment should be of utmost importance in determining its efficacy and the duration of the protective efficacy of vaccines should include the length of time the vaccine prevented the shedding of the organism to the environment (Kaiser et al., 2019). Booster inoculations significantly reduced shedding and were significantly better than non-booster inoculation. However, this was contrary to the report of Music et al. (2019) that although booster vaccination reduced shedding of H3N2 influenza virus, it did not do particularly better than non-booster vaccination. In conclusion, the bioreactor propagated attenuated UPM1142P20B1 isolate induced both humoral and cell-mediated immune responses that protected commercial broiler chickens from pathogenic FAdV 8b when administered singly or with booster. The inoculated groups were protected from histopathologic changes in the liver, spleen, and thymus and produced higher BW. The inoculated groups also recorded significantly reduced viral load in the liver and shedding in the cloaca than the uninoculated control and is recommended as a vaccine for the prevention of IBH disease caused by FAdV serotype 8b. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsMohd Hair Bejo, Abdul Rahman Omar and Aini Ideris conceived the research; Chidozie Ugwu carried out the experiments; Chidozie Ugwu and Nurulfiza Mat Isa analyzed the data; Mohd Hair Bejo acquired the Funding; Mohd Hair Bejo, Abdul Rahman Omar, Nurulfiza Mat Isa and Aini Ideris supervised the research; Chidozie Ugwu Wrote the original draft and Chidozie Ugwu, Mohd Hair Bejo, Abdul Rahman Omar, Nurulfiza Mat Isa and Aini Ideris reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript. FundingThis research was funded by Universiti Putra Malaysia, grant number vot 6300899. Data availabilityThe data presented in this study are available in the manuscript and any further clarifications can be obtained on reasonable request from the corresponding author. ReferencesAbd El-Ghany, W.A. 2021. A comprehensive review on adenoviruses infections in fowl: epidemiology, forms, diagnosis, and control. J. World Poult. Res. 11(2), 151–167. Betts, M.J. and Russell, R.B. 2003. Amino acid properties and consequences of subsitutions. In Bioinformatics for geneticists. Eds., Barnes M.R. and Gray I.C. Hoboken, NJ: Wiley. Crhanova, M., Havlickova, H., Sisak, F. and Rychlik, I. 2011. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infec. Immun. 79, 2755–2763. Dai, X., Xiong, Y., Li, N. and Jian, C. 2019. Vaccine types. Vaccines—the history and future. London, UK: IntechOpen, doi:10.5772/intechopen.84626. Deng, L., Sharif, S. and Nagy, É. 2013. Oral inoculation of chickens with a candidate fowl adenovirus 9 vector. Clin. Vaccine Immunol. 20(8), 1189–1196. Gibbs, J.S., Chiou, H.C., Bastow, K.F., Cheng, Y. and Coen, D.M. 1988. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc. Natl. Acad. Sci. USA 85, 6672–6676. Grgić, H., Sharif, S., Haghighi, H.R. and Nagy, É. 2013. Cytokine patterns associated with a serotype 8 fowl adenovirus infection. Viral Immunol. 26, 143–149. Hair Bejo, M. 2010. Poultry vaccines: an innovation for food safety and security (inaugural lecture). Serdang, Malaysia: Universiti Putra Malaysia Press. Isakova-Sivak, I. and Rudenko, L. 2015. Safety, immunogenicity and infectivity of new live attenuated influenza vaccines. Expert Rev. Vaccines 14(10), 1313–1329. Islam, M.N., Rahman, M.M., Rahman, M.K. and Alam, J. 2023. First evidence of fowl adenovirus induced inclusion body hepatitis in chicken in Bangladesh. Can. J. Infect. Dis. Med. Microbiol. 3, 7253433. Kaiser, T.J., Smiley, R.A., Fergen, B., Eichmeyer, M. and Genzow, M. 2019. Influenza A virus shedding reduction observed at 12 weeks post-vaccination when newborn pigs are administered live-attenuated influenza virus vaccine. Influenza Other Respir. Viruses 13(3), 274–278. Kim, D., Huey, D., Oglesbee, M. and Niewiesk, S. 2011. