| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 500-511 Original Research Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus ratsZeinab Z. Khater1* and Hend Sh. Ghareeb21Department of Zoology, Faculty of Science, Zagazig University, Zagazig, Egypt 2Plant Protection Research Institute, Dokki, Giza, Egypt *Corresponding Author: Zeinab Z. Khater. Department of Zoology, Faculty of Science, Zagazig University, Egypt. Email: z_sci.egy [at] zu.edu.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
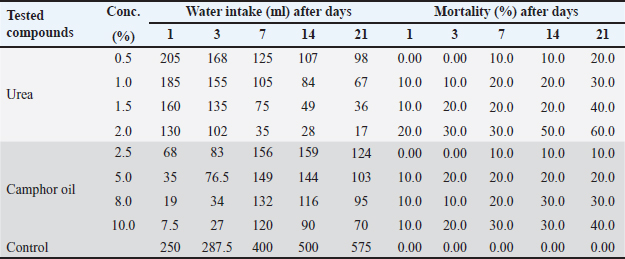
AbstractBackground: One of the most challenging pests to control is the wild rat (Rattus norvegicus), which poses serious risks to both human health and the economy. Fertilizers are a more recent method of pest management with various action modes and are considered safe control agents when applied at low doses. Aim: The present study aimed to examine the toxicological impacts of the contaminated water with urea and camphor oil individually, post-treatment of rats with camphor oil after the pre-treatment with urea and post-treatment of rats with urea mixed with camphor oil after urea pre-treatment against the wild rats (R. norvegicus). Methods: The study extends to explore the influence of these treatments on the physicochemical parameters of the water administered by rats. Moreover, the effect of the most three toxic treatments was studied on the blood and renal functional parameters and the kidney tissue of rats after 21 days of treatment. Results: The study showed that urea was more potent than camphor oil when applied individually and increasing the concentration of urea in the pre-treatment or when combined with camphor oil in the post-treatment caused a significant increase in the mortality of rats. The post-treatment of rats with camphor oil only or camphor oil mixed with urea after the pre-treatment with urea induced a synergistic activity against rats. In addition, the exposed water to urea and camphor oil has been modified in physicochemical parameters and formed ulcers and harm to the kidneys of the exposed wild rats. Conclusion: This study significantly contributes to the ecological and toxicological potential risk indexes of urea and camphor oil together, which are restricted on the perceptible value relevance in the literature of water quality and renal pathology. Therefore, urea and camphor oil represent successful agents for the wild rat's control. Keywords: Urea and camphor oil, Toxic influence, Physicochemical parameters of water, Hematological parameters, Electron microscopy of rat kidney. IntroductionThe wild rat is one of the most dangerous species of rodents that attack crops (Dedovic et al., 2012). It is known for infesting and harming property and it is an important vector of numerous diseases (Kosoy et al., 2015). It causes serious damage to crops and eats any available food including seeds, fruits, and plant shoots, which lead to incalculable economic losses (Bonnefoy et al., 2008). In addition, the urine and feces of rats contaminate forage that has been poorly stored which shows a threat to animal health (Daniels et al., 2003). Thus, rats are the target of a large number of pest control strategies. The excessive use of pesticides in the control of wild rats (R. norvegicus) is far from ideal because it has great hurtful impacts on human and environmental health (Beshay, 2005). Currently, there is a strong interest in developing new environmentally friendly agents for controlling pests (Dedovic et al., 2012). Fertilizers represent a new effective means for pest control instead of pesticides. It is considered safer than pesticides for use around pest and vertebrate wildlife when applied at low rates (Speiser and Kistler, 2002). It is mostly used in agriculture to improve plant growth and similarly, it has a successful impact on the rodent monitor (Testud, 2004). Urea is one of the most poisonous fertilizers to rats due to its release of ammonia, known as hyper ammonia syndrome, which directly affects the nervous system forms oxidative stress, and harms the rat organs causing death (Siva et al., 2017). Several plant products also have properties that make them efficient pest control agents. Camphor can be used as a potent rodenticide; it causes irritability in rats, neuromuscular, blurred vision, colitis, contraction of heart muscles, difficulty breathing, seizures, and death (Shahabi et al., 2014). It has a toxic influence on the R. norvegicus rat and causes significant damage to its internal organs (Mourad, 2010). Moreover, the combination of two control agents is also a successful method for pest management and this combination is more effective in controlling pests than the effect of each agent individually (Hend, 2018). The liver, kidneys, gastrointestinal tract, and lungs, are the organs of the drug metabolism, with the liver being the main organ of the biotransformation of drugs (Neal, 2012). Therefore, the kidneys, liver, and blood cells are mostly impacted by drug toxicity (Orinya et al., 2016). The main objective of this investigation was to indicate the variations between the impacts of urea and camphor oil individually, pre-treatment with urea and camphor oil post-treatment, pre-treatment with urea followed by post-treatment with the mixture of urea and camphor oil on the mortality, biochemical parameters and kidney tissues of R. norvegicus rat. Also, the influence of these treatments on the physical and chemical properties of the administered water by rats was determined. Materials and MethodsAnimalsAdult males of the wild rat were trapped in maize fields located in Zagazig district, Sharkia province, Egypt. The animals were carefully transferred to the laboratory, and then housed in metallic cages supplied with water and crushed maize to acclimatize for 15 days before starting the experiments. Tested materialsUrea [CO (NH2)2] (46.5% N) was obtained from El–Gomhouria Company, Egypt. While, the camphor oil [C10H16O] was purchased from Abu Zaid Company, Egypt. Each of them was dissolved in distilled water to obtain the required final test concentrations of 0.5%, 1%, 1.5%, and 2% for urea and 2.5%, 5%, 8%, and 10% for camphor oil. Experimental setupControl wild rats (healthy rats) were randomly distributed into five batches. The first batch was untreated, containing ten rats orally