| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 316–323 Original Research Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino ratsMirna Aboshama*, Walied Abdo, Ahmed Elsawak and Abdelrahman KhaterDepartment of Veterinary Pathology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El Sheikh, Egypt *Corresponding Author: Mirna Aboshama. Department of Veterinary Pathology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El Sheikh, Egypt. Email: mirnakamal55 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
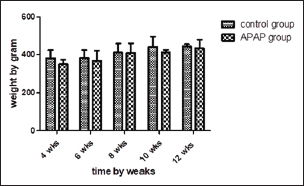
ABSTRACTBackground: Paracetamol is one of the most popular drugs; it is used daily by many people especially the elderly, without a limitation on the length of the period allowed for continuous use. Harms from long-term use are less clear, particularly in extrahepatic regions. Aim: This study aimed to investigate whether using paracetamol at a non-observable adverse effect level dose, known not to cause toxic effects, for a long period can induce toxicity in aged male albino rats. Methods: A daily dose of 500 mg per kg body weight of paracetamol was given to adult male albino rats for 12 weeks. During this period, rats were sacrificed at 4, 6, 8, 10, and 12 weeks to evaluate the toxic changes at several time intervals. Results: Chemical analysis revealed elevated serum alanine transaminase, aspartate transaminase, alkaline phosphatase, urea, creatinine, and declined level of total protein in N-acetyl-p-aminophenol (APAP)-treated group; it also caused oxidative stress, as shown by decreased glutathione, superoxide dismutase, and elevated malondialdehyde in the liver, kidney, and brain. Histopathological examination demonstrated cytoplasmic vacuolation and sinusoidal congestion with the development of single-cell necrosis in the liver. Renal tubular necrosis, glomerular atrophy, and ischemic neuronal injury, especially in the hippocampus were observed. the deleterious effects of APAP were increased in severity with increasing the period of treatment. Conclusion: Our results suggest that acetaminophen in a subtoxic dose for a long period could result in mild toxic effects on the liver but more serious lesions in the kidney and brain. Keywords: Drug side effects, Paracetamol toxicity, Prolonged drug use, Analgesics. IntroductionOne of the most used medications in the world is acetaminophen, N-acetyl-p-aminophenol (APAP), which is also called paracetamol. It is easily accessible without prescription in the majority of countries (Brune et al., 2015) and is included on the WHO’s list of essential medications (World Health Organization, 2021). However, paracetamol poisoning is among the most frequent causes of hepatic dysfunction worldwide and in the USA. In the US, it causes 56,000 admissions to the emergency department, 2,600 cases are getting hospitalized, and 500 fatalities per year. Unintentional overdoses account for 50% of these cases(Caparrotta et al., 2018). Acetaminophen use by the elderly is an area of concern; however, it is used by approximately 90% of older adults at an average dose of 3–4 g per day (Blieden et al., 2014). It is important to assess the impact of prolonged APAP use. This enables public officials to approve its availability in over-the-counter medications and aids doctors in evaluating patient benefits and risks. The toxic outcomes of acute APAP overdosing have been extensively explored (Ferner et al., 2011). More attention must be paid to the deleterious consequences of prolonged usage at nontoxic levels. Our objective of this study is to investigate the consequences of treating aged rats with a no observed adverse effect level dose of APAP (500 mg per kilogram body weight) (Venkatesan et al., 2014) daily for a long period (up to 12 weeks) on many organs and if the toxic effect of acetaminophen would increase with increasing period of treatment through assessment of toxicity at several time intervals (after 4, 6, 8, 10, and 12 weeks of APAP administration). Materials and MethodsAnimalsFifty male Wistar rats, 24 months old, with a median body weight of 380 g, obtained from “Vacsera Farm,” Helwan, were randomly distributed in cages (5 rats per cage) in an adequately ventilated house that had a 