| Short Communication | ||
Open Vet. J.. 2024; 14(2): 738-742 Open Veterinary Journal, (2024), Vol. 14(2): 738-742 Short Communication The impact of Apis dorsata forest honey administration on follicle-stimulating hormone and luteinizing hormone levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal modelWidjiati Widjiati1*, Epy Muhammad Luqman1, Suryo Kuntjorodjakti1, Aulanni’am Aulanni’am2, Zahra Shabira3, Sultan Fadhilla Taqwa3, Riski Lesta Mega3, Dean Chou4, Ahmad Shofy Mubarak5 and Viski Fitri Hendrawan61Department of Veterinary Science, Veterinary Medicine Faculty, Airlangga University, Mulyorejo, Surabaya, East Java, Indonesia 2Department of Chemistry, Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia 3Magister Student of Veterinary Reproduction, Veterinary Medicine Faculty, Airlangga University, Mulyorejo, Surabaya, East Java, Indonesia 4Department of Biomedical Engineering National Cheng Kung University, Tainan City, Taiwan 5Department of Marine, Faculty of Fisheries and Marine Sciences, Airlangga University, Mulyorejo, Surabaya, East Java, Indonesia 6Departement of Veterinary Reproduction, Veterinary Medicine Faculty, Brawijaya University, Puncak Dieng, Dau, Malang, East Java, Indonesia *Corresponding Author: Widjiati Widjiati. Department of Veterinary Science, Veterinary Medicine Faculty, Airlangga University, Mulyorejo, Surabaya, East Java, Indonesia. Email: widjiati [at] fkh.unair.ac.id Submitted: 24/10/2023 Accepted: 27/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
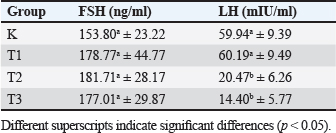
AbstractBackground: Chronic physical stress has many effects on the nervous system and can cause structural changes in different parts of the brain and hemomodulatory, including hormonal. Current pharmacotherapeutic treatments have limited efficacy and are associated with many deleterious side effects Aim: The aim of this research is to determine how Apis dorsata forest honey administration affects follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats who are subjected to forced swim tests as a model of chronic physical stress placed in a container filled with water from which it cannot escape. Methods: This was an experimental laboratory study with 32 rats divided into four treatment groups: control (C), Treatment 1 (T1) with a forced swim test + honey (2 g/rat/day), Treatment 2 (T2) with a forced swim test + honey (4 g/rat/day), and Treatment 3 (T3) with a forced swim test + honey (6 g/rat/day). All treatments were administered for 14 days. Then, blood was taken for FSH and LH serum tests, and a one-way ANOVA and Duncan test were used to statistically test the data analysis. Results: The results of this study indicate that the administration of forest honey had no significant effect (p > 0.05) on the FSH parameter, but there was a significant decrease in LH levels in the T2 and T3 groups (p < 0.05). Conclusion: It can be concluded that giving forest honey to rats who were subjected to a 14-day forced swim test had no effect on FSH and LH levels. In rats given a forced swim test as a model of chronic stress, administration at doses of 4 and 6 g/rat/day reduced LH serum levels. Thus, giving forest honey could maintain reproductive health in rat that experience chronic stress. Keywords: Rattus norvegicus, Forced swim test, Apis dorsata forest honey, FSH and LH, Reproductive health. IntroductionThe presence of stress-inducing stimuli in the environment, which pose a threat to both cattle and humans, can lead to a decline in the overall well-being of organisms. This is mostly attributed to the disturbance of homeostatic mechanisms within the body (Verma et al., 2011). The primary stressors experienced by livestock are influenced by various elements, including environmental temperature, weather conditions, feed-related factors, and excessive physical exertion. In times of stress, the human body initiates a physiological response by releasing glucocorticoids, primarily cortisol, from the adrenal cortex. This release is triggered by the presence of corticotropin releasing hormone and adrenocorticotropic hormone (Whirledge and Clidowski, 2010). The release of cortisol serves to control metabolism, blood pressure, and blood sugar levels in response to stress. Cortisol further serves the purpose of promoting gluconeogenesis during the physiological response known as the fight or flight response (Whirledge and Clidowski, 2010). Nevertheless, an overabundance of corticosteroid secretion adversely impacts the reproductive system as it diverts energy allocation toward other organ systems. The stimulation of the glucocorticoid receptor located on gonadotropin-releasing hormone (GnRH) neurons inside the hypothalamus leads to the initiation of programmed cell death in neuronal cells. This process ultimately results in hypogonadism, mostly attributed to the diminished pulsatile secretion of GnRH (Nono et al., 2017). The absence of pulsatility in the release of GnRH has an impact on the production of follicle stimulating hormone (FSH) and luteinizing hormone (LH), leading to disruptions in the hypothalamus-pituitary-gonadal axis (HPG) signaling pathway and a decrease in reproductive efficiency in livestock. According to the findings, stress has been found to be linked to sporadic anovulatory episodes due to its impact on reducing total LH levels. The available evidence suggests that stress has a negative impact on reproductive performance through its influence on the pulsatility of GnRH and the release of FSH and LH (Mohamad et al., 2019). The utilization of natural constituents has become a prevalent phenomenon on a global scale, driven by the desire to discover novel active chemicals that can enhance the well-being of both humans and animals. This movement has extended to other domains, including reproductive health (Moniruzzaman et al., 2013). Forest honey is a naturally occurring substance that exerts a beneficial impact on the reproductive system. Forest honey is known to possess significant quantities of phenolic compounds and flavonoids due to its classification as multiflora honey. This particular type of honey has been found to have the ability to decrease cortisol levels in the face of physical stress, as demonstrated in studies. Forest honey, due to its antioxidant properties, can potentially mitigate the necrotic effects on Leydig cells in mice exposed to monosodium glutamate, hence exhibiting a beneficial association with the reproductive system (Mohamad et al., 2019). It is anticipated that incorporating forest honey as a natural component may lead to a decrease in corticosteroid levels, which are released in response to physical stress induction. This reduction in corticosteroid levels is expected to enhance reproductive efficiency, specifically in terms of folliculogenesis and ovulation, as indicated by the parameters of FSH levels and LH levels. In light of the aforementioned context, an investigation was undertaken to examine the impact of administering forest honey on the FSH and LH levels in Rattus novergicus subjected to the forced swim test as a representative model of chronic physical stress (Schliep et al., 2015). Materials and MethodsMaterialsThe research employed a range of instruments and supplies, including ZD® forest honey, distilled water, oral gavage, Eppendorf tube, centrifuge, Olympus® microscope, NBF 10%, tissue pot, Onemed® 5cc syringe, scalpel, surgical scissors, anatomical tweezers, ketamine (Ket-A-100®), and xylazine (Xyla®). The mice are provided with Hi-Pro-Vit Medicated 593® as their meal Stramek et al. (2019). Experimental animal preparationThe present study employs a laboratory experimental approach, specifically utilizing a Completely Randomized Design research design. The research is conducted at the Embryology Laboratory, located inside the Faculty of Veterinary Medicine at Airlangga University. The present investigation included a sample of 32 female rats (R. novergicus), which were allocated into four distinct treatment groups, with each group consisting of eight rats. Before the commencement of the treatment, the experimental animals had an acclimatization period during which they were allowed to relax and provided with an ample supply of food and water for 7 days (Yankelevitch-Yahav et al., 2015). This study consisted of four distinct treatment groups, specifically the control positive group (C) which received forced swim test treatment, and treatment group 1 (T1) which received forced swim test treatment in combination with honey administration. The recommended dosage for oral administration is 2 g honey/rat/day PO. The treatment protocol, referred to as T2, involves the utilization of the forced swim test in combination with the administration of honey. The recommended dosage for oral administration is 4 g honey/rat/day PO. In addition, the treatment protocol involves three sessions of forced swim testing, referred to as T3, in combination with the administration of honey. The recommended dosage is 6 g honey/rat/day PO. The duration of all treatments spanned a period of 14 days, with termination occurring on the 15th day (Yankelevitch-Yahav et al., 2015). Treatment of rats in the forced swim testThe present study employed the forced swim test paradigm as a means to simulate chronic physical stress in mice, building upon the methodology. To execute this experimental procedure, mice were subjected to a specialized enclosure measuring 50 × 30 × 25 cm, which was filled with water to a height of 18 cm. The water temperature was maintained within the range of 24oC–28oC. The mice were then required to engage in a five-minute swimming session of moderate intensity once daily, followed by placement in a darkened enclosure. The exposure period occurs daily at 09:00 Western Indonesian Time (WIB) for a duration of 14 consecutive days. Following the swimming therapy, three groups, namely T1, T2, and T3, were administered forest honey orally (PO) at varying doses of 2, 4, and 6 g honey/rat/day PO, respectively, over a period of 14 days (Shirvani