administered with tape water and served as control. The second batch of wild rats was supplied orally with urea at four concentrations (0.5%, 1%, 1.5%, and 2%), each concentration containing ten rats. The third batch was orally supplemented with camphor oil in four concentrations (2.5%, 5%, 8%, and 10%) of ten animals each. The fourth batch was treated orally with urea at the same concentrations given to the second batch and after seven days, the rats were treated with the highest concentration of camphor oil (10%) as a post-treatment; to obtain four treatments each of them containing ten rats. The fifth batch was administered orally with urea at the same concentrations given to the second batch and after seven days, rats were post-treated with the same pre-treated concentration of urea mixed with camphor oil at its highest tested concentration to get four treatments, each one containing ten rats. Water consumption and the mortality percent of both treated and untreated rats were recorded daily during the experimental period, which was 21 days. At the end of the experiment, rats treated with the highest concentration of each of urea and camphor oil and also the animals that pre-treated with urea at the highest concentration and then post-treated with urea mixed with camphor oil also at the highest concentration were anesthetized and sacrificed by decapitation. The experiment was repeated for 21 days with 25 rats for each concentration of 2% urea, 10% camphor oil, and 2% urea pre-treatment then post-treatment of 2% urea + 10% camphor oil to recollect blood samples from these rats for the biochemical analysis and histological studies of the kidney. Sampling and analytical methodsTap water samples were taken from the laboratory of Zoology Department, Faculty of Science, Zagazig University, Egypt. Tap water samples were collected from the investigated area before (10 samples) and after (50 samples, 10 samples for each concentration) subjection to urea at 1%, 1.5%, and 2% concentrations separately, camphor oil at the highest concentration 10% and the mixture of urea at the concentration of 2% with camphor oil at 10% concentration. Water physicochemical propertiesThe water samples were filtered, and placed in clean sampling glass bottles till used (Boyd, 1990). Color and odorThe color and odor of the water samples were determined according to Ambasht and Ambasht (1990). pH-valueDigital mini-pH-meter model 55 was used to measure the pH value of different water samples (Khater and Ibraheim, 2016). Nitrates and ammoniumNitrates and ammonium can be determined according to (Khater, 2011). AlkalinityThe alkalinity of different concentrations of water samples with urea and camphor oil was determined according to (Khater, 2011). Histopathological studiesEstimation of rat blood parametersForty blood samples were taken from the control and the rats were administered with urea at 2% concentration, camphor oil at a concentration of 10%, and urea at 2% mixed with camphor oil at a concentration of 10% after decapitation after 21 days in EDTA tubes for estimation of hematological parameters, and Weatherman tubes for estimation of blood urea and serum creatinine (Ibraheim and Khater, 2013). The serum was separated by centrifugation from the blood cells at 3,000 rpm for ten minutes at room temperature. Furthermore, the serum was stored in the freeze at −20ºC in Eppendorf tubes until utilized (Khater and Ibraheim, 2016). Ultra-structural studies on the rats kidneyForty kidney specimens were collected from the control and the exposed wild rats to urea (2%), camphor oil (10%), and a mixture of urea (2%) with camphor oil (10%) for 21 days, subjected to histological methods, and then set for the electron microscopic examination by JeolJem100CX transmission electron microscope of Zagazig University, Sharkia province, Egypt (Khater and Ibraheim, 2016). Statistical AnalysisThe analysis of difference was performed using one-way ANOVA at the significant level of 0.05 (p < 0.05) by the SPSS program and means of data and standard error were compared by using the Microsoft Excel program. Data analysis, tables, and graphics were done using Microsoft Word (2007) and Origin 8. Ethical approvalThe current investigation was accepted by The Institutional Animal Care and Use Committee at Zagazig University (ZU-IACUC/1/F/250/2023). ResultsEfficacy of urea and camphor oil individually against R. norvegicus ratsThe influence of urea at the tested concentrations (0.5%, 1%, 1.5%, and 2%) and camphor oil at the concentrations (2.5%, 5%, 8%, and 10%) was evaluated individually against R. norvegicus rats and the amount of water consumed by rats from each treatment was also calculated, as shown in Table 1. Urea at all tested concentrations recorded the highest water intake on the first day of the experiment and then the amount of water administered continued to decrease. On the contrary, camphor oil was less palatable to rats at the beginning of the experiment. At all concentrations of camphor oil, the lowest intake of water was recorded on the first day of the treatment. This may be due to the rats being surprised by the pungent smell of oil, but then the rate of water administered increased gradually with the increase in the experimental period. In addition, by increasing the concentration of each urea and camphor oil the water intake by rats decreased. Thus, urea at the highest concentration of 2% recorded the least water consumption with a value of 17 ml at the end of the experiment on day 21, and camphor oil at the highest concentration of 10% achieved the lowest water administration with value of 7.5 ml on the first day of the experiment. The rat's consumption of untreated water in the control was significantly higher than those treated with both tested compounds. Regarding the efficacy of urea and camphor oil against rats, by increasing the concentrations of each compound and increasing the experimental period, the mortality of rats increased. But urea more effective than camphor oil, it was recorded 60% mortality of rats at its highest concentration of 2% compared to camphor oil which achieved 40% mortality at its highest concentration of 10% on the 21 days of the experiment. On the other hand, all control rats were survived. Table 1. Efficacy of urea and camphor oil individually against Rattus norvegicus rats.