12:12 hours light/dark cycle and unlimited accessibility to ration and water. Rats were left to adapt to their environment for 2 weeks. Experimental designRats have been separated into an APAP-treated group (25 rats) and a control group (25 rats). APAP was obtained from Amon Pharmaceutical Co., (Egypt). The APAP–treated group received 500 mg per kg b.w.t (Venkatesan et al., 2014) daily. After 4, 6, 8, 10, and 12 weeks of administration, 10 rats (5 APAP-treated and 5 control rats) were sacrificed each time. Blood and tissue samplingBefore sacrifice, animals were anesthetized using ketamine 125 mg/kg and xylazine 10 mg/kg b.w.t injected intraperitoneally (Veilleux-Lemieux et al., 2013). Blood samples were collected from the retro-orbital plexus and allowed to clot. After that, the blood was centrifugated at 3,500 rounds/minute for 10 minutes to separate serum which was kept at −20°C until analyzed. Hepatic, renal, and brain tissue specimens were fixed in a 10% buffered formalin for histological analysis. In addition, liver, kidney, and brain tissue specimens were immediately placed in liquid nitrogen then samples were kept at −80°C. The body weights of all rats were recorded before each sacrifice. Blood serum biochemical analysisKits for the enzymes aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), creatinine, and urea were purchased from Biodiagnostic Co., Egypt. The transaminase activities were determined calorimetrically as a change in absorbance/minute at the wavelength 505 nm, as described by Reitman and Frankel (1957). Serum ALP activity was detected colorimetrically at the wavelength 510 nm according to Belfield and Goldberg (1971). Creatinine was detected according to Bartels et al. (1972) and urea levels were measured using the method of Fawcett and Scott (1960). Serum total protein kits were obtained from Spectrum Diagnostics., Al-Obour city, Cairo, Egypt, and determined by the colorimetric method at 546 nm wavelength according to Sapan et al. (1999). AntioxidantsLiver, kidney, and brain tissue that were preserved at −80°C were used to estimate levels of reduced glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) using commercial kits. GSH kits were purchased (CAT No. GR 2511, Biodiagnostic Co., Egypt) and activities were determined by colorimetric method at 405-nanometer wavelength according to Beutler et al. (1963), SOD kits were obtained as (CAT No. SD 2521, Biodiagnostic Co., Egypt) and were determined colorimetrically at the wavelength of 560 nm according to Nishikimi et al. (1972), MDA kits were also obtained (CAT No. MD 2529, Biodiagnostic Co., Egypt) and levels were detected by colorimetry at 534 nm wavelength according to Kei (1978). Histopathological examinationSamples were fixed in formalin for at least 24 hours and dehydrated in graded concentrations of ethanol, followed by xylene clearing, impregnation in soft paraffin, and embedded in hard paraffin wax. Blocks were sectioned at 5 μm and mounted on slides, then slides were prepared and stained routinely with hematoxylin and eosin stain (Bancroft and Gamble, 2008) for histopathological examination. Histopathological lesions in the liver, kidney, and brain of each group were graded and scored according to a three-point score depending on vascular changes (0–4), degeneration (0–4), and necrosis (0–4). Statistical analysisGraphPad Prism 5 was used to calculate the means and SD of the means of each group for all generated data. One-way analysis of variance and Tukey’s multiple comparison tests were applied to determine the significant difference between the treated groups and the control group and between all treated groups at the five points of sacrifice. p-values <0.05 were regarded as a significant difference. A two-way analysis of variance was used to analyze data on body weights. Ethical approvalAll procedures were ethically approved by the institutional animal care and use committee (IACUC), Kafrelsheikh University, approval number: KFS-IACUC/147/2023. ResultsNo mortalities or abnormal clinical signs were noticed during the study. The mean body weights of treated rats were not significantly different from the control group at any of the sacrifice points, according to statistics (Fig. 1).
