| Research Article | ||
Open Vet. J.. 2023; 13(11): 1400-1408 Open Veterinary Journal, (2023), Vol. 13(11): 1400–1408 Original Research Molecular detection of Babesia ovis and blood parameters’ investigation reveal hematological and biochemical alterations in babesiosis-infected Lohi sheep in Multan, PakistanMuhammad Sajid1*, Syed Atif Hasan Naqvi2, Muhammad Riaz1, Ummad Ud Din Umar2, Nasreen Nasreen3, Adil Khan4,5 and Mourad Ben Said6,71Zoology Division, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan 2Department of Plant Pathology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan 3Department of Zoology, Khyber Pakhtunkhwa, Abdul Wali Khan University, Mardan, Pakistan 4Department of Zoology, Bacha Khan University, Charsadda, Pakistan 5Department of Biology, Mount Allison University, Sackville, Canada 6Laboratory of Microbiology, National School of Veterinary Medicine of Sidi Thabet, University of Manouba, Manouba, Tunisia 7Department of Basic Sciences, Higher Institute of Biotechnology of Sidi Thabet, University of Manouba, Manouba, Tunisia *Corresponding Author: Muhammad Sajid. Zoology Division, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan. Email: sajidmultani [at] hotmail.com Submitted: 10/07/2023 Accepted: 02/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
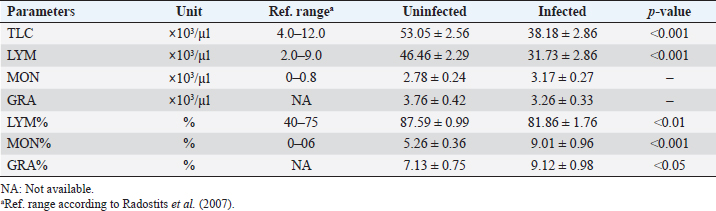
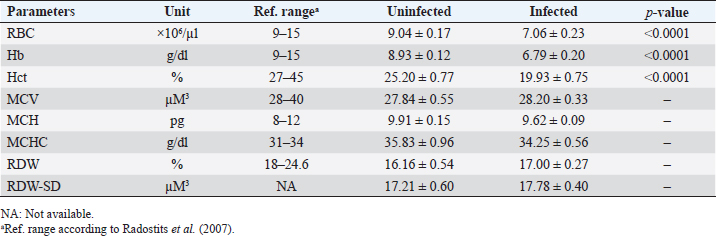
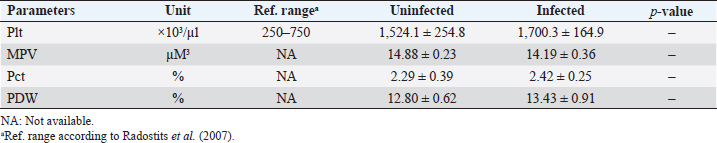
AbstractBackground: Babesia infections in sheep can cause a wide range of clinical and laboratory presentations. Changes in blood parameters are a meaningful manifestation of physiological and pathological changes in an organism. Aim: Therefore, the present study was conducted to analyze and compare hematological and biochemical parameters between blood profiles of Lohi sheep naturally infected and uninfected with Babesia ovis, the main causative agent of ovine babesiosis. Methods: Initially, blood and serum samples from 67 Lohi sheep were collected, DNA was extracted and babesial infection was detected through polymerase chain reaction. The overall infection rate of B. ovis was 37% (25/67). Sixteen infected (experiment group) and 16 uninfected (control group) sheep that were apparently healthy with no history of previous treatment for babesiosis, were selected for hemato-biochemical analysis. Blood samples were analyzed through an automatic CBC analyzer, while serum collected from gel vacutainers was analyzed for blood urea, blood urea nitrogen (BUN), creatinine, and total bilirubin. Each parameter was compared between infected and uninfected animals using a paired t-test in Minitab Express™ software for statistical analyses. Results: Erythron comparison showed a highly significant ( p < 0.0001) decrease in RBC, hemoglobin, and Hct. A nonsignificant increase in mean corpuscular volume (MCV), red cell distribution width (RDW), and RDW–standard deviation (RDW-SD), while a nonsignificant decrease in mean corpuscular haemoglobin (MCH) and MCH concentration (MCHC) values was recorded in infected sheep. Leukon comparison showed a significantly low level of total leukocyte (p < 0.001) in infected sheep. Platelet (Plt) along with platelet crit (Pct) and platelet distribution width (PDW) were nonsignificantly higher, whereas a nonsignificant decrease in mean Plt volume was recorded in infected sheep as compared to uninfected animals. Among biochemical parameters, blood urea, BUN, and total bilirubin showed significant differences (p < 0.05), while creatinine showed a nonsignificant difference. Conclusion: To the best of our knowledge, this is the first report on hemato-biochemical changes associated with babesiosis in the Lohi breed. Consistent with hemolytic anemia, these data would justify physical examination and, together with the medical history, would provide an excellent basis for the diagnosis of babesiosis. Keywords: Ovine babesiosis, Molecular detection, Blood parameters, Lohi sheep breed, Pakistan. Introduction“Blood urination” currently known as “babesiosis” in “Ovine Hosts” was first described in 1880 by Focsa who observed similarities with cattle blood urination in a sheep flock, near Constanţa, Romania (Mihalca, 2010). The disease spectrum ranges from a deceptively quiescent infection to fulminant, malaria-like episodes that are often life-threatening (Aguilar-Delfin et al., 2001). Clinical symptoms of acute disease include pyrexia, hemolytic anemia, pale mucous membrane, and hemoglobinuria. Naturally infected sheep with an intact spleen show more placid symptoms of babesial infection than splenectomized sheep (Yeruham et al., 1998; Sevinc et al., 2007). However, they may have a significant impact on sheep’s productive and reproductive capabilities (Islam et al., 2018). Ovine babesiosis, caused by Babesia ovis, has a significant impact on national sheep production, as it ranks as the third most epidemiologically important sheep disease in Pakistan (Rashid et al., 2010). Pathological changes can only be better assessed when normal blood values are available for comparison (Njidda et al., 2014). The current study focused on “Lohi,” which is the largest and most productive sheep breed in Pakistan (Nouman and Abrar, 2014); however, published references for frequently measured hematological and biochemical parameters for this breed are limited. Although considerable information is available on normal blood parameters of domestic animals, it is difficult to be convinced that the use of such reference intervals is consistent across breeds of sheep (Lepherd et al., 2009) given that many genetic and nongenetic parameters, including breed, genotype, management system, drugs, nutrition, and climate, influence the hematology and serum biochemistry (Onasanya et al., 2015). Very limited data are available on hemato-biochemical indices of sheep breeds indigenous to the Indian subcontinent (Rahman et al., 2018). Although ovine babesiosis is an old disease, the literature provides little information on the hematological and biochemical profiles of victims (Sevinc et al., 2013). To the best of our knowledge, there are no published reports on the complete