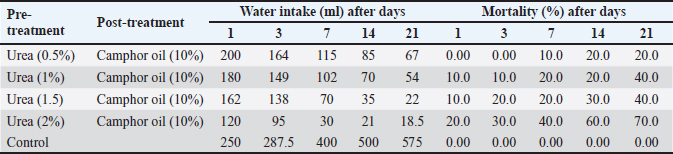
Effect of the pre-treatment with urea and camphor oil post-treatment against R. norvegicus ratsThe effect of camphor oil at the highest concentration 10% post-treatment after the pre-treatment with urea at the concentrations (0.5%, 1%, 1.5%, and 2%) separately was illustrated in Table 2. The obtained results showed that the highest water intake at all tested treatments was recorded on the first day of the experiment. Whereas, with an increase in the experimental time and an increase in the concentration of urea in the pre-treatment before camphor oil post-treatment, the water administered by rats was decreased. Thus, the highest concentration of urea 2% pre-treatment before camphor oil post-treatment caused the lowest intake of water (18.5 ml) at the end of the experiment on day 21. Water consumption rates for all tested treatments were below that of untreated rats in control. Furthermore, increasing the concentration of urea in the pre-treatment before the treatment with camphor oil led to an increase in the mortality of rats. The highest mortality value 70% was achieved by urea at the highest concentration of 2% pre-treatment followed by camphor oil post-treatment after 21 days of the experiment. The lowest concentration of 0.5% of urea followed by camphor oil as post-treatment markedly reduced the mortality of rats to 20% after the same experimental time. In contrast, there is no mortality of untreated rats was recorded in the control. It seems clear from our results that increasing the urea concentration in the pre-treatment induced an observed reduction in the water intake by rats and increased at the same time mortality rate. Table 2. Effect of the pre-treatment with urea and camphor oil post-treatment against Rattus norvegicus rats.
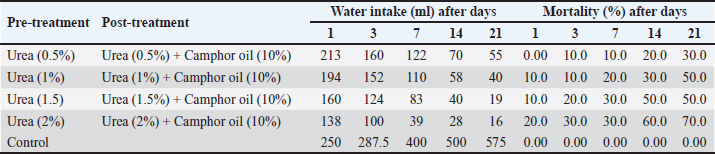
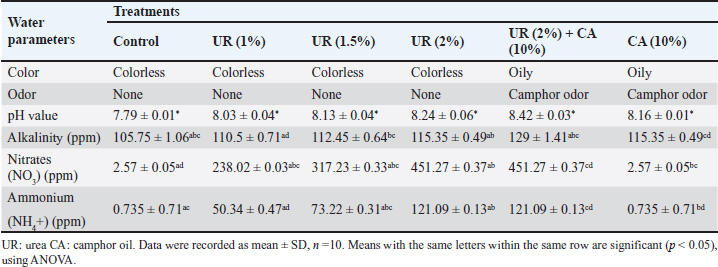
Efficiency of the pre-treatment with urea and mixing of urea with camphor oil post-treatment against R. norvegicus ratsThe effect of camphor oil at a concentration of 10% mixed with each tested concentration of urea against rats previously treated with urea concentrations individually was investigated, as indicated in Table 3. The highest water consumption by rats for all treatments was recorded on the first day of the treatment and then it decreased continually throughout the experiment. The pre-treatment of rats with urea at the highest concentration 2% followed by treatment with the same concentration of urea mixed with camphor oil achieved only 16 ml of water administered on the 21st day of the treatment. In contrast, after the same experimental period, the pre-treatment of rats with urea at the lowest concentration of 0.5% followed by the post-treatment with urea at the same concentration mixed with camphor oil induced the highest water intake with value 55 ml. On the other hand, the same treatment showed the lowest efficacy against rats with a mortality rate of 30% after 21 days of the experiment. It was followed by urea pre-treatment at each of 1% and 1.5% concentrations then post-treatment with the same concentrations separately combined with camphor oil, which recorded 50% mortality after the same period of the experiment. The mixing of urea at the highest concentration of 2% with camphor oil post-treatment for rats pre-treated with the same concentration of urea alone achieved the highest mortality with a value of 70% compared to the control which did not show any mortality. These results can be reflected by increasing the concentration of urea individually in the pre-treatment or when combined with camphor oil in the post-treatment, the water intake decreases and the mortality rate significantly increases. There is no information or published studies about the post-treatment of R. norvegicus rats with camphor oil or by mixing camphor oil with urea after the pre-treatment of rats only. Physico-chemical parameters of the waterThe physico-chemical parameters of water samples treated with urea at 1%, 1.5%, and 2% concentrations, camphor oil at the concentration of 10%, and a mixture of urea at 2% concentration with camphor oil at the concentration of 10% were recorded in Table 4. There were changes in the color and odor of the water samples between the exposed and the control ones. The pH value lies within the international permissible limits except the group four while, the alkalinity, nitrates, and ammonium values do not lie within it. Histopathological studiesBlood parameters estimation of wild ratsModifications in wild rat blood parameters such as hematological and kidney functional parameters (blood urea and serum creatinine) were determined for control and exposed wild rats. The results observed distinct modifications in all the blood parameters (Table 5). Significantly different (p < 0.05) values were found between the blood parameters of the exposed wild rats and control. Ultra-structural studies on the wild rats' kidneyStudies with electron microscopy showed serious effects of urea (2%) + camphor oil (10%), urea (2%), and camphor oil (10%) on the kidneys of the examined wild rats drinking it (Figs. 1–3). The intensity of histopathological alterations in the kidney cortex was raised in the exposed wild rats to urea and camphor oil compared to the control. DiscussionEfficacy of urea and camphor oil individually against R. norvegicus ratsThe present results reported that rats treated with urea take a large amount of water, and this may be due to the fact that urea exerts direct biogenic activity, which can be explained as a result of the osmotic gradient between extracellular and intracellular fluid. Whereas, when this is positive, as in the case of a rapid rise in urea levels in the blood, it leads to dehydration of cells and thus stimulates thirst in rats. Similarly, Dedovic et al. (2012) revealed that the palatability of R. norvegicus rats to sodium selenite was highest in the first two days of treatment, but after the next several days, the rats' consumption of it continued to decrease. The consumption of sodium selenite by rats decreased on the seventh day of the treatment, and the highest mortality rate of rats was achieved by this compound between the fourth and