et al., 2019). Sample collectionFollowing a treatment period of 14 days, euthanasia on all animals using a combination of Ket-A-100® and Xyla® for anesthesia. Then, a surgical incision was performed in the midline ventral region using a scalpel. Subsequently, the incision site was prepared by employing scissors on both the thoracic and abdominal regions. The process of blood collection was conducted by use of a 5cc syringe administered intra-cardiacly. The obtained blood is permitted to undergo coagulation to get the serum, which is subsequently subjected to centrifugation at 2,500 revolutions per minute for the purpose of assessing levels of FSH and LH using enzyme-linked immunosorbent assay (ELISA) (Rendevski et al., 2017). FSH and LH serum testThe analysis of FSH and LH levels was conducted at the Biology Laboratory of the Faculty of Science and Technology, Airlangga University. The ELISA method was employed, utilizing the Bioss (cat: BSKR 60303) for FSH and (cat: BSKR 633005) for LH. Then obtain FSH levels reported in units of nanograms per milliliter (ng/ml), and LH levels in milli-international units per milliliter (mIU/ml). Statistical analysisA computer application was employed to conduct statistical analysis, One-Way ANOVA with post-hoc Duncan, to examine potential disparities among groups in the variables of FSH and LH levels. Statistically significant differences are shown by a p-value ≥ 0.05) (Mazzanti and Di, 2016). Ethical approvalExperimental animals have gained acceptance certificate of conduct from the Ethical Clearance from the Faculty of Veterinary Medicine, Airlangga University, number 1.KEH.041.2022. Results and DiscussionTable 1 and Figure 1 displays the outcomes obtained from the analysis of FSH and LH levels utilizing the colorimetric ELISA technique. The findings indicate that there were no statistically significant changes in the FSH levels across the treatment groups, which included C, T1, T2, and T3 (p ≥ 00.05). Nevertheless, the group denoted as T2, which underwent the forced swim test procedure and then received daily treatment of 4 g/head of forest honey, had the greatest levels of FSH, specifically measuring 181.71 ± 28.17 ng/ml. The group denoted as K had the lowest levels of FSH, specifically measuring 153.80 ± 23.22. Table 1. FSH (ng/ml) and LH (mIU/ml) levels in each treatment group using ELISA.
ELISA method was employed to analyze LH levels, and the obtained results indicated a consistent decrease in LH levels with increasing dosages of forest honey, as presented in Table 1. T1 group exhibited the highest LH levels, measuring at 60.19 ± 9.49 mIU/ml, which did not demonstrate a statistically significant difference when compared to group K (p-value ≥ 0.05). In T2 and T3, there was a notable reduction in LH levels when compared to T1 and C (p-value < 0.05). Among these groups, T3 exhibited the lowest LH levels, specifically measuring 14.40 ± 5.77 mIU/ml. This study found no statistically significant differences in the FSH levels between the control group and the treatment group; however, there was a noticeable trend toward an increase. The results align with the findings of a previous study conducted by Schliep et al. (2015), which observed an elevation in FSH levels among women who experience environmental stress (Han et al., 2019). The observed phenomenon can be attributed to the stimulation of the hypothalamic-pituitary-adrenal signaling system (HHA axis), which results in the suppression of the LH surge, hence preventing ovulation and impacting the levels of FSH. The observed elevation of FSH in this study is hypothesized to result from impaired folliculogenesis in the ovaries, potentially caused by the inhibition of the signal pathway mediated by the hormone HHA. An explanation regarding the elevation of FSH levels, which initiates the synthesis of estrogen, followed by a subsequent decline as the secretion of inhibitory factors commences (Bakier, 2017; Luqman et al., 2022). Elevated levels of FSH are indicative of a perturbation in the hormonal cascade, potentially suggesting an impairment in the process of folliculogenesis or anovulation disease. A significant positive association between stress levels and FSH levels. Specifically, the findings indicated that elevated cortisol levels were associated with increased FSH levels. Despite the presence of a significant amount of antioxidants in forest honey and its ability to reduce reactive oxygen species (ROS) levels in the oxidative stress model (Ackerman et al., 2012), as demonstrated in the findings of this study do not indicate any direct impact of forest honey on the enhancement of FSH levels (Stuart and Robinson, 2015). The measured LH levels in this study exhibit a downward trajectory in the T2 and T3, and they display a statistically significant difference when compared to the control group (C). Demonstrated that heightened corticosteroid levels resulting from HHA activation lead to the suppression of hypothalamus-pituitary-gonad signaling (Du et al., 2009).