hemato-biochemical changes associated with ovine babesiosis in the Lohi breed. Therefore, the present study involved uninfected sheep, from the same herd, as a control group; these animals had been reared under similar feeding, management, and environmental conditions throughout the study period. The data generated in this study would justify the physical examination and that when combined with medical history would provide an excellent basis for diagnosis. It would also help in determining the measure of tissue/organ damage, immune status, and the type of anemia, which is a logical outcome of the intra-erythrocytic nature of B. ovis infection. Materials and MethodsStudy areaDistrict Multan has an arid climate with very hot summers and mild winters. The city witnesses some of the most extreme weather in the country. The highest recorded temperature is approximately 54°C (129°F), and the lowest recorded temperature is approximately −1°C (30°F). The average rainfall is roughly 127 mm (5.0 in). Dust storms are a common occurrence within the city. Animal selectionThe study included 67 Lohi sheep that resided in a private sector breeding farm located in Qasba Marral, district Multan. All sheep appeared to be healthy and did not show any signs of babesiosis, nor had they been previously treated for it. To eliminate the potential influence of age, gender, and pregnancy on the results, only nonpregnant females between 1 and 3 years old were selected for the final comparison, with a total of 32 sheep included, and equally divided between 16 infected and 16 uninfected animals. Blood and serum samplingEach time, 2 ml out of 4 ml of sheep jugular vein blood was collected in EDTA-containing vacutainers (blood collection tubes), while the remaining 2 ml into serum separator tubes-BD SST™ gel tubes commonly referred to as “gel vials” specifically designed for serum collection. Vacutainers were gently inverted 5–6 times to thoroughly mix the blood with the anticoagulant, while the gel vials after inverting 5–6 times to activate the “clot activator” were kept standing for 30 minutes to allow time for blood to clot and serum to separate that was then collected in serum cups. Kept in the ice box, all samples (whole blood and sera) were transferred to a well-equipped Laboratory. DNA extraction and polymerase chain reaction (PCR) amplificationA total of 200 μl of whole blood samples was used for DNA extraction with the Favor Prep Blood Genomic DNA Extraction Mini Kit from Favorgen Biotech Corp., according to the manufacturer’s instructions. DNA was eluted in a final volume of 100 μl. DNA extracts were then stored at −20°C until use. Babesia ovis infection was screened in all samples with PCR amplification of fragment (549 bp) of the ssu rRNA sequence specific for B. ovis by using specific primers BboF (5’- TGGGCAGGACCTTGGTTCTTCT-3’) and BboR (5’-CCGCGTAGCGCCGGCTAAATA-3’), as described by Aktas et al. (2005). The PCR reaction was performed in a final volume of 25 µl containing 12.5 µl of Green Master Mix® (Promega, Madison, WI), 1.0 µl of 10 µM primer mix (BboF + BboR), and 0.5 µl of 1 M betaine and 5 µl of template DNA solution. Green Master Mix® is a premixed ready-to-use solution containing DNA polymerase along with dNTPs and MgCl2 in reaction buffer. The thermal cycling profile was described by Aktas et al. (2005). Distilled water and DNA extracted from B. ovis were used as negative and positive controls, respectively. PCR products were electrophoresed in 1.5% agarose gel and sized with 100 bp DNA ladder (Fermentas). Hemato-biochemical analysisAll infected and uninfected samples were analyzed by using the automatic CBC analyzer Mythic™-18, Orphee SA, Switzerland, while sera collected from gel vacutainers were analyzed for blood urea, blood urea nitrogen (BUN), creatinine, and total bilirubin through Rayto-9200 semi-auto chemistry analyzer. Statistical analysisAs all parameters had a co-variant effect, we therefore used adjusted means. Each parameter was statistically compared between B. ovis infected and uninfected animals by using a paired t-test in Minitab Express™ software (Minitab LLC, Pennsylvania, USA) with a threshold value of 0.05. Ethical approvalAll animal experiments were approved by the State Committee on Animal Ethics, Bahauddin Zakariya University, Pakistan. The recommendations of the European Council Directive (86/609/EC) of November 24, 1986, regarding the standards in the protection of animals used for experimental purposes were also followed. ResultsThe overall infection rate of B. ovis was 37% (25/67). Thirty-two animals (half infected and half uninfected with B. ovis) selected for hemato-biochemical comparison revealed the following results. Leukon comparisonLeukon comparison showed a significantly low level of total leukocyte (TLC) (p < 0.001) in infected than uninfected. Similarly, lymphocyte (LYM) was found to be significantly lower (p < 0.001) than uninfected sheep. Monocyte (MON) and granulocyte (GRA) showed a nonsignificant (p > 0.05) difference between infected and uninfected. However, MON% and GRA% were found to be significantly decreased in infected sheep with p-value of <0.001 and <0.05, respectively (Table 1). Erythron comparisonErythron comparison showed a highly significant ( p < 0.0001) decrease in RBC, hemoglobin (Hb), and Hct. in infected than uninfected. Mean corpuscular volume (MCV), red cell distribution width (RDW), and RDW–standard deviation (RDW-SD) showed a minor, nonsignificant (p > 0.05) increase in infected sheep while mean corpuscular volume (MCH) and MCH concentration (MCHC) showed a minor, nonsignificant (p > 0.05) decrease in infected sheep (Table 2). Platelet (Plt) comparisonPlt count was found nonsignificantly (p > 0.05) higher in infected sheep. Similarly, platelet crit (Pct) and platelet distribution width (PDW) were nonsignificantly (p > 0.05) higher in infected animals than uninfected. Whereas, a nonsignificant (p > 0.05) decrease was observed in mean Plt volume (MPV) (Table 3). Table 1. Means (±standard error) of TLC, LYM, MON, GRA, LYM%, MON%, and GRA% in B. ovis-infected Lohi sheep compared to uninfected.