seventh day of consumption. Changes in the amount of water intake by rats can be used as indicators for assessing the public health case of the experiential animals (El-Hilaly et al., 2004). Moreover, Testud (2004) showed that nitrogen fertilizers have a successful effect on the control of rodents. Hydrolysis of urea leads to the release of ammonia, which has a toxic influence on rats due to an increase in the level of ammonium in the blood. This leads to hyperammonia, which causes neurological alterations and oxidative stress and also damages the organs of rats, leading to mortality (Siva et al., 2017). Another similar study demonstrated that ammonia is poisonous; it caused functional disorders in the brains of albino rats, which contributed to coma and death (Priyadarshini and Neeraja, 2015). In addition, Omar et al. (2007) indicated that ammonia causes the rapid death of rats as a result of brain edema. In the same trend, Hend (2017) showed that ammonium nitrate caused a highly significant toxic effect on R. norvegicus rats, and by increasing the concentrations of this fertilizer and increasing the experimental time, the mortality of rats increased. It caused 53.33% mortality in rats at a concentration of 1.5% after only one day of treatment. Then the mortality rate of wild rats rose with the increase in the experimental period, reaching 80% after 21 days of the experiment. After the same period, the same fertilizer recorded 86.66% mortality in R. norvegicus rats at the highest tested concentration of 2% (Khater and Hend, 2018). On the other hand, plant products contain toxic substances, which makes them effective against rats (Tan et al., 2008). Edwards et al. (1993) confirmed that camphor oil was toxic to rats. The toxicity of this oil is due to its cineole content, which ranges from 13% to 78% of the total oil. Furthermore, it caused inhibition of the secretion of catecholamine by blocking the nicotinic acetylcholine receptors, which negatively affected the nervous system and thus the rat's behavior and survival (Park et al., 2000). Moreover, it contains about 60 monoterpenoids in addition to 1,8-cineole as its major component and is responsible for its toxic behavior (Bignell and Dunlope, 1996). Similarly, Fakir et al. (2021) revealed that Berberis hispanica extract at high doses showed an observed mortality in rats. A significant decrease was also shown in water intake by animals treated with these doses compared to the untreated rats. This decrease in water administration by treated animals is due to the deregulation of appetite under the influence of this extract. On the contrary, the administration of albino rats daily with Abrus precatorius extract for 28 days did not induce any toxicity in rats, so no mortality was recorded. No significant change in the water consumption of the treated rats was recorded compared to the control. The lack of significant alteration in water intake by the treated rats indicates the non-toxic effect of the extract (Tabasum et al., 2019). This can be summarized by saying that mortality and water consumption represent very sensitive indicators to evaluate the toxicity of a substance (Kebede et al., 2016). Table 3. Efficacy of the pre-treatment with urea and mixing of urea with camphor oil post-treatment against Rattus norvegicus rats.
Table 4. Water physico-chemical properties (mean ± SD) of control and the exposed ones.
Table 5. Hematological and kidney functional parameters (mean ± SD) of control and exposed wild rats to the polluted water.
Effect of urea and camphor oil against R. norvegicus ratsIn our study, with an increase in the concentration of urea in the pre-treatment before the post-treatment with camphor oil, the water administered by rats decreased and the mortality rate increased. Consequently, the highest concentration of urea (2% pre-treatment) before the post-treatment with camphor oil at a concentration of 10% caused the lowest water intake (18.5 ml) and the highest rate of mortality (70%) at the last experiment on day 21. Furthermore, increasing the urea concentration in the pre-treatment of rats and also in the post-treatment when mixed with camphor oil led clearly to an increase in the mortality rate of rats. These findings were in agreement with a related earlier study that showed that the efficacy of the post-treatment depends mainly on the substance used in the pre-treatment and the dosage of the other substance used in the post-treatment (Inns and Leadbeater, 1983). Similar findings revealed that in the post-treatment of rats with K-27 after the pre-exposure to paraoxon, the survival of treated animals depends on the substance used in the pre-treatment and also on the dose of paraoxon. Additional administration of K-27 after the pre-treatment with paraoxon caused a considerable rise in the mortality of wild rats compared to those exposed to paraoxon alone. Moreover, the pre-treatment with physostigmine or K-27 before the treatment with the sublethal dose of paraoxon caused an increase in the mortality rate of rats (Dietrich et al., 2020). On the contrary, Nurulain et al. (2009) reported that sometimes the post-treatment reduces the toxic influence of the pre-treatment against rats and thus reduces the mortality rate. The post-treatment of male Wistar rats orally with Vernonia amygdalina extract after the pre-administration of dimethyl nitrosamine significantly reduced the toxic effect of dimethyl nitrosamine (Usunobun and Anyanwu, 2016). In addition, Fanucchi et al. (2012) indicated that the pre-exposure of Sprague–Dawley adult rats to chlorine caused severe injury to the airway and pulmonary edema, which led to mortality. The post-treatment of rats with a mixture of ascorbate and deferoxamine after the pre-exposure to chlorine can reduce the severity of acute lung injury induced by chlorine and so decrease mortality. In the same trend, Annateresa et al. (2013) demonstrated that the combination of canola oil with Nacl enhances the oxidative stress of spontaneously hypertensive stroke prone (SHRSP) rats. However, the amount of water intake by rats treated with canola oil combined with salt was significantly higher than that taken by rats administered with canola oil alone.