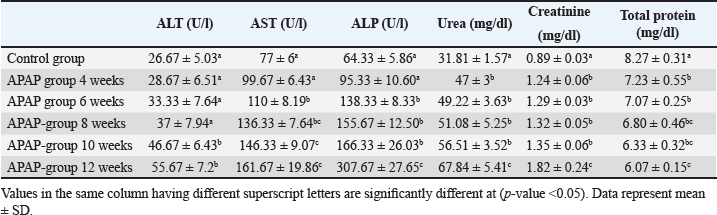
Fig. 1. The bars represent the means of rat weights, and the lines represent the SD of the mean. Biochemical findingsAn elevated levels of transaminases, ALP, urea, and creatinine, and declined levels of total protein were observed in the APAP-treated group, with an increasing of their significance levels. The difference from the control was statistically significant starting from 10 weeks of APAP treatment in ALT and 6 weeks in the case of AST and ALP (Table 1). Urea, creatinine, and total protein were significantly higher than the control group after APAP treatment throughout the experiment. Table 1. Serum biochemistry parameters in the control and APAP-treated groups at different time intervals.
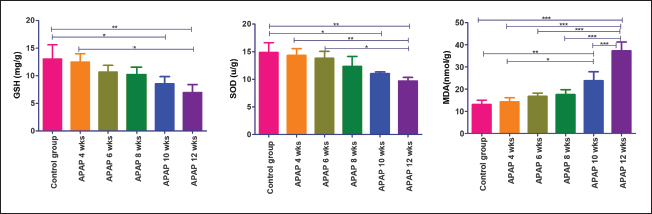
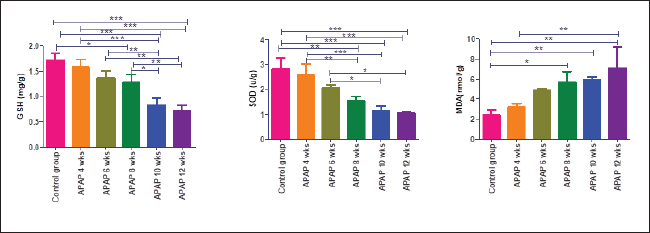
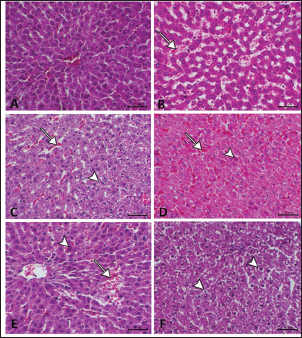
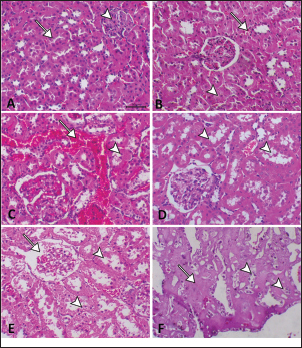
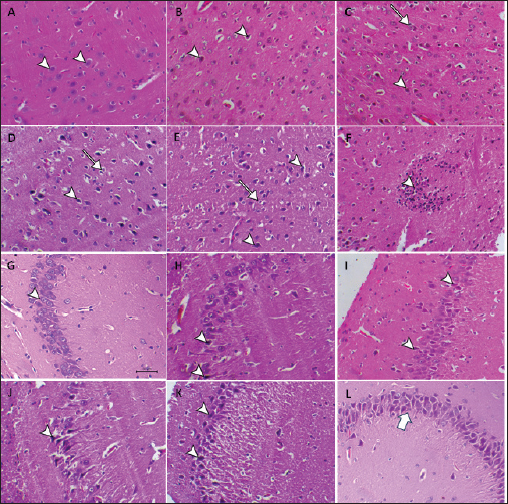
Fig. 2. Hepatic GSH, SOD, and MDA at different periods of treatment. data indicates the mean ± SD, (n=5). Means are represented in bars, and SD is represented in T-shaped bars. Horizontal line matching two groups that are significantly different. * p-value p-value p-value <0.001. Activities of hepatic, renal, and brain GSH and SOD were decreased, whereas MDA levels were increased with increasing periods of APAP treatment. A statistically significant difference in hepatic GSH, SOD, and MDA started in the 10th week after APAP administration; however, they started in the sixth week in the kidney and brain tissues (Figs. 2–4). Histopathological findingliverThe livers of APAP-treated rats in comparison with controls revealed only mild congestion after 4 weeks and mild cytoplasmic vacuolation after 6 weeks. Single-cell necrosis was found after 8 weeks. The degree of vacuolar degeneration, single-cell necrosis, and sinusoidal congestion increased in the following sacrifices, but the picture of pericentral hepatocytic necrosis, known to occur with high doses, was not found (Fig. 5). Statistical analysis of the hepatic lesion scoring in each group indicated that only the last three-point sacrifices are significantly different from the control group. KidneyLesions in the kidney started with congested capillaries and cloudy swelling in the epithelium of renal tubules after 4 weeks. Tubular epithelial degeneration and nuclear pyknosis after 6 weeks. The severity of lesions increased with time with the development of glomerular atrophy, widening of Bowman’s space with obvious degeneration, and sloughing of the lining epithelium of tubules, ending up with severe renal tubular necrosis, especially in the papillary region after the last sacrifice (Fig. 6). Histopathological lesion scoring of all the points of sacrifice were significantly different from the control group. BrainIschemic neuronal injury, neuronophagia, gliosis, and capillary congestion were found in brain tissue, especially in the hippocampus region. Lesions begin mild in the first sacrifice but as the length of treatment increases, the severity of the lesions increases, ending with focal malacia after 12 weeks (Fig.7). Lesion scoring in all APAP-groups showed statistically significant difference from the control group values (Fig. 8).