Table 2. Means (±standard error) of RBC, Hb, Hct., MCV, MCH, MCHC, RDW, and RDW-SD in B. ovis-infected Lohi sheep compared to uninfected.
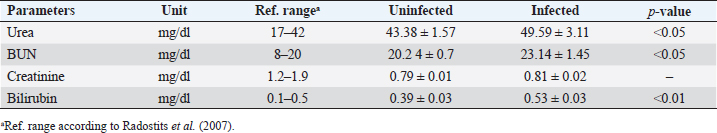
Biochemical comparisonAmong biochemical parameters, blood urea, BUN, and total bilirubin showed significant differences (p < 0.05), while creatinine showed a nonsignificant difference (Table 4). DiscussionBabesia infections in sheep can cause a wide range of clinical and laboratory presentations. Blood parameters are a meaningful manifestation of physiological and pathological changes in an organism. Therefore, the present study was conducted to analyze and compare hematological and biochemical parameters between blood profiles of Lohi sheep naturally infected and uninfected with B. ovis. Leukopenia, a significant decrease in total TLC with a marked decrease in GRAs and LYMs, but an increase in MONs observed in infected animals are in partial agreement with Sevinc et al. (2013) who found leukopenia with decreased GRA and LYM in infected sheep with very high parasitemia in all infected sheep. An increase in MON was found in sheep with low and moderate parasitemia that gradually declined in sheep with a high parasitemic load. The difference probably resulted from infection of animals with different subspecies of B. ovis (Esmaeilnejad et al., 2012a). Monocytosis, i.e., increase in MON is also consistent with the findings of Esmaeilnejad et al. (2012a) who observed monocytosis in ovine babesiosis, and Wright et al. (1998) who reported similar results in bovine babesiosis. El-Sifi et al. (1990) suggested a response of monocytosis as the body’s defense against infection. The marked elevation in the number of MON in Babesia infection is due to their role as active mediators in the natural, innate, nonspecific immune response that involves the activation of macrophages (Court et al., 2001). A protective role is known for macrophages during infection by several Babesia species (Homer et al., 2000). Adejinmi et al. (2004) found a similar reduced TLC response in West African Dwarf sheep infected with Babesia sp. Rahbari et al. (2008) also observed a significant decrease in the TLCs; however, the LYM count was higher than normal. Present findings of a significant decrease in Hb, Hct., and total RBC in B. ovis infected sheep clearly indicated “hemolytic anemia” as previously described in ovine babesiosis by Kozat et al. (2003), Adejinmi et al. (2004), Bicek et al. (2005), Rahbari et al. (2008), Rashid et al. (2010), Sulaiman et al. (2010), Esmaeilnejad et al. (2012a, 2012b), Ijaz et al. (2013), Sevinc et al. (2013), Esmaeilnejad et al. (2014), and Orunç-Kilinç et al. (2015). Statistically significant low values observed Hb, Hct., and total RBC were expected because Babesia destroys erythrocytes (Sevinc et al., 2013). Numerous mechanisms link infection and hemolysis (Berkowitz, 1991) that may be extravascular mediated by the mononuclear phagocytic system, or intravascular. Babesia causes extravascular hemolysis by direct invasion of RBCs and membrane alteration. Cellular invasion and metabolic activity of the parasite alter the cell membrane, leading to splenic sequestration (Dhaliwal et al., 2004). MCV and MCHC are the necessary investigations to codify anemia on a morphological basis (Wiwanitkit, 2007). In this study, the MCV was slightly higher, and the MCHC was lower than in healthy animals, although both were within the reference ranges described by Radostits et al. (2007). Increased MCV (macrocytosis) and low MCHC (hypochromia) are due to reticulocytosis (Latimer, 2011) in which reticulocytes are large, young, and anucleate that lack their full concentration of Hb. Reticulocytosis causes an increase in red cell distribution width-RDW (Brockus, 2011), which is in accordance with present findings and leads to the conclusion that it is “macrocytic hypochromic anemia.” Hypochromic macrocytes indicate either dyserythropoiesis or an increased percentage of reticulocytes (Bain, 2006). Macrocytic hypochromic anemia may be classified as reticulocytosis with regenerative anemia as the classification systems overlap (Tvedten, 2010). The type of anemia may vary in Babesia infection (Kaur, 2014). Rahbari et al. (2008), Sulaiman et al. (2010), and Esmaeilnejad et al. (2012a) described ovine babesiosis associated with microcytic hypochromic anemia. Table 3. Means (±standard error) of Plt, MPV, Pct, and PDW in B. ovis-infected Lohi sheep compared to uninfected.