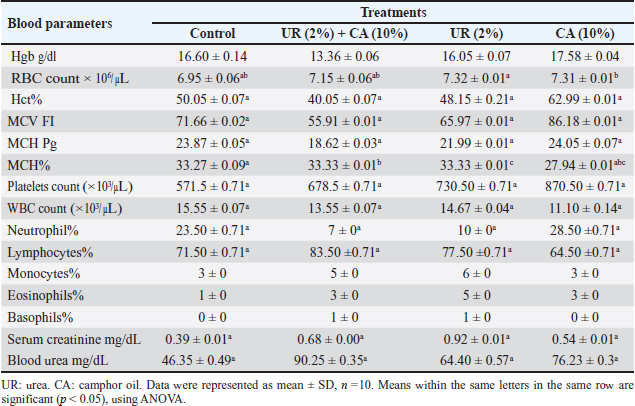
Fig. 1. Electron micrograph of the kidney cortex components of a normal wild rat indicating Malpighian corpuscle which explains the general morphology of glomerular capillary and podocyte. The podocyte cell body (Pc) has an irregular-shaped nucleus (N) with batches of chromatine materials (Ch), centrally placed in the cytoplasm packed with various inclusions and organelles, and peripheral nucleolus (Ne). The glomerular capillary has filtration slit (FS), primary process (PP), secondary foot processes (SPs), subpodocyte space (SPS), blood capillary (BC), and red blood cells (RBCs) (X 1500) (A). The general morphology of proximal convoluted tubule (PCT) which has round nuclei (N) with peripherally located nucleoli (Ne) and homogenously distributed chromatine (Ch), peripherally placed in a cytoplasm packed with different inclusions and organelles, long microvilli (Mv), and central small lumen (L). These organelles comprise lysosomes (Ly) appear as small rounded vesicles bounded by a single membrane, mitochondria (M) which are numerous exhibiting elongated or round shapes, and abundant glycogen particles (Gl) in the form of rosette-shaped particles (X 4000) (B). The distal convoluted tubule has basal infoldings (IFs), short microvilli (Mv), central large lumen (L), and the same inclusions of PCT (X4000) (C). Physico-chemical parameters of the waterAs is clear in the results, there is no change in the color and odor of the water samples except for the mixing of urea (2%) with camphor oil (10%) and camphor oil (10%) only due to the addition of the camphor oil (Gupta, 2000). The present investigation indicated that the pH values of different treatments were alkaline, and additional impacts of urea to camphor oil were found. This is in agreement with that of Davies (2005) and Meloni and Oy (2013). The results of this study showed that pH values at different examined limits lie within the favorable levels (6.2–8.3) recommended for the survival and growth of living creatures except for the mixture of urea with camphor oil, and this complies with the results of Korai et al. (2008) and Pandey and Tiwari (2009).
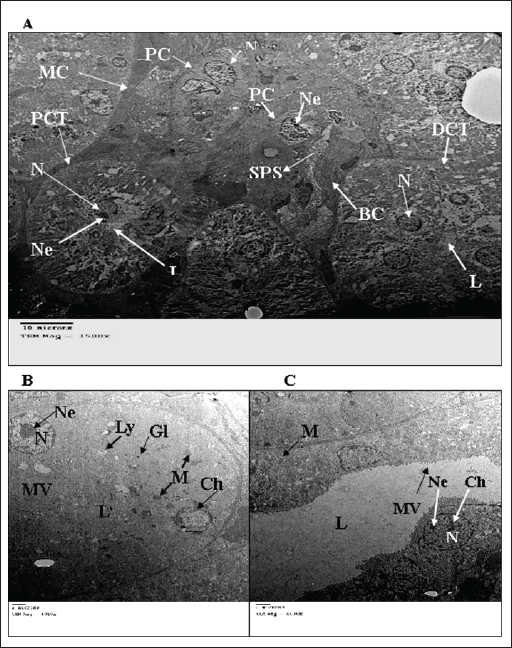
Fig. 2. TEM micrograph of the kidney cortex components of a normal wild rat indicating the general morphology of Malpighian corpuscle showing the general morphology of glomerular capillary and podocyte. The Pc has an irregular-shaped nucleus (N) with batches of chromatin materials (Ch), centrally placed in a cytoplasm packed with different inclusions and organelles, and peripheral nucleolus (Ne). Glomerular capillary has FS, PP, SPS, SP, BC, and RBC (X 2500) (A). PCT has a round nucleus (N) with homogenous distributed chromatine (Ch), peripherally placed in a cytoplasm packed with different inclusions and organelles, and peripherally located nucleolus (Ne), and long microvilli (Mv). These organelles consist of smooth endoplasmic reticulum (SER) and rough endoplasmic reticulum (RER). Lysosomes (Ly) appear as small rounded vesicles bounded by a single membrane. Abundant glycogen particles (Gl) in the form of rosette-shaped particles. Mitochondria (M) are numerous showing elongated or round shapes. (X 6000) (B). The distal convoluted tubule cell has basal IF, short microvilli (Mv), and the same inclusions of PCT (X10000) (C).
