| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 90-107 Original Research The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virusAmal A. M. Eid1*, Ali M. Mahmoud2, Esraa E. Hamouda1, Mohamed Metwally3, Rasha M.M. Ezz-Eldin4 and Reham M. ElBakrey11Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Veterinary Medicine Directorate, Mansoura, Egypt 3Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Amal A.M. Eid. Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: amalaeidvet [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
AbstractBackground: Being a ubiquitous, highly contagious virus with a continuous mutation and a large number of evolutions worldwide, the infectious bronchitis virus (IBV) continues to wreak problems among Egyptian chickens and generate economic losses. The commonly applied IBV vaccination protocols in broilers include alternatives to classic and/or variant attenuated live virus vaccines. Aim: The current study targeted to assess the protective efficacy of concurrent and successive Ma5 and 4/91 vaccine strain regimens against the field variant II IBV strain (IBV-EGY-ZU/Ck-127/2021) in chickens. Methods: Commercial broiler chickens were vaccinated with Ma5 and 4/91 strains simultaneously at 1 and 14 days of age. The evaluation parameters included clinical protection and humoral and early innate immunity aspects in the renal tissues of vaccinated and infected birds. Results: The vaccine regimen ameliorated the clinical and histopathological lesions against variant II IBV and enhanced body gain as well as succeeded in preventing tracheal shedding and minimizing cloacal shedding of the field virus. The IL-1β mRNA gene expression was evident as early as 24 hours, with highly significant upregulation at 48 hours post vaccination and 24 hours post challenge (PC) in vaccinated birds. Remarkable upregulation was observed in oligoadenylate synthetases (OAS) expression 48 hours PC in vaccinated and unvaccinated infected birds. The vaccinated birds developed a significant antibody titer of 704.0 ± 111.98 at 28 days of age, with a consistent antibody titer increase after the challenge. Conclusion: Overall, a combination of heterologous protectotype commercial vaccines achieved good protection against the Egyptian variant II IBV strain. This vaccine program could be an effective protocol against the threat posed by IBV viruses circulating in the Egyptian field. Keywords: Infectious bronchitis virus (IBV), Variant II, Vaccine efficacy, Pathology, Immunity. IntroductionThe infectious bronchitis virus (IBV) has garnered increased attention because of its impact on productivity and commerce. The avoidance of infectious bronchitis (IB) in chickens is attained by utilizing live and inactivated vaccines, which deliver defense against virulent field IBVs in the case of exposure. Despite such precautionary measures, IBV outbreaks often occur in many countries producing poultry (Cook et al., 1999; Cavanagh, 2007; Jackwood, 2012; Sultan et al., 2019). Persistent IBV infections occur because of many factors, including impaired biosecurity, vaccine application and programs as well as continued mutation, and the emergence of new variants of the IBV (Jackwood and De Wit, 2013). Despite the widespread use of vaccination as a control measure (Yu et al., 2001; Xu et al., 2007), the coronavirus (ACoV) prototype–IBV has consequence economic effects on poultry production globally (Cavanagh, 2007), as a result of low weight gain, decreased feeding effectiveness and deaths in broilers, and decreased egg production and quality in layers and breeders (Yu et al., 2001; Xu et al., 2007). IBV targets the epithelial cells of the upper respiratory, urinary, and reproductive tracts and other epithelial tissues, which cause clinical manifestations based on its tropism affinity in the domestic chicken (Cavanagh et al., 1997; Cavanagh, 2007; Cook et al., 2012; Jackwood and De Wit, 2013). The causative agent is an enveloped, single-stranded positive-sense RNA virus belonging to the family Coronaviridae, genus Gammacoronavirus in the order Nidovirales (Miłek and Blicharz-Domanska, 2018). The viral genome consists of structural and nonstructural protein-coding gene segments. The structural protein contains nucleocapsid (N) proteins, matrix (M), envelope (E), and spikes S1 and S2 (King and Cavanagh, 1991). The spike S1 is crucial in the attachment of the virus, the fusion of the virus with the cell membrane, host cell specificity, diversity, and antibody neutralization (Jackwood, 