Table 4. Means (±standard error) of urea, BUN, creatinine, and bilirubin in B. ovis-infected Lohi sheep compared to uninfected.
Anemia may also be attributed to immune-mediated phenomena by autoantibodies against the membrane component of Babesia infected and uninfected red blood corpuscles (Rubino et al., 2006), the generation of toxic hemolytic factors of the parasite (Rafaj et al., 2007), mechanical desolation by trophozoite intra-cellular binary fission (Zobba et al., 2008), or via the release of vasoactive molecules such as kallikrein-a subgroup of serine proteases (Schetters et al., 2009). In addition, oxidative shocks on RBCs may play a dominant role in the pathogenesis of anemia (Esmaeilnejad et al., 2014). Succinctly, the measurement of hematological parameters is important in determining the severity of Babesia infection in sheep, as no correlation between the level of parasitemia and the degree of anemia could be found in the detailed clinical and laboratory observations made by Sevinc et al. (2013). The interpretation of Plt count becomes more difficult for animals in which laboratory-specific intervals are not available. This is because the methods at hand for measuring Plt, err in precision and accuracy, and results from the same animal may differ between methods (Koplitz et al., 2001; Tasker et al., 2001). Thus, veterinary laboratories enact reference intervals specific to their own equipment. In this study, Plt counts in both B. ovis infected and uninfected sheep were found to be suggestive of thrombocytosis compared to the reference range provided by Radostits et al. (2007). Though nonsignificant, a comparison of the Plt values of the infected with the uninfected group suggests thrombocytosis in the infected group. Altered Plt trafficking (physiologic thrombocytosis) and inflated Plt production (enhanced thrombopoiesis including reactive thrombocytosis) could be the main causes (Boudreaux et al., 2011). Iron deficiency could be another possible cause of thrombocytosis, as moderate to marked thrombocytosis is a common finding in iron deficiency (Weiss, 2010). Previous studies on babesiosis (Bicek et al., 2005; Col and Uslu, 2007; Askar et al., 2008; Chaudhuri et al., 2008; Lotfollahzadeh et al., 2012; Swelum et al., 2014) described a marked decrease in serum iron as a frequent finding in different animal species. In addition, higher Plt values in both groups could be an analyzer-dependent artifact. When small, shredded, or haemolyzed RBCs, leukocyte slivers, or particulate cellular debris are erroneously counted as Plts, it shows pseudo-thrombocytosis and can potentially occur with any counting method (Stokol, 2010). MPV meditates the average size of Plt in circulation and generally decreases with increasing Plt (Boudreaux et al., 2011). This is consistent with present findings of lower MPV in infected compared with uninfected sheep. MPV and Plt allow the derivation of an equivalent Plt variable, the Pct. As Hct. is an indicator of total erythrocyte mass in the body, Plt mass in the body translates to Pct (Tvedten et al., 2008). Pct is the physiologically most relevant parameter for hemostasis and regulation of thrombopoiesis (Butkiewicz et al., 2006). The increase in PDW in the present study implies Plt anisocytosis. Analogous to RDW, PDW, i.e., Plt distribution width increases with variability in Plt size and is a measure of Plt anisocytosis (Bain, 2006). Plt findings, including Plt, Pct, MPV, and PDW in ovine babesiosis during the present study, are in contrast to those of Sevinc et al. (2013) who observed thrombocytopenia with a decrease in Pct and PDW while increasing MPV. Although used as a diagnostic sign of babesiosis, changes in Plt index values: Pct and MPV in response to Babesia parasites and their treatment are even less understood (Zvorc et al., 2010). Babesiosis in dromedary camels was found to induce a nonsignificant increase in Plt and Pct, while a decrease in Hct. and PDW (Swelum et al., 2014) supported the present findings in infected sheep. Babesia-infected RBCs are reported to congest renal, hepatic, and pancreatic capillaries and reduce blood flow, which can lead to ischemic injury to such vital organs (Mathe et al., 2007). The higher the parasitemia, the greater the likelihood of ischemic injury; therefore, blood levels of urea, creatinine, and bilirubin are reliable parameters for evaluating hepatic and renal function (Camacho et al., 2005). Azotemia and increased creatinine suggest decreased glomerular filtration, hence altered renal function in most animal species (Rosenfeld and Dial, 2010). Previous findings of mild to significantly higher levels of BUN have been reported in babesial infection of sheep (Rahbari et al., 2008; Rasheed and Al-Fetly, 2012; Esmaeilnejad et al., 2012a; Sevinc et al., 2013), goats (Sulaiman et al., 2010), camels (Swelum et al., 2014), dogs (Crnogaj et al., 2010), and cattle (Kaur, 2014) confirm the current results. Induced immune complexes in piroplasm infections