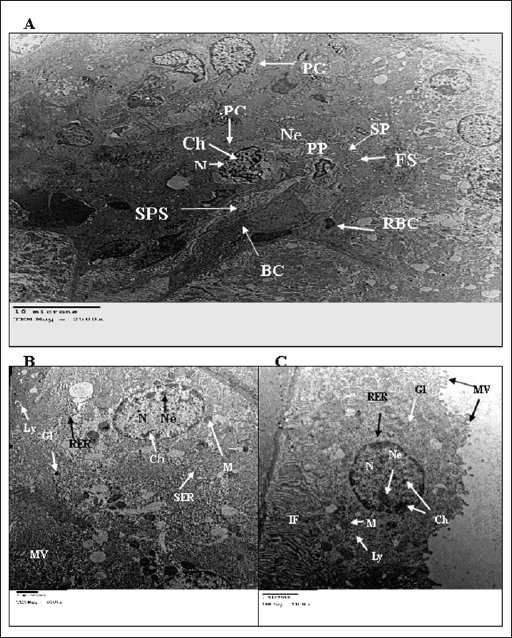
Fig. 3. TEM micrograph of the kidney cortex components of a treated wild rat indicating elongated nucleus (EN), necrotic nucleus (NN), vesicular granules (VG), condensed chromatine (CCh), swollen mitochondria (SM), Damaged mitochondria (DM), lytic cytoplasm (LC), degeneration of the cytoplasmic organelles (DO), marked dilation with inflammatory cells (IC), vacuolation of the cytoplasm (VC), ulcers (U), pigments (P), enlarged PC (EPc), glomerular capillary comprises enlarged FS (EFS), enlarged BC (EBC), enlarged PP (EPP), enlarged SP (ESP) and enlarged fenestrated endothelium (EFE). (A) and (B) shows the kidney cortex of a treated rate exposed to 2% urea + camphor oil at 10% (X 1,400, 2,700, respectively). (C) and (D) shows the kidney cortex of a treated rate exposed to 2% urea (X 1,400, 2,700, respectively). (E) and (F) shows the kidney cortex of a treated rate exposed to 10% camphor oil (X 1,400, 2,700, respectively). Alkalinity, nitrates, and ammonium are raised in the water samples that contain urea and camphor oil addition, and these are by Davies (2005) and Peterson (2008). The alkalinity range (20 mg/l) in the present investigation was not advised, as recommended by ANZECC and ARMCANZ (2000), Sithik et al. (2009), and Khater and Ibraheim (2016). Histopathological studiesEstimation of blood parameters of wild ratsThe present investigation indicated slight modifications in all blood parameters for control and exposed rats, and this coincided with the results of Hanafy et al. (2013), Khater and Ibraheim (2016), Hamed et al. (2023), and Oktanella et al. (2023). Ultra-structural studies on the wild rats' kidneySuch studies indicated the hazardous effects of urea and camphor oil on the experimental wild rats, as they damaged the kidney tissues of the investigated rats, causing dysfunction and kidney ulcers. They harm the renal tissues, forming kidney disappointment and putrefaction, and this concurred with the results of Dar et al. (2017) and Kong et al. (2017). ConclusionThe present investigation indicated that increasing the concentration of urea and camphor oil reduced the amount of water intake by R. norvegicus rats and caused an increase in the mortality of wild rats. Moreover, the post-treatment of rats by mixing urea with camphor oil after the treatment with urea only induced the highest effect on the mortality of rats, which indicates a synergistic activity of the two agents compared to the treatment with each agent separately. AcknowledgmentsThe authors greatly thank Prof. Dr. Magdy Wilson, Professor in the Harmful Animals Research Department, Plant Protection Research Institute, Egypt, for his support and encouragement during the completion of the manuscript. The authors greatly thank Prof. Dr. Abdel Monem Khalil for his critical reading of this paper. Data availabilityAll data will be made available upon a reasonable request. Conflict of interestThe authors have no competing interests. FundingThere is no funding for authors. Author contributionsZeinab Z.K. Khater and Hend Sh. Ghareeb are responsible for everything in the research. ReferencesAmbasht, R.S. and Ambasht, P.K. 1990. Environment & Pollution: An Ecological Approach. Book, CBS Publishers & Distributors (4) Pvt. Ltd., ISBN 13: 9788123912233, pp: 323. Annateresa, P., Xavier, A.C., Louise, L., Fadi, J.C. and Paul, A.L. 2013. Salt loading in canola oil fed SHRSP rats induces endothelial dysfunction. PLoS One 8(6), e66655. ANZECC and ARMCANZ. 2000. Australian and New Zealand Guidelines for Fresh and Marine Water Quality. National Water Quality Management Strategy (NWQMS), pp: 314. Beshay, R.M. 2005. Biological and toxicological studies on some rodents. Ph.D. Thesis, Faculty of Agriculture, Benha University, pp: 173. Bignell, C.M. and Dunlope, P.J. 1996. Volatile leaf oils of some South Western and Southern Australian species of the genus Eucalyptus. Part VII. Subgenus Symphyomyrtus section. Excert. Flav. Fragr. J. 11, 35–41. Bonnefoy, X., Kampen, H. and Sweeney, K. 2008. Public health significance of urban pests. World Health Organization, Regional office for Europe, pp: 387–419. Boyd, C.E. 1990. Water quality in ponds for aquaculture. USA: Alabama Agriculture Experiment Station, Auburn University. Daniels, M.J., Hutschings, M.R. and Greig, A. 2003. The risk of disease to livestock posed by contamination of farm stored feed by wildlife excreta. Epidemiol. Infect. 