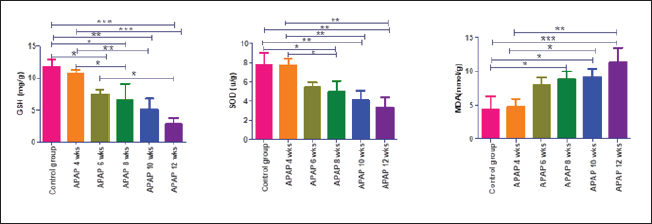
Fig. 3. Renal GSH, SOD, and MDA at different periods of treatment. Data indicates the mean ± SD, (n=5). Means are represented in bars, and SD is represented in T-shaped bars. Horizontal line matching two groups that are significantly different. * p-value p-value p-value <0.001.
Fig. 4. GSH, SOD, and MDA in brain tissue at different periods of treatment. Data indicates the mean ± SD, (n=5). Means are represented in bars, and SD is represented in T-shaped bars. Horizontal line matching two groups that are significantly different. * p-value p-value p-value <0.001. DiscussionWhen acetaminophen is taken in therapeutic doses, 80%–90% of it is conjugated with glucuronic acid or sulfate and eliminated via the kidneys (McGill and Jaeschke, 2013). The cytochrome P450 enzymes react with a small amount of the drug, generating the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Mazaleuskaya et al., 2015). The resulting metabolite is rarely harmful at therapeutic doses since it binds to GSH and is eliminated via bile. However, APAP overdosing leads to saturation of the other metabolic pathways (McGill and Jaeschke, 2013), and NAPQI synthesis rises significantly (Xie et al., 2015). NAPQI produced in excessive amounts results in a vigorous reaction with hepatic GSH reserves and a rapid depletion of GSH. This results in free reactive NAPQI that can combine with protein sulfhydryl groups to generate APAP protein adducts, especially the mitochondrial protein (McGill and Jaeschke, 2013). Causing oxidative stress, formation of peroxynitrite, mitochondrial membrane permeability transition pores, and cellular death (Jaeschke et al., 2021). This also explains our results of elevated MDA, a sign of oxidative stress, and decreased SOD and GSH levels, which are antioxidants, in the hepatic, renal, and brain tissues. APAP has a low (25%) affinity for plasma protein, allowing easy distribution of the drug throughout the body (Prescott, 1980). Thus, lesions appeared in various organs being mild in the liver but increasing in severity in the brain, and kidney. The toxic effect of the used APAP dose on the liver was mild, according to our results. This confirmed the result of Venkatesan et al. (2014) using the same dose for 28 days. This could be explained by one of the following hypotheses: prolonged APAP use causes induction of its metabolism in the liver by glucuronidation and sulfation pathways, which are considered defense mechanisms (Poulsen and Thomsen, 1988). In addition, enhanced APAP clearance and diminished transport within hepatic tissue due to decreased fenestration of endothelial cells lining hepatic sinusoids in aged rats (Mitchell et al., 2011). Another explanation is that mitochondrial protein adducts occurring in the liver due to acetaminophen toxicity are much fewer in rats than in mice. Therefore, rats exhibited lower oxidative damage (McGill et al., 2012). However, necrotic changes were found in livers of female rats using the same dose for full-term pregnancy (20 days) (Neto et al., 2004). This could be explained by the faster and more prominent depletion of liver GSH during pregnancy (Larrey et al., 1986).