can cause mechanical damage to glomeruli resulting in higher levels of urea in sheep (Elsadig et al., 2013). Creatinine is a more accurate measure of glomerular filtration rate (GFR) than BUN, especially in ruminants (Carlson, 2009). Nevertheless, a small increase in creatinine can be attributed to progressively compromised renal function, as 65%–75% of the total nephrons must be nonfunctional before the serum creatinine level exceeds the normal range (Gibson et al., 2006, 2007). Saliva, gastrointestinal tract, and sweat are routes of urea excretion in addition to its renal clearance, but there is no secondary cause of creatinine elevation unlike BUN (Tripathi, 2011). A slight increase in serum creatinine level was also observed by Rahbari et al. (2008) in ovine babesiosis. Significantly higher creatinine concentrations following Babesia infection were observed in sheep (Yeruham et al., 1998; Uilenberg, 2006; Esmaeilnejad et al., 2012a), dogs (Furlanello et al., 2005; Crnogaj et al., 2010), camels (Swelum et al., 2014), and in dairy animals (Talkhan et al., 2010; Kaur, 2014). Whereas, a decrease in serum creatinine has been also reported by Sevinc et al. (2013) and Konto et al. (2014) in sheep and dogs, respectively. The decrease in creatinine values may be the result of the initial blood dilution, as few dogs showed an increase in creatinine levels during this study (Konto et al., 2014). Unconjugated bilirubin produced in the spleen is eliminated in the bile from the liver after being conjugated with glucuronides (Olver et al., 2010). The retrograde flow of bile pigments in serum and tissue produces a yellow coloration termed as icterus or jaundice which is the prominent clinical sign of ovine babesiosis (Uilenberg, 2006; Sevinc et al., 2013). Other symptoms such as lethargy, anorexia, vomiting, and diarrhea (Krause et al., 1996) are due to increased levels of bilirubin or hyperbilirubinemia (Rosenfeld and Dial, 2010). The total bilirubin concentration (TBIC) increases following intemperate erythrolysis and hepatic injury, but when these oddities occur together, the concentration increases dramatically (Sevinc et al., 2013). If the conversion of Hb to bilirubin exceeds the ability of the liver to conjugate and excrete bilirubin, then the total bilirubin level becomes elevated in the blood (Turgut, 2000). Therefore, in this study, the elevated TBIC was most likely due to the hemolytic breakdown of RBCs. Present findings of hyperbilirubinemia are consistent with those of Rahbari et al. (2008) and Sevinc et al. (2013) who reported a significant increase in bilirubin associated with ovine babesiosis. Results on babesiosis in goats (Sulaiman et al., 2010), cattle (Yeruham et al., 2003; Alam and Nasr, 2011; Kaur, 2014), and camels (Swelum et al., 2014) are similar to the present study. ConclusionBabesia ovis infection induced leukopenia with monocytosis, anemia with hypochromic macrocytosis, thrombocytopenia, and azotemia with hyperbilirubinemia. Although asymptomatic, the altered blood parameters reduce vitality and lead to a decrease in animal production, and therefore, an increase in economic losses. Current data will provide insight into the pathogenicity of subclinical infections. AcknowledgmentsThe authors would like to thank all veterinarians for helping with sample collection. The authors declare that there is no conflict of interest. FundingThis project was financially supported by the Higher Education Commission, Islamabad, Pakistan, under a 5,000 indigenous PhD fellowship program. Conflict of interestThe authors declare no conflict of interest. Author contributionsMS designed this study, collected samples and epidemiological data, performed the molecular and microscopic diagnosis, and performed statistical analysis. MS and MBS wrote the manuscript and SAHN, MR, UUDU, NN, and AK edited it. MBS finalized the manuscript and all the authors approved the final version. Data availabilityThe data that support the findings of this study are available from the corresponding author upon request. ReferencesAdejinmi, J.O., Sadiq, N.A., Fashanu, S.O., Lasisi, O.T. and Ekundayo, S. 2004. Studies on the blood parasites of sheep in Ibadan, Nigeria. Afr. J. Biomed. Res. 7, 41–43. Aguilar-Delfin, I., Homer, M.J., Wettstein, P.J. and Persing, D.H. 2001. Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immunol. 69, 7955–7958. Aktas, M., Altay, K. and Dumanli, N. 2005. Development of polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goat. Vet. Parasitol. 