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood 117(23), 6143–6151. Mittal, D., Jindal, N., Tiwari, A.K. and Khokhar, R.S. 2014. Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. Virus Dis. 25, 114–119. Mohamed Sohaimi, N.M., Bejo, M.H., Omar, A.R., Ideris, A. and Mat Isa, N. 2019. Molecular characterization of fowl adenovirus isolate of Malaysia attenuated in chicken embryo liver cells and its pathogenicity and immunogenicity in chickens. PLoS One, 14(12), e0225863. Molnár, J., Szakács, G. and Tusnády, G.E. 2016. Characterization of disease-associated mutations in human transmembrane proteins. PLoS One 11(3), e0151760. Music, N., Tzeng, W., Liaini Gross, F., Levine, M.Z., Xu, X., Shieh, W.J., Tumpey, T.M., Katz, J.M. and York, I.A. 2019. Repeated vaccination against matched H3N2 influenza virus gives less protection than single vaccination in ferrets. NPJ Vaccines 4, 28. Naveed, M., Tehreem, S., Mubeen, S., Nadeem, F., Zafar, F. and Irshad, M. 2016. In-silico analysis of non-synonymous-SNPs of STEAP2: to provoke the progression of prostate cancer. Open Life Sci. 11, 402–416. Norina, L., Norsharina, A., Nurnadiah, A.H., Redzuan, I., Ardy, A. and Nor-Ismaliza, I. 2016. Avian adenovirus isolated from broiler affected with inclusion body hepatitis. Malaysian J. Vet. Res. 7(2), 121–126. Pallister, J., Wright, P. and Sheppard, M. 1996. A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. J. Virol. 70, 5115–5122. Pikuła, A., Lisowska, A., Jasik, A. and Smietanka, K. 2018. Identification and assessment of virulence of a natural reassortant of infectious bursal disease virus. Vet. Res. 49, 89. Rauw, F., Gardin, Y., Palya, V., van Borm, S., Gonze, M., Lemaire, S., van den Berg, T. and Lambrecht, B. 2009. Humoral, cell-mediated and mucosal immunity induced by oculo-nasal vaccination of one-day-old SPF and conventional layer chicks with two different live Newcastle disease vaccines. Vaccine 27, 3631–3642. Reed, L.J. and Muench, H.A. 1938. Simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27, 493–497. Redondo, H., Fragoso, J.S., Tahala, M.A., Bensassi, Y., Gil, I., Elbachir, E. and Abad Moreno, J.C. 2018. Characterization of strain of fowl adenoviruses circulating in Morocco. Poult. Sci. 97(11), 4057–4062. Revajova, V., Herich1, R., Seman, V., Levkut, M. Jr., Levkutova1, M., Karaffova1, M. and Levkut, M. 2017. An unusual outbreak of inclusion body hepatitis on a broiler chicken farm: a case report. Veterinarni Medicina 62(11), 6. Rosendahl Huber, S., van Beek, J., de Jonge, J., Luytjes, W. and van Baarle, D. 2014. T cell responses to viral infections—opportunities for Peptide vaccination. Front. Immunol. 5, 171. Sohaimi, N.M., Bejo, M.H., Omar, A.R., Ideris, A. and Isa, N.M. 2018. Hexon and fiber gene changes in an attenuated fowl adenovirus isolate from Malaysia in embryonated chicken eggs and its infectivity in chickens. J. Vet. Sci. 19(6), 759–770. Soumyalekshmi, S., Ajith, M.K. and Chandraprakash, M. 2014. Isolation of fowl adenovirus in chicken embryo liver cell culture and its detection by hexon gene-based PCR. Ind. J. Sci. Res. Tech. 2(3), 33–36. Steer-Cope, P.A., Sandy, J.R., O’Rourke, D., Scott, P.C., Browning, G.F. and Noormohammadi, A.H. 2019. Vaccination with FAdV-8a induces protection against inclusion body hepatitis caused by homologous and heterologous strains. Avian Pathol. 48(5), 396–405. Thimme, R., Wieland, S., Steiger, C., Ghrayeb, J., Reimann, K.A., Purcell, R.H. and Chisari, F.V. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77(1), 68–76. Ugwu, C.C., Hair-Bejo, M., Nurulfiza, M.I., Omar, A.R. and Ideris, A. 2023. TaqMan probe-based qPCR method for specific detection and quantification of fowl adenovirus 8b challenge from chickens inoculated with live attenuated or inactivated virus. Open Vet. J. 13(2), 171–178. Ugwu, C.C., Hair-Bejo, M., Nurulfiza, M.I., Omar, A.R. and Ideris, A. 2022. Efficacy, humoral, and cell-mediated immune response of inactivated fowl adenovirus 8b propagated in chicken embryo liver cells using bioreactor in broiler chickens, Vet. World 15(11), 2681–2692. Ugwu, C.C., Hair-Bejo, M., Nurulfiza, M.I., Omar, A.R. and Ideris, A. 2020. Propagation and molecular characterization of fowl adenovirus serotype 8b isolates in chicken embryo liver cells adapted on Cytodex™ 1 microcarrier using stirred tank bioreactor. Processes 8(9), 1065. Veno, J., Rahman, R.N.Z.R.A., Masomian, M., Ali, M.S.M. and Kamarudin, N.H.A. 2019. Insight into improved thermostability of cold-adapted staphylococcal lipase by glycine to cysteine mutation. Molecules 24, 3169. Wang, C., Yu, S., Aorigele Jia, D., Yao, H., Zhao, H., Lillehoj, H.S., Simujide Postnikoff, A.C.L. and Xu, S. 2012. Regulation of T lymphocyte subpopulations in specific pathogen-free chickens following experimental fowl adenovirus-VIII infection. Braz. J. Microbiol. 43(4), 1281–1290. Wu, E., Pache, L., Von Seggern, D.J., Mullen, T.M., Mikyas, Y., Stewart, P.L., Nemerow, G.R. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 77(13), 7225-7235. Wu, X., Wu, P., Shen, Y., Jiang, X. and Xu, F. 2018. CD8+ resident memory T Cells and viral infection. Front. Immunol. 9, 2093. Yosipovich, R., Aizenshtein, E., Shadmon, R., Krispel, S., Shuster, E. and Pitcovski, J. 2015. Overcoming the susceptibility gap between maternal antibody disappearance and auto-antibody production. Vaccine 33, 472–478. Zarubaev, V.V., Pushkina, E.A., Borisevich, S.S., Galochkina, A.V., Garshinina, A.V., Shtro, A.A., Egorova, A.A., Sokolova, A.S., Khursan, S.L., Yarovaya, O.I. and Salakhutdinov, N.F. 2018. Selection of influenza virus resistant to the novel camphor-based antiviral camphecene results in loss of pathogenicity. Virology 524, 69–77. Zhao, J., Zhong, Q., Zhao, Y., Hu, Y. and Zhang, G. 2016. Correction: pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One 11(8), e0161744. | ||
| How to Cite this Article |
| Pubmed Style Ugwu CC, Bejo MH, Isa NM, Omar AR, Ideris A. Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Vet J. 2024; 14(2): 617-629. doi:10.5455/OVJ.2024.v14.i2.2 Web Style Ugwu CC, Bejo MH, Isa NM, Omar AR, Ideris A. Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. https://www.openveterinaryjournal.com/?mno=170090 [Access: April 27, 2024]. doi:10.5455/OVJ.2024.v14.i2.2 AMA (American Medical Association) Style Ugwu CC, Bejo MH, Isa NM, Omar AR, Ideris A. Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Vet J. 2024; 14(2): 617-629. doi:10.5455/OVJ.2024.v14.i2.2 Vancouver/ICMJE Style Ugwu CC, Bejo MH, Isa NM, Omar AR, Ideris A. Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Vet J. (2024), [cited April 27, 2024]; 14(2): 617-629. doi:10.5455/OVJ.2024.v14.i2.2 Harvard Style Ugwu, C. C., Bejo, . M. H., Isa, . N. M., Omar, . A. R. & Ideris, . A. (2024) Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Vet J, 14 (2), 617-629. doi:10.5455/OVJ.2024.v14.i2.2 Turabian Style Ugwu, Chidozie Clifford, Mohd Hair Bejo, Nurulfiza Mat Isa, Abdul Rahman Omar, and Aini Ideris. 2024. Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Veterinary Journal, 14 (2), 617-629. doi:10.5455/OVJ.2024.v14.i2.2 Chicago Style Ugwu, Chidozie Clifford, Mohd Hair Bejo, Nurulfiza Mat Isa, Abdul Rahman Omar, and Aini Ideris. "Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor." Open Veterinary Journal 14 (2024), 617-629. doi:10.5455/OVJ.2024.v14.i2.2 MLA (The Modern Language Association) Style Ugwu, Chidozie Clifford, Mohd Hair Bejo, Nurulfiza Mat Isa, Abdul Rahman Omar, and Aini Ideris. "Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor." Open Veterinary Journal 14.2 (2024), 617-629. Print. doi:10.5455/OVJ.2024.v14.i2.2 APA (American Psychological Association) Style Ugwu, C. C., Bejo, . M. H., Isa, . N. M., Omar, . A. R. & Ideris, . A. (2024) Efficacy, immunogenicity, and virus shedding in broiler chickens inoculated with live attenuated fowl adenovirus serotype 8b propagated a bioreactor. Open Veterinary Journal, 14 (2), 617-629. doi:10.5455/OVJ.2024.v14.i2.2 |