Fig. 5. A: The liver of the nontreated group has a normal hepatic lobule with a central vein of normal size. B: The liver of the APAP-treated group after 4 weeks showed congested and dilated sinusoids with blood (arrow). C: liver of the APAP-treated group after 6 weeks showing congestion of sinusoids (arrow) associated with mild cytoplasmic vacuolation (arrowhead). D: Liver of the APAP-treated group after 8 weeks showing hepatic sinusoidal congestion (arrow) and single-cell necrosis associated with acidophilic cytoplasm and pyknotic nucleus (arrowhead). E: liver of the APAP-treated group after 10 weeks showing a dilated central vein surrounded by activated fibroblasts, sinusoidal congestion (arrow), focal necrosis associated with focal hemorrhage, and single-cell necrosis with cytoplasmic eosinophilia and nuclear pyknosis( arrowhead). F: liver of the APAP-treated group after 12 weeks showing a severe degree of perinuclear cytoplasmic vacuolation (arrowhead) with nuclear pyknosis.
Fig. 6. A: Kidney of the control group showed normal renal tubules (arrow) and a normal glomerulus (arrowhead). B: Kidney of the APAP-treated group after 4 weeks showed capillary congestion (arrow) and the tubular epithelium showed cloudy swelling (arrowhead). C: Kidney of the APAP-treated group after 6 weeks showed severe congestion (arrow) and marked tubular epithelial degeneration with nuclear pyknosis (arrowhead). D: Kidney of the APAP-treated group after 8 weeks showed a severe degree of renal tubular vacuolation with sloughing of tubular epithelium into the lumen of the tubule (arrowheads). E: Kidney of the APAP-treated group after 10 weeks showing tubular epithelial necrosis with nuclear pyknosis and loss (arrowhead) and shrunken glomeruli with widening of Bowman’s space (arrow). F: Kidney of the APAP-treated group after 12 weeks showing severe tubular necrosis of the renal papilla forming eosinophilic structureless masses (arrow) with cytoplasmic vacuolation (arrowhead). Histopathological examination of the kidneys of the APAP group revealed necrotic changes, parallel with rising seral urea and creatinine. Renal biopsy is rarely done for diagnostic purposes in cases of APAP-induced nephropathy. However, a moderate overdose of paracetamol could result in acute renal dysfunction, even without serious hepatic damage (Björck et al., 1988). Other studies also reported acute renal damage (Mazer and Perrone, 2008). The mechanism of renal toxicity of paracetamol involves the production of NAPQI in the kidney in high amounts, causing GSH depletion, and covalently binding to tissue nucleophiles (Mudge et al., 1978). However, administration of N-acetylcysteine did not affect the peak creatinine level, indicating that kidney damage is not just caused by the depletion of GSH (Eguia and Materson, 1997). GSH conjugates produced by the liver may contribute to the renal damage caused by APAP (Trumper et al., 1996). APAP can pass through the blood–brain barrier (Mallet et al., 2010) resulting in ischemic neuronal injury and oxidative stress, found in our study, this result agrees with a previous finding (Lalert et al., 2020). Lesions were very clear and more prominent in the hippocampus region of the brain, which is responsible for forming new memories, learning, and emotions (Tyng et al., 2017). The damage to this area of the brain supports the results of prior investigations showing that long-term paracetamol use has a negative impact on cognition as well as memory (Brandlistuen et al., 2013; Blecharz-Klin et al., 2014) and also decreased empathy (Kandis et al., 2018). APAP is known to be a selective COX-2 inhibitor preventing the formation of prostaglandin from arachidonic acid (Hinz et al., 2008), COX-2 is mainly concentrated in the hippocampus (Tocco et al., 1997). Arachidonic acid metabolites generated by COX-2 play an important function in the regulation of blood circulation in the brain (Stefanovic et al., 2006). Therefore, we can explain the greater harmful effect of paracetamol in the hippocampus by the increased COX-2 level there.