133, 277–281. Alam, T.H. and Nasr, S.M. 2011. Hematological and biochemical investigation in bovine babesiosis and theileriosis. Benha Vet. Med. J. 22, 118–126. Askar, T.K., Slamanoglu, B., Cakmak, A. and Beskaya, A. 2008. Relative changes in serum lipid-lipoprotein and trace element levels in cattle babesiosis. Medycyna Weterynaryjna 64, 1104–1106. Bain, B.J. 2006. Blood cells: a practical guide. Malden, MA: Blackwell Publishing. Berkowitz, F.E. 1991. Hemolysis and infection: categories and mechanisms of their interrelationship. Rev. Infect. Dis. 13, 1151–1162. Bicek, K., Deger, Y. and Deger, S. 2005. Some biochemical and haematological parameters of sheep infected with Babesia species. Yüzüncü Yıl Üniv. Vet. Fak. Derg. 16, 33–35. Boudreaux, M.K., Spangler, E.A. and Welles, E.G. 2011. Hemostasis. In Duncan and Prasse’s veterinary laboratory medicine clinical pathology. Ed., Latimer, K.S. Ames, IA: Iowa State University Press. Brockus, C.W. 2011. Erythrocytes. In Duncan and Prasse’s veterinary laboratory medicine clinical pathology. Ed., Latimer, K.S. Ames, IA: Iowa State University Press. Butkiewicz, A.M., Kemona, H., Dymicka-Piekarska, V., Matowicka-Karna, J., Radziwon, P. and Lipska, A. 2006. Platelet count, mean platelet volume and thrombocytopoietic indices in healthy women and men. Thrombosis Res. 118, 199–204. Camacho, A.T., Guitian, F.J., Palla, E., Gestal, J.J., Olmeda, A.A., Habela, M.A., Telford III, S.R. and Spielman, A. 2005. Theileria (Babesia) equi and Babesia caballi infection in horses in Galicia, Spain. Trop. Anim. Health Prod. 37, 293–302. Carlson, G. 2009. Clinical chemistry tests. In Large animal internal medicine. Ed., Smith, B.P. Philadelphia, PA: Mosby. Chaudhuri, S., Varshney, J.P. and Patra, R.C. 2008. Erythrocytic antioxidant defence, lipid peroxides level and blood iron, zinc and copper concentrations in dogs naturally infected with Babesia gibsoni. Res. Vet. Sci. 85, 120–124. Col, R. and Uslu, U. 2007. Changes in selected serum components in cattle naturally infected with Theileria Annulata. Bull. Vet. Institute Pulawy 51, 15–18. Court, R., Jackson, L. and Lee, R. 2001. Elevated anti-parasitic activity in peripheral blood monocytes and neutrophils of cattle infected with Babesia bovis. Int. J. Parasitol. 31, 29-37. Crnogaj, M., Petlevski, R., Mrljak, V., Kis, I., Torti, M., Kucer, N., Matijatko, V., Sacer, I. and Stokovic, I. 2010. Malondialdehyde levels in serum of dogs infected with Babesia canis. Vet. Med. (Praha), 4, 163–171. Dhaliwal, G., Cornett, P.A. and Tierney, Jr. L.M. 2004. Hemolytic anemia. Am. Fam. Phys. 69, 2599–2606. Elsadig, A.A., Elmansoury, Y.H.A., Elbasheir, H.M., Saad, M.B., Elhussein, A.M. and Babeker, E.A. 2013. Effect of experimental infection with Theleria lestoqurdi on some haematological and biochemical parameters in desert ewes during pregnancy. J. Vet. Med. Anim. Prod. 4, 88–95. El-Sifi A., Degheidy, N., Neweehy, T., Abdu, O. and Abdon Zeina, H. 1990. Studies on blood picture of sheep naturally infected with piroplasmosis in Egypt. In 4th Science Congress, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. Esmaeilnejad, B., Tavassoli, M. and Asri-Rezaei, S., 2012a. Investigation of hematological and biochemical parameters in small ruminants naturally infected with Babesia ovis. Vet. Res. Forum 3, 31–36. Esmaeilnejad, B., Tavassoli, M., Asri-Rezaei, S., Dalir-Naghadeh, B. and Pourseyed, S.H. 2012b. Evaluation of serum total protein concentration and protein fractions in sheep naturally infected with Babesia ovis. Comp. Clin. Pathol. 23, 151. Esmaeilnejad, B., Tavassoli, M., Asri-Rezaei, S., Dalir-Naghadeh, B., Malekinejad, H., Jalilzadeh-Amin, G., Arjmand, J., Golabi, M. and Hajipour, N. 2014. Evaluation of antioxidant status, oxidative stress and serum trace mineral levels associated with Babesia ovis parasitemia in sheep. Vet. Parasitol. 205, 38–45. Furlanello, T., Caldin, M. and Fiorio, F. 2005. Primary hyperfibrinolysis in dogs. Vet. Clin. Pathol. 34, 293. Gibson, K.J., Boyce, A.C., Karime, B.M. and Lumbers, E.R. 2007. Maternal renal insufficiency alters plasma composition and renal function in the fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1204–R1211. Gibson, K.J., Thomson, C.L., Boyce, A.C., Karime, B.M. and Lumbers, E.R. 2006. Effects of a reduction in maternal renal mass on pregnancy and cardiovascular and renal function of the pregnant ewe. Am. J. Physiol. Renal Physiol. 290, F1153–F1162. Homer, M.J., Aguilar-Delfin, I., Telford III, S.R., Krause, P.J. and Persing1, D.H. 2000. Babesiosis. Clin. Microbiol. Rev. 13, 451–469. Ijaz, M., Rehman, A., Ali, M.M., Umair, M., Khalid, S., Mehmood, K. and Hanif, A. 2013. Clinico-epidemiology and therapeutical trials on babesiosis in sheep and goats in Lahore, Pakistan. J. Anim. Plant Sci. 23, 666–669. Islam, S., Rahman, M.K., Ferdous, J., Hossain, M.B., Hassan, M.M. and Islam, A. 2018. Haematological reference values for healthy fat-tailed sheep (Dhumba) in Bangladesh. J. Adv. Vet. Anim. Res. 5, 481–484. Kaur, P. 2014. Studies on molecular based diagnostic assay and clinico-pathobiochemical findings in Babesia bigemina infection in dairy animals. PhD dissertation, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India. Konto, M., Biu, A.A., Ahmed, M.I., Mbaya, A.W. and Luka, J. 2014. Clinico-biochemical responses of dogs to experimental infection with Babesia canis. Vet. World 7, 113–118. Koplitz, S.L.S., Scott, M.A. and Cohn, L.A. 2001. Effects of platelet clumping on platelet concentrations measured by use of impedance or buffy coat analysis in dogs. J. Am. Vet. Med. Assoc. 