130, 561–568. Dar, M.A., Sultana, M., Mir, A.H., Raina, R. and Prawez, S. 2017. Effect of repeated oral administration of roundup and ammonium nitrate on liver of wistar rats. Proc. Natl. Acad. Sci. 89, 505–510. Davies, P.S. 2005. The biological basis of wastewater treatment. Strathkelvin Instruments Ltd., pp: 1–20. Dedovic, S., Vuksa, M., Petrovic, M.M., Bojkovski, J., Pavlovic, I., Jokic, G. and Stojnic, B. 2012. Control of brown rat (Rattus norvegicus) on a dairy farm in Serbia. Biotechnol. Anim. Husband. 28(3), 623–633. Dietrich, E.L., Syed, M.N., Mohamed, Y.H., Kamil, K. and Georg, A.P. 2020. Combined pre-and post-treatment of paraoxon exposure. Molecul. 25, 1521. Edwards, P.B., Wanjura, W.J. and Brown, W.V. 1993. Selective herbivore by Christmas beetles in response to intraspecific variation in Eucalptus terpenoids. Oecol. 4, 551–557. El-Hilaly, J., Israili, Z.H. and Lyoussi, B. 2004. Acute and chronic toxicological studies of Ajugaiva in experimental animals. J. Ethnopharmacol. 91(1), 43–50. Fakir, L., Bourhia, M., Salamatullah, A., Alzahrani, A., Ullah, R., Ezzeldin, E., Mostafa, G. A., Bari, A., Alaoui, T., Gmouth, S., Benbacer, L. and Abdelhamid, Z. 2021. Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats. Open Chem. 19, 686–695. Fanucchi, M.V., Bracher, A., Doran, S.F., Giuseppe, L.S., Fernandez, S., Edward, M.P., Larry, B. and Sadis, M. 2012. Post- exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am. J. Respir. Cell Mol. Biol. 46(5), 599–606. Gupta, P.K. 2000. Methods in environmental analysis water, soil and air. Agrobios 5, 1–400. Hamed, I., Sherif, R.M., El-Sheikh, E.A., Aldawek, A.M., and Shalaby, A.A. 2023. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J. 13(10), 1334–1345. Hanafy, M., Hussein, M. and Hashem, M. 2013. Biophysical and biological studies on the effect of electromagnetic field on the ehrlich tumor cells implanted in mice. J. Am. Sci. 9(12), 833–840. Hend, Sh. Gh. 2017. Toxic effect of ammonium nitrate and Bacillus subtilis on the wild rat, Rattus norvegicus. J. Am. Sci. 13(12), 119–127. Hend, Sh. G. 2018. Plants as promising safe molluscicides for control Monacha cartusiana snail. Nat. Sci. 16(12), 133–141. Ibraheim, M.H. and Khater, Z.Z.K. 2013. The effect of electromagnetic field on water and fish Clarias garpienus, Zagazig, Egypt. Life Sci. J. 10(4), 3310–3324. Inns, R.H. and Lead beater, L. 1983. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J. Pharm. Pharmacol. 35, 427–433. Kebede, S., Afework, M., Debella, A., Ergete, W. and Makonnen, E. 2016. Toxicological study of the butanol fractionated root extract of Asparagus africanus Lam., on some blood parameter and histopathology of liver and kidney in mice. BMC Res. Not. 9, 49. Khater, Z.Z.K. 2011. Ecological and biological studies on the effect of some water pollutants on some fishes. M. Sc. Thesis. Faculty of Science, Zagazig University, Egypt. Khater, Z.Z.K. and Hend, Sh. G. 2018. Ecotoxicological effects of ammonium nitrate and Bacillus subtilis on the wild rat, Rattus norvegicus. Egypt. J. Zool. 69(69), 147–162. Khater, Z.Z.K. and Ibraheim, M.H. 2016. Some ecological studies on the impact of magnetic field on the tap water. Egypt. J. Aquat. Biol. Fish. 20(2), 51–60. Kong, W., Huang, C., Tang, Y., Zhang, D., Wu, Z. and Chen, X. 2017. Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella). Sci. Rep. 2017, 1588. Korai, A.L., Sahato, G.A., Lashari, K.H. and Arbani, S.N. 2008. Biodiversity in relation to physicochemical properties of Keenjhar Lake, Thatta District, Sindh, Pakistan. Turk. J. Fish. Aquat. Sci. 8, 259–268. Kosoy, M., Khlyap, L., Cosson, J. and Morand, S. 2015. Aboriginal and invasive rats of genus Rattus as hosts of infectious agents. Vect. Born. Zoon. Dis. 15, 3–12. Meloni, E. and Oy, F. 2013. The NWF water purification process—freshwater in a natural way. Lahti, pp:1–32. Mourad, A.A. 2010. Toxicity effect of ethanolic Eucalyptus leaves extract on white Norway rat, (Rattus norvegicus Var. Albus). J. Plant Prot. Pathol. 1(11), 877–884. Neal, M.J. 2012. Drug metabolism. Medical pharmacology at a Glance (7th ed). London, UK: John Wiley and Sons Inc. Nurulain, S.M., Lorke, D.E., Hasan, M.Y., Shafiullah, M., Kuca, K., Musilek. K. and Petroianu, G.A. 2009. Efficacy of eight experimental bis pyridinium oximes against paraoxon-induced mortality: comparison with the conventional oximes pralidoxime and obidoxime. Neurotox. Res. 16, 60–67. Oktanella, Y., Untari, H., Wuragil, D.K., Ismiawati, H., Hasanah, N.A., Agustina, G.C. and Pratama, D.A.O. 2023. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet. J. 13(8), 1003–1011. Omar, C., Pilar, L., Tiago, B.R. and Vicente, F. 2007. Magnetic resonance analysis of the effects of acute ammonia intoxication on rat brain. Role of NMDA receptors. J. Neuro. Chem. 103(4), 1334–1343. Orinya, O.A., Adenkola, A.Y. and Ogbeo, R.J. 2016. Haematological and biochemical studies on the effect of diclofenac sodium on wistar Rattus norvegicus. Int. J. Biol. Chem. Sci. 10(5), 2231–2242. Pandey, S.K. and Tiwari, S. 2009. Physico- chemical analysis of ground water of selected area of Ghazipurcity-A case study. Nat. Sci. 7(1), 17–20. Park, T.J., Seo, H.K., Kang, B.J. and Kim, K.T. (2000). Noncompetitive inhibition by camphor of nicotinic acetylcholine receptors. Biochem. Pharmacol. 61, 787–793. Peterson, H. 2008. A biological treatment process for better water and improved working conditions. Canad. Water Treat. 8(1), 18–20. Priyadarshini, J. and Neeraja, P. 2015. Impact of ammonia on oxidative metabolism in certain tissues of albino rat. Int. J. Pharma. Bio. Sci. 6(3), B215–B221. Shahabi, S., Ali, S.G., Moghadamnia, A.A., Barghi, E., Zabihi, E., Amiri, M.G., Maliji, G., Faraji, A.S., Boora, M.A., Ghazinejad, N. and Shamsai, H. 2014. The effect of camphor on sex hormones levels in rats. Cell J. 16(2), 231–234. Sithik, A.M.A., Thirumaran, G., Arumugam, R., Kannan, R.R.R. and Anantharaman, P. 2009. Physico-chemical parameters of holy places Agnitheertham and Kothandaramar Temple; southeast coast of India. American-Euras. J. Sci. Res. 4(2), 108–116. Siva, K.T., Shobha, R.A., Sujatha, K., Purushotham, B. and Neeraja, P. 2017. Toxicity evaluation of ammonium sulfate to albino rat. Asia. J. Pharm. Clin. Res. 10(1), 313–316. Speiser, B. and Kistler, C. 2002. Field tests with a molluscicides containing iron phosphate. Crop Prot. 21, 389–394. Tabasum, S., Khare, S. and Jain, K. 2019. Subchronic toxicity assessment of orally administered methanol (70%) seed extract of Abrus precatorius L. in Wistar albino rats. Turk. J. Pharm. Sci. 16(1), 88–95. Tan, P.V., Mezui, C., Enow-Orock, G., Njikam, N., Dimo, T. and Bitolog, P. 2008. Teratogenic effects, acute and sub-chronic toxicity of the leaf aqueous extract of Ocimum suave wild (Lamiaceae) in rats. J. Ethnopharmacol. 115, 232–237. Testud, F. 2004. Engrais mineraux inorganic fertilizers. EMC Toxicol. Pathol. 1(1), 21–28. Usunobun, U. and Anyanwu, G.O. 2016. Dimethyl nitrosamine (DMN) pre-treated rats and protective effect of Vernonia amygdalina post-treatment on liver function. Int. J. Pharmacol. Toxicol. 4(1), 74–77. | ||
| How to Cite this Article |
| Pubmed Style Khater ZZ, Ghareeb HS. Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 500-511. doi:10.5455/OVJ.2024.v14.i1.45 Web Style Khater ZZ, Ghareeb HS. Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. https://www.openveterinaryjournal.com/?mno=182393 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.45 AMA (American Medical Association) Style Khater ZZ, Ghareeb HS. Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 500-511. doi:10.5455/OVJ.2024.v14.i1.45 Vancouver/ICMJE Style Khater ZZ, Ghareeb HS. Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 500-511. doi:10.5455/OVJ.2024.v14.i1.45 Harvard Style Khater, Z. Z. & Ghareeb, . H. S. (2024) Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 500-511. doi:10.5455/OVJ.2024.v14.i1.45 Turabian Style Khater, Zeinab Z., and Hend Sh. Ghareeb. 2024. Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 500-511. doi:10.5455/OVJ.2024.v14.i1.45 Chicago Style Khater, Zeinab Z., and Hend Sh. Ghareeb. "Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats." Open Veterinary Journal 14 (2024), 500-511. doi:10.5455/OVJ.2024.v14.i1.45 MLA (The Modern Language Association) Style Khater, Zeinab Z., and Hend Sh. Ghareeb. "Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 500-511. Print. doi:10.5455/OVJ.2024.v14.i1.45 APA (American Psychological Association) Style Khater, Z. Z. & Ghareeb, . H. S. (2024) Ecological and oral toxicity assessment of urea and camphor oil against Rattus norvegicus rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 500-511. doi:10.5455/OVJ.2024.v14.i1.45 |