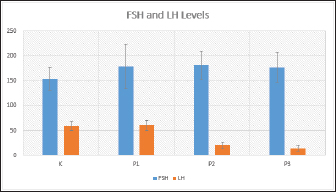
Fig. 1. FSH (ng/ml) and LH (mIU/ml) levels in each treatment shown as Graph. Consequently, this suppression leads to a decrease in LH secretion and inhibits the process of ovulation. An anovulation is a condition characterized by distinct patterns of increased FSH levels and decreased LH levels (Dutta et al., 2022). The present study observed that groups T2 and T3 exhibited a significant elevation in FSH levels and a corresponding reduction in LH levels, thereby satisfying the predetermined criterion. The researchers hypothesize that the viscosity and prolonged delivery of forest honey, as well as the delayed response to stress induction, may diminish the efficacy of honey therapy and result in adverse effects. ConclusionIn conclusion, it can be inferred that the aforementioned points collectively support the notion that the administration of forest honey to mice subjected to a forced swim test for a duration of 14 days did not elicit any discernible impact on the concentrations of FSH and LH. The administration of 4 and 6 g honey/rat/day dosages resulted in a reduction in LH levels in mice subjected to a forced swim test, which serves as a chronic stress paradigm. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo external fund. Authors contributionsAll authors contributed to this study. All authors read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAckerman, K.E., Patel, K.T., Guereca, G., Pierce, L., Herzog, D.B. and Misra, M. 2012. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin. Endocrinol. 78(1), 114–119. Bakier, S. 2017. Rheological properties of honey in a liquid and crystallized state. In InTech eBooks. Available via https://doi.org/10.5772/67035. Du, J., Wang, Y., Hunter, R.G., Wei, Y., Blumenthal, R., Falke, C., Khairova, R., Zhou, R., Yuan, P., Machado-Vieira, R., McEwen, B.S. and Manji, H.K. 2009. Dynamic regulation of mitochondrial function by glucocorticoids. PNAS USA, 106(9), 3543–3548. Dutta, S., Sengupta, P., Roychoudhury, S., Chakravarthi, S., Wang, C.W. and Sláma, P. 2022. Antioxidant paradox in male infertility: “a blind eye” on inflammation. Antioxidants, 11(1), 167. Han, Y., Wang, S., Wang, Y. and Zeng, S. 2019. IGF-1 inhibits apoptosis of porcine primary granulosa cell by targeting degradation of BIMEL. Int. J. Mol. Sci, 20(21), 5356. Luqman, E.M., Ananda, A.T., Widjiati, W. and Hendrawan, V.F. 2022. Protective effect of Apis dorsata honey on chronic monosodium glutamate-induced testicular toxicity in mus musculus mice. J. Pharm. Sci. 19(3), 246–250. Mazzanti, G. and Di Giacomo, S. 2016. Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Mol. 21(9), 1243. Mohamad, T.A.S.T., Islahudin, F., Jasamai, M. and Jamal, J.A. 2019. Preference, perception and predictors of herbal medicine use among Malay women in Malaysia. Patient Pref. Adher. 13, 1829–1837. Moniruzzaman, M., Khalil, I., Sulaiman, S.A. and Gan, S.H. 2013. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement Altern. Med. 13, 43. Nono, F., Yulianti, D.L. and Krisnaningsih, A.T.N. 2017. Pengaruh penggunaan ramuan herbal sebagai feed additive terhadap in come over feed cost ayam broiler. J. Anim. Sci. 5(2), 100–105. Rendevski, V., Aleksovski, B., Stojanov, D., Mihajlovska-Rendevska, A., Aleksovski, V., Baneva–Dolnenec, N., Nikodijevic, D. and Gudeva-Nikovska, D. 2017. Validation of the ELISA method for quantitative detection of TNF-α in patients with intracerebral hemorrhage. Open Access Maced. J. Med. Sci. 5(6), 703–707. Schliep, K.C., Mumford, S.L., Vladutiu, C.J., Ahrens, K.A., Perkins, N.J., Sjaarda, L.A., Kissell, K., Prasad, A., Wactawski-Wende, J. and Schisterman, E.F. 2015. Perceived stress, reproductive hormones, and ovulatory function. Epidemiol, 26(2), 177–184. Shirvani, H., Aslani, J., Mohammadi, Z.F. and Arabzadeh, E. 2019. Short-term effect of low-, moderate-, and high-intensity exercise training