219, 1552–1556. Kozat, S., Yüksek, N., Altuğ, N., Ağaoğlu, Z.T. and Erçin, F. 2003. Studies on the effect of iron (Fe) preparations in addition to Babesiosis treatment on the haematological and some mineral levels in sheep naturally infected with Babesia ovis. YYU Vet. Fak. Derg. 14, 18–21. Krause, P.J., Telford III, S.R., Spielman, A., Sikand, V., Ryan, R., Christianson, D., Burke, G., Brassard, P., Pollack, R., Peck, J. and Persing, D.H. 1996. Concurrent lyme disease and babesiosis: evidence for increased severity and duration of illness. J. Am. Med. Assoc. 275, 1657–1660. Latimer, K.S. 2011. Hemorrhagic anemia, case 1. Duncan and Prasse’s veterinary laboratory medicine clinical pathology. Ames, IA: Iowa State University Press. Lepherd, M.L., Canfield, P.J., Hunt, G.B. and Bosward, K.L. 2009. Hematological, biochemical and selected acute phase protein reference intervals for weaned female merino lambs. Aust. Vet. J. 87, 5–11. Lotfollahzadeh, S., Rahmani, M., Mohri, M. and Madadgar, O. 2012. Changes in serum iron concentration and hepatic enzyme activities in cattle infected with Theileria annulata and Babesia bigemina. Comp. Clin. Pathol. 21, 829–832. Mathe, A., Dobos-Kovacs, M. and Voros, K. 2007. Histological and ultra-structural studies of renal lesions in Babesia canis infected dogs treated with Imidocarb. Acta Vet. Hung. 55, 511–523. Mihalca, A.D. 2010. The quest for piroplasms: from Babes to Smith to molecules. Sci. Parasitol. 11, 14–19. Njidda, A.A., Shuai’bu, A.A. and Isidahomen, C.E. 2014. Haematological and serum biochemical indices of sheep in semi-arid environment of northern Nigeria. Glob. J. Sci. Front. Res. D Agric. Vet. 14, 49–56. Nouman, S. and Abrar, Y. 2014. Estimates of phenotypic and genetic parameters for ewe productivity traits of Lohi sheep in Pakistan. Glob. J. Anim. Breed. Genet. 2, 70–74. Olver, C.S., Andrews, G.A., Smith, J.E. and Kaneko, J.J. 2010. Erythrocyte structure and function. In Schalm’s veterinary hematology. Eds., Weiss, D.J. and Wardrop, K.J. Ames, IA: Blackwell Publishing. Onasanya, G.O., Oke, F.O., Sanni, T.M. and Muhammad, A.I. 2015. Parameters influencing haematological, serum and bio-chemical references in livestock animals under different management systems. Open J. Vet. Med. 5, 181–185. Orunç-Kilinç, O., Göz, Y., Yüksek, N., Başbuğan, Y., Yilmaz, A.B. and Ataş, A.D. 2015. Determination of serum cardiac biomarkers and plasma D-dimer levels in anemic sheep with babesiosis. Turk. J. Vet. Anim. Sci. 39, 606–610. Radostits, O.M., Gay, C.C., Hinchcliff, K.W. and Constable, P.T. 2007. Veterinary medicine: a textbook of the diseases of cattle, sheep, goats, pigs and horses. London, UK: Saunders. Rafaj, R.B., Mrljak, V., Kucer, N., Brkljačić, M. and Matijatko, V. 2007. Protein C activity in babesiosis of dogs. Vet. Arhiv 77, 1–8. Rahbari, S., Nabian, S., Khaki, Z., Alidadi, N. and Ashrafihelan, J. 2008. Clinical, hematological and pathologic aspects of experimental ovine babesiosis in Iran. Iran. J. Vet. Res. 9, 59–64. Rahman, M.K., Islam, S., Ferdous, J., Uddin, M.H., Hossain, M.B., Hassan, M.M. and Islam, A. 2018. Determination of hematological and serum biochemical reference values for indigenous sheep (Ovis aries) in Dhaka and Chittagong Districts of Bangladesh. Vet. World. 11, 1089-1093. Rasheed, D. and Al-Fetly, H. 2012. Detection of Theileria spp. in blood samples and estimation of haematological and biochemical changes in sheep in Al-Diwaniya Province. Kufa J. Vet. Sci. 2, 45–53. Rashid, A., Khan, J.A., Khan, M.S., Rasheed, K., Maqbool, A. and Iqbal, J. 2010. Prevalence and chemotherapy of babesiosis among Lohi sheep in the livestock experiment station, Qadirabad, Pakistan and environs. J. Venom. Anim. Toxins Incl. Trop. Dis. 16, 587–591. Rosenfeld, A.J. and Dial, S.M. 2010. Clinical pathology for the veterinary teams. New Delhi, India: Wiley-Blackwell. Rubino, G., Cito, A.M., Lacinio, R., Bramante, G., Caroli, A., Pieragostini, E. and Petazzi, F. 2006. Hematology and some blood chemical parameters as a function of tick-borne disease (TBD) signs in Horse. J. Equine Vet. Sci. 26, 475–480. Schetters, T.P.M., Kleuskens, J.A., Van De Crommert, J., De Leeuw, P.W., Finizio, A.L. and Gorenflot, A. 2009. Systemic inflammatory response in dogs experimentally infected with Babesia canis, a hematological study. Vet. Parasitol. 162, 7–15. Sevinc, F., Sevinc, M., Ekici, O.D., Yildiz, R., Isik, N. and Aydogdu, U. 2013. Babesia ovis infections: detailed clinical and laboratory observations in the pre- and post-treatment periods of 97 field cases. Vet. Parasitol. 