Fig. 7. A: Brain (cerebral cortex) of the control group shows normal neurons (arrowhead). B: Brain of APAP treated group after 4 weeks showing ischemic neuronal injury (arrowhead). C: Brain of APAP treated group after 6 weeks showing ischemic neuronal injury (arrowhead), and neuronophagia (arrows). D: Brain of APAP treated group after 8 weeks showed severe ischemic neuronal injury (arrowhead), and neuronophagia (arrows). E: Brain of APAP treated group after 10 weeks showing ischemic neuronal injury (arrowhead) neuronophagia (arrow) with satelatosis. F: Brain of APAP treated group after 12 weeks, showing malacia associated with focal gliosis (arrowhead). G: Brain (hippocampus region) of the control group shows normal neurons within the granular layer (arrowhead). H: Brain of APAP treated group after 4 weeks showing ischemic neuronal injury in the hippocampus region (arrowheads). I: Brain of APAP treated group after 6 weeks showing ischemic neuronal injury in the hippocampus region (arrowheads). J: Brain of APAP treated group after 8 weeks showed ischemic neuronal injury in the hippocampus region (arrowhead) also the presence of perivascular oedema. K: Brain of APAP-treated group after 10 weeks showed a large number of ischemic neuronal injuries in the hippocampus region (arrowheads). L: Brain of APAP treated group after 12 weeks showed severe ischemic neuronal injury in the hippocampus region (arrow).
Fig. 8. Scoring histopathological lesions in the liver (A), kidney (B), and brain (C) data indicates the mean ± SD, (n=5). Means are represented in bars, and SD is represented in T-shaped bars. Horizontal line matching two groups that are significantly different. * p-value p-value p-value <0.001. ConclusionIn this study, acetaminophen treatment, even in a dose known to be nontoxic, for a long period resulted in hepatotoxicity, nephrotoxicity, and brain toxicity. Toxicity level increased with Increasing the period of treatment. Present results also declare that the liver is not the primary organ in APAP toxicity as a mild toxic effect was found in the liver but more serious lesions and changes in biochemical parameters were found in the kidney and brain. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsMirna Aboshama and Walied Abdo participated in the study design, conducted the experiment, were involved in the methodology, and analyzed and interpreted the data. Ahmed Elsawak and Abdelrahman Khater suggested the research idea and prepared the text to be published. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesBancroft, J.D. and Gamble, M. 2008. Theory and practice of histological techniques. 6th Edition, Churchill Livingstone, Elsevier, China. Bartels, H., Böhmer, M. and Heierli, C. 1972. Serum creatinine determination without protein precipitation. Clin. Chim. Acta. Inter. J. Clin. Chem. 37, 193–197. Belfield, A. and Goldberg, D. 1971. Colorimetric determination of alkaline phosphatase activity. Enzyme 12, 561–568. Beutler, E., Duron, O. and Kelly, B.M. 1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888. Björck, S., Svalander, C.T. and Aurell, M. 1988. Acute renal failure after analgesic drugs including paracetamol (acetaminophen). Nephron 49, 45–53. Blecharz-Klin, K., Joniec-Maciejak, I., Piechal, A., Pyrzanowska, J., Wawer, A. and Widy-Tyszkiewicz, E. 2014. Paracetamol impairs the profile of amino acids in the rat brain. Environ. Toxicol. Pharmacol. 37, 95–102. Blieden, M., Paramore, L.C., Shah, D. and Ben-Joseph, R. 2014. A perspective on the epidemiology of acetaminophen exposure and toxicity in the united states. Expert. Rev. Clin. Pharmacol. 