on cerebral dopamine neurotrophic factor (CDNF) and oxidative stress biomarkers in brain male Wistar rats. Comp. Clin. Path. 28(2), 369–376. Stramek, A.K., Johnson, M.L. and Taylor, V.J. 2019. Improved timed-mating, non-invasive method using fewer unproven female rats with pregnancy validation via early body mass increases. Lab. Anim. 53(2), 148–159. Stuart, S.A. and Robinson, E. 2015. Reducing the stress of drug administration: implications for the 3Rs. Sci. Rep. 5, 14288 Verma, R., Balhara, Y.P.S. and Gupta, C.S. 2011. Gender differences in stress response: role of developmental and biological determinants. Ind. Psychiatry J. 20(1), 4. Whirledge, S. and Cidlowski, J.A. 2010. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 35(2), 109–125. Yankelevitch-Yahav, R., Franko, M., Huly, A. and Doron, R. 2015. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 97, 52587. | ||
| How to Cite this Article |
| Pubmed Style Widjiati W, Luqman EM, Kuntjorodjakti S, Aulanni'am A, Shabira Z, Taqwa SF, Mega RL, Chou D, Mubarak AS, Hendrawan VF. The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Vet. J.. 2024; 14(2): 738-742. doi:10.5455/OVJ.2024.v14.i2.14 Web Style Widjiati W, Luqman EM, Kuntjorodjakti S, Aulanni'am A, Shabira Z, Taqwa SF, Mega RL, Chou D, Mubarak AS, Hendrawan VF. The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. https://www.openveterinaryjournal.com/?mno=174251 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i2.14 AMA (American Medical Association) Style Widjiati W, Luqman EM, Kuntjorodjakti S, Aulanni'am A, Shabira Z, Taqwa SF, Mega RL, Chou D, Mubarak AS, Hendrawan VF. The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Vet. J.. 2024; 14(2): 738-742. doi:10.5455/OVJ.2024.v14.i2.14 Vancouver/ICMJE Style Widjiati W, Luqman EM, Kuntjorodjakti S, Aulanni'am A, Shabira Z, Taqwa SF, Mega RL, Chou D, Mubarak AS, Hendrawan VF. The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Vet. J.. (2024), [cited January 25, 2026]; 14(2): 738-742. doi:10.5455/OVJ.2024.v14.i2.14 Harvard Style Widjiati, W., Luqman, . E. M., Kuntjorodjakti, . S., Aulanni'am, . A., Shabira, . Z., Taqwa, . S. F., Mega, . R. L., Chou, . D., Mubarak, . A. S. & Hendrawan, . V. F. (2024) The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Vet. J., 14 (2), 738-742. doi:10.5455/OVJ.2024.v14.i2.14 Turabian Style Widjiati, Widjiati, Epy Muhammad Luqman, Suryo Kuntjorodjakti, Aulanni'am Aulanni'am, Zahra Shabira, Sultan Fadhilla Taqwa, Riski Lesta Mega, Dean Chou, Ahmad Shofy Mubarak, and Viski Fitri Hendrawan. 2024. The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Veterinary Journal, 14 (2), 738-742. doi:10.5455/OVJ.2024.v14.i2.14 Chicago Style Widjiati, Widjiati, Epy Muhammad Luqman, Suryo Kuntjorodjakti, Aulanni'am Aulanni'am, Zahra Shabira, Sultan Fadhilla Taqwa, Riski Lesta Mega, Dean Chou, Ahmad Shofy Mubarak, and Viski Fitri Hendrawan. "The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model." Open Veterinary Journal 14 (2024), 738-742. doi:10.5455/OVJ.2024.v14.i2.14 MLA (The Modern Language Association) Style Widjiati, Widjiati, Epy Muhammad Luqman, Suryo Kuntjorodjakti, Aulanni'am Aulanni'am, Zahra Shabira, Sultan Fadhilla Taqwa, Riski Lesta Mega, Dean Chou, Ahmad Shofy Mubarak, and Viski Fitri Hendrawan. "The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model." Open Veterinary Journal 14.2 (2024), 738-742. Print. doi:10.5455/OVJ.2024.v14.i2.14 APA (American Psychological Association) Style Widjiati, W., Luqman, . E. M., Kuntjorodjakti, . S., Aulanni'am, . A., Shabira, . Z., Taqwa, . S. F., Mega, . R. L., Chou, . D., Mubarak, . A. S. & Hendrawan, . V. F. (2024) The impact of Apis dorsata forest honey administration on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in rats (Rattus norvegicus) forced swim test as a chronic physical animal model. Open Veterinary Journal, 14 (2), 738-742. doi:10.5455/OVJ.2024.v14.i2.14 |