191, 35–43. Sevinc, F., Turgut, K., Sevinc, M., Ekici, O.D., Coskun, A., Koc, Y., Erol, M. and Ica, A. 2007. Therapeutic and prophylactic efficacy of Imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 149, 65–71. Stokol, T. 2010. Essential thrombocythemia and reactive thrombocytosis. In Schalm’s veterinary hematology. Eds., Weiss, D.J. and Wardrop, K.J. Ames, IA: Blackwell Publishing. Sulaiman, E.G., Arslan, S.H., Al-Obaidi, Q.T. and Daham, E. 2010. Clinical, haematological and biochemical studies of babesiosis in native goats in Mosul. Iraqi J. Vet. Sci. 24, 31–35. Swelum, A.A., Ismael, A.B., Khalaf, A.F. and Abouheif, M.A. 2014. Clinical and laboratory findings associated with naturally occurring babesiosis in dromedary camels. Bull. Vet. Inst. Pulawy 58, 229–233. Talkhan, O.F.A., Mervat, E.I.M. and Ali, M.A. 2010. Cattle babesiosis and associated biochemical alteration in Kalubyia Governorate. J. Nat. Sci. 8, 29–36. Tasker, S., Cripps, P.J. and Mackin, A.J. 2001. Evaluation of methods of platelet counting in the cats. J. Small Anim. Pract. 42, 326–332. Tripathi, N.K. 2011. Urinary system. In Duncan and Prasse’s veterinary laboratory medicine clinical pathology. Ed., Latimer, K.S. Ames, IA: Iowa State University Press. Turgut, K. 2000. Veterinary clinic laboratory diagnosis [in Turkish]. Konya, Turkey: Bahcivanlar Press. Tvedten, H. 2010. Laboratory and clinical diagnosis of anemia. In Schalm’s veterinary hematology. Eds., Weiss, D.J. and Wardrop, K.J. Ames, IA: Blackwell Publishing. Tvedten, H., Lilliehook, I., Hillstrom, A. and Haggstrom, J. 2008. Plateletcrit is superior to platelet count for assessing platelet status in Cavalier King Charles Spaniels. Vet. Clin. Pathol. 37, 266–271. Uilenberg, G. 2006. Babesia—a historical overview. Vet. Parasitol. 138, 3–10. Weiss, D.J. 2010. Iron and copper deficiencies and disorders of iron metabolism. In Schalm’s veterinary hematology. Eds., Weiss, D.J. and Wardrop, K.J. Ames, IA: Blackwell Publishing. Wiwanitkit V. 2007. Tropical anemia. New York, NY: Nova Science Publications. Wright, I.G., Goodger, B.V. and Clark, I.A. 1998. Immuno-pathophysiology of Babesia bovis and Plasmodium falciparum infection. Infect. Immun. 4, 214–218. Yeruham, I., Avidar, Y., Aroch, I. and Hadani, A. 2003. Intra-uterine infection with Babesia bovis in a 2-day-old calf. J. Vet. Med. B Infect. Dis. Vet. Public Health, 50, 60–62. Yeruham, I., Hadani, A. and Galker, F. 1998. Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis—a review. Vet. Parasitol. 74, 153–163. Zobba, R., Ardu, M., Niccolini, S., Chessa, B., Manna, L., Cocco, R. and Parpaglia, M.L.P. 2008. Clinical and laboratory finding in equine piroplasmosis. J. Equine Vet. Sci. 28, 301–308. Zvorc, Z., Rafaj, R.B., Kuleš, J. and Mrljak, V. 2010. Erythrocyte and platelet indices in babesiosis of dogs. Vet. Arch. 80, 259-267. | ||
| How to Cite this Article |
| Pubmed Style Sajid M, Naqvi SAH, Riaz M, Umar UUD, Nasreen N, Khan A, Said MB. Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Vet. J.. 2023; 13(11): 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 Web Style Sajid M, Naqvi SAH, Riaz M, Umar UUD, Nasreen N, Khan A, Said MB. Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. https://www.openveterinaryjournal.com/?mno=160504 [Access: January 24, 2026]. doi:10.5455/OVJ.2023.v13.i11.2 AMA (American Medical Association) Style Sajid M, Naqvi SAH, Riaz M, Umar UUD, Nasreen N, Khan A, Said MB. Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Vet. J.. 2023; 13(11): 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 Vancouver/ICMJE Style Sajid M, Naqvi SAH, Riaz M, Umar UUD, Nasreen N, Khan A, Said MB. Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Vet. J.. (2023), [cited January 24, 2026]; 13(11): 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 Harvard Style Sajid, M., Naqvi, . S. A. H., Riaz, . M., Umar, . U. U. D., Nasreen, . N., Khan, . A. & Said, . M. B. (2023) Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Vet. J., 13 (11), 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 Turabian Style Sajid, Muhammad, Syed Atif Hasan Naqvi, Muhammad Riaz, Ummad Ud Din Umar, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. 2023. Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Veterinary Journal, 13 (11), 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 Chicago Style Sajid, Muhammad, Syed Atif Hasan Naqvi, Muhammad Riaz, Ummad Ud Din Umar, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. "Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan." Open Veterinary Journal 13 (2023), 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 MLA (The Modern Language Association) Style Sajid, Muhammad, Syed Atif Hasan Naqvi, Muhammad Riaz, Ummad Ud Din Umar, Nasreen Nasreen, Adil Khan, and Mourad Ben Said. "Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan." Open Veterinary Journal 13.11 (2023), 1400-1408. Print. doi:10.5455/OVJ.2023.v13.i11.2 APA (American Psychological Association) Style Sajid, M., Naqvi, . S. A. H., Riaz, . M., Umar, . U. U. D., Nasreen, . N., Khan, . A. & Said, . M. B. (2023) Molecular detection of Babesia ovis and blood parameters’ investigation reveal haematological and biochemical alterations in babesiosis infected Lohi sheep in Multan, Pakistan. Open Veterinary Journal, 13 (11), 1400-1408. doi:10.5455/OVJ.2023.v13.i11.2 |