7, 341–348. Brandlistuen, R.E., Ystrom, E., Nulman, I., Koren, G. and Nordeng, H. 2013. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Inter. J. Epidemiol. 42, 1702–1713. Brune, K., Renner, B. and Tiegs, G. 2015. Acetaminophen/paracetamol: a history of errors, failures and false decisions. Eur. J. Pain. 19, 953–965. Caparrotta, T.M., Antoine, D.J. and Dear, J.W. 2018. Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature. Eur. J. Clin. Pharmacol. 74, 147–160. Eguia, L. and Materson, B.J. 1997. Acetaminophen-related acute renal failure without fulminant liver failure. Pharmacother. J. Human Pharmacol. Drug. Ther. 17, 363–370. Fawcett, J. and Scott, J. 1960. A rapid and precise method for the determination of urea. J. Clin. Pathol. 13, 156–159. Ferner, R.E., Dear, J.W. and Bateman, D.N. 2011. Management of paracetamol poisoning. BMJ 342, d2218. Hinz, B., Cheremina, O. and Brune, K. 2008. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB. J. 22, 383–390. Jaeschke, H., Adelusi, O.B. and Ramachandran, A. 2021. Ferroptosis and acetaminophen hepatotoxicity: are we going down another rabbit hole? Gene. Express. 20, 169. Kandis, S., Ates, M., Kizildag, S., Camsari, G.B., Yuce, Z., Guvendi, G., Koc, B., Karakilic, A., Camsari, U.M. and Uysal, N. 2018. Acetaminophen (paracetamol) affects empathy-like behavior in rats: dose-response relationship. Pharmacol. Biochem. Behav. 175, 146–151. Kei, S. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 90, 37–43. Lalert, L., Ji-Au, W., Srikam, S., Chotipinit, T., Sanguanrungsirikul, S., Srikiatkhachorn, A. and Maneesri-Le Grand, S. 2020. Alterations in synaptic plasticity and oxidative stress following long-erm paracetamol treatment in rat brain. Neurotox. Res. 37, 455–468. Larrey, D., Letteron, P., Foliot, A., Descatoire, V., Degott, C., Geneve, J., Tinel, M. and Pessayre, D. 1986. Effects of pregnancy on the toxicity and metabolism of acetaminophen in mice. J. Pharmacol. Exper. Therap. 237, 283–291. Mallet, C., Barrière, D.A., Ermund, A., Jönsson, B.A., Eschalier, A., Zygmunt, P.M. and Högestätt, E.D. 2010. Trpv1 in brain is involved in acetaminophen-induced antinociception. PLoS One 5, E12748. Mazaleuskaya, L.L., Sangkuhl, K., Thorn, C.F., Fitzgerald, G.A., Altman, R.B. and Klein, T.E. 2015. Pharmgkb summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogen. Genom. 25, 416. Mazer, M. and Perrone, J. 2008. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J. Med. Toxicol. 4, 2–6. Mcgill, M.R. and Jaeschke, H. 2013. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 30, 2174–2187. Mcgill, M.R., Williams, C.D., Xie, Y., Ramachandran, A. and Jaeschke, H. 2012. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 264, 387–394. Mitchell, S.J., Huizer-Pajkos, A., Cogger, V.C., Mclachlan, A.J., Le Couteur, D.G., Jones, B., De Cabo, R. and Hilmer, S.N. 2011. Age-related pseudocapillarization of the liver sinusoidal endothelium impairs the hepatic clearance of acetaminophen in rats. J. Gerontol. Series A. Biomed. Sci. Med. Sci. 66(4), 400–408. Mudge, G., Gemborys, M.W. and Duggin, G. 1978. Covalent binding of metabolites of acetaminophen to kidney protein and depletion of renal glutathione. J. Pharmacol. Exper. Therap. 206, 218–226. Neto, J.A., Oliveira-Filho, R.M., Simoes, M., Soares Jr, J. and Kulay Jr, L. 2004. Long-term acetaminophen (paracetamol) treatment causes liver and kidney ultra-structural changes during rat pregnancy. Clin. Exper. Obst. Gynecol. 31, 221–224. Nishikimi, M., Rao, N.A. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. Poulsen, H.E. and Thomsen, P. 1988. Long-term administration of toxic doses of paracetamol (acetaminophen) to rats. Liver 8, 151–156. Prescott, L. 1980. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 10, 291s–298s. Reitman, S. and Frankel, S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56–63. Sapan, C.V., Lundblad, R.L. and Price, N.C. 1999. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 29, 99–108. Stefanovic, B., Bosetti, F. and Silva, A.C. 2006. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage 32, 23–32. Tocco, G., Freire-Moar, J., Schreiber, S.S., Sakhi, S., Aisen, P. and Pasinetti, G. 1997. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: Implications for alzheimer’s disease. Exper. Neurol. 144, 339–349. Trumper, L., Monasterolo, L.A. and Elías, M.M. 1996. Nephrotoxicity of acetaminophen in male wistar rats: role of hepatically derived metabolites. J. Pharmacol. Exper. Therap. 279, 548–554. Tyng, C.M., Amin, H.U., Saad, M.N. and Malik, A.S. 2017. The influences of emotion on learning and memory. Front. Psychol. 8, 1454. Veilleux-Lemieux, D., Castel, A., Carrier, D., Beaudry, F. and Vachon, P. 2013. Pharmacokinetics of ketamine and xylazine in young and old sprague–dawley rats. J. Am. Assoc. Lab. Anim. Sci. 52, 567–570. Venkatesan, P.S., Deecaraman, M., Vijayalakshmi, M. and Sakthivelan, S.M. 2014. Sub-acute toxicity studies of acetaminophen in sprague dawley rats. Biol. Pharm. Bull. 37, 1184–1190. World Health Organization. 2021. Who model list of essential medicines-22nd list, 2021. Geneva, Switzerland: WHO. Xie, Y., Mcgill, M.R., Cook, S.F., Sharpe, M.R., Winefield, R.D., Wilkins, D.G., Rollins, D.E. and Jaeschke, H. 2015. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in heparg cells. Xenobiotica 45, 921–929. | ||
| How to Cite this Article |
| Pubmed Style Aboshama M, Abdo W, Elsawak A, Khater A. Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 316-323. doi:10.5455/OVJ.2024.v14.i1.28 Web Style Aboshama M, Abdo W, Elsawak A, Khater A. Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. https://www.openveterinaryjournal.com/?mno=177429 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.28 AMA (American Medical Association) Style Aboshama M, Abdo W, Elsawak A, Khater A. Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 316-323. doi:10.5455/OVJ.2024.v14.i1.28 Vancouver/ICMJE Style Aboshama M, Abdo W, Elsawak A, Khater A. Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 316-323. doi:10.5455/OVJ.2024.v14.i1.28 Harvard Style Aboshama, M., Abdo, . W., Elsawak, . A. & Khater, . A. (2024) Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 316-323. doi:10.5455/OVJ.2024.v14.i1.28 Turabian Style Aboshama, Mirna, Walied Abdo, Ahmed Elsawak, and Abdelrahman Khater. 2024. Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 316-323. doi:10.5455/OVJ.2024.v14.i1.28 Chicago Style Aboshama, Mirna, Walied Abdo, Ahmed Elsawak, and Abdelrahman Khater. "Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats." Open Veterinary Journal 14 (2024), 316-323. doi:10.5455/OVJ.2024.v14.i1.28 MLA (The Modern Language Association) Style Aboshama, Mirna, Walied Abdo, Ahmed Elsawak, and Abdelrahman Khater. "Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 316-323. Print. doi:10.5455/OVJ.2024.v14.i1.28 APA (American Psychological Association) Style Aboshama, M., Abdo, . W., Elsawak, . A. & Khater, . A. (2024) Effect of the long-term use of a NOAEL dose of acetaminophen (paracetamol) on hepatic, renal, and neural tissues of aged albino rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 316-323. doi:10.5455/OVJ.2024.v14.i1.28 |