2012). The S1 glycoprotein’s variations are essential to identifying new virus genotypes and possibly antiviral responses. Based on the sequencing study of the full-length S1 gene, the IBVs were categorized into 6 genotypes and 32 lineages (Valastro et al., 2016). Since the first case of IBV was isolated from chickens and recorded as a coronavirus in the USA in the 1930s (Schalk and Hawn, 1931) and the detection of the first variants of IBV in the 1950s (Jungherr et al., 1956), many outbreaks of IB and numerous IBV serotypes and/or genotypes have been identified worldwide. In Egypt, the first report of IBV was in 1954 (Ahmed, 1954). After that, various IBV strains were recognized and interrelated to the classic strain M41, and the variant strains such as D274, D1466, D3898, D3128, D08880, and 793B (Davelaar et al., 1984; Taha et al., 1991; Eid, 1998; Madbouly et al., 2002; Abdel-Moneim et al., 2006). The Egyptian variant-1 strain (Abdel-Moneim et al., 2002) and Egyptian variant-2 (Abdel-Moneim et al., 2012) were identified and classified as the GI-23 lineage. The GI-23 is considered the most prevalent lineage in Egypt as a result of its circulating in all flocks of chicken up till now (Ghetas et al., 2016; Abdel-Sabour et al., 2017; Moharam et al., 2020; Sabra et al., 2020). The most efficient strategy for preventing and controlling IBV is using vaccines as well as biosecurity (Jackwood et al., 2015; Zhao et al., 2015). There are several studies indicating that using a vaccine with a homologous strain can produce good protection against the challenge of IBVs (Lee et al., 2010; Zhao et al., 2015). But the recombination and mutation that occur in the IBV genome and variation in the antigenic epitope of the S1 protein contribute to the change of serotype and the emergence of new variant strains (Bande et al., 2017; Moreno et al., 2017; Franzo et al., 2019), and this leads to IB outbreaks in vaccinated chicken flocks (Xu et al., 2007; De Wit et al., 2011; Ismail et al., 2020). Therefore, the control of IB through vaccines is problematical, and certain strains failed to induce protection (Jackwood et al., 2015; Lin and Chen, 2017; Yan et al., 2017). The evaluation of the efficacy and safety of different available vaccine combinations against the circulating IBV serotypes is essential (Awad et al., 2016). Also, the assessment of the cross-protection efficacy of IBV vaccine combinations against heterologous strains of virulent IBV is an alternative strategy for controlling IBV because chickens are exposed at the same time to different IBV variant strains under the field conditions (Awad et al., 2016; Habibi et al., 2017). It has been demonstrated previously that the use of combined classic and variant vaccines has succeeded in reducing the negative impact of heterologous challenges from different variant stains and consequently providing good protection (Chhabra et al., 2015; Pohuang et al., 2016; Yang et al., 2023). The situation on Egyptian poultry farms in terms of problems and losses as a result of the outbreak of IBV, despite the application of vaccination programs, is not much different from what is happening around the world. Identification of the prevalent genotypes in the region is fundamental for the effective protection of chickens against infection, optimizing and assessing different IB vaccination combinations to induce adequate immune responses against these IBV variants and minimize their impact. Here, the protective efficacy of the combination of commercially available lives IB vaccines, as Ma5 and 4/91 strains were assessed in experimental chickens against the recently circulating IBV strain by observing the clinical and pathological findings, detecting the virus shedding, analyzing the early cytokine response, and evaluating humoral immunity. Materials and MethodsVirus and vaccinesThe challenge virus strain IBV-EGY-ZU/CK/Dak-127/2021, which was a neuropathogenic strain, was isolated from commercial broiler farms in Egypt in 2021. The S1 gene of the IBV strain was partially sequenced using IBV S1 HVR1-2 primers, as mentioned by Naguib et al. (2017). DNA sequences were obtained by an Applied Biosystems 3130 genetic analyzer (HITACHI, Japan). The IBV strain was deposited in GenBank under accession number OQ730214. For genetic analysis of the field IBV strain, the phylogenetic tree was performed via neighbor-joining and maximum parsimony in MEGA 6 (Tamura et al., 2013). Two commercial IBV vaccines were used to evaluate their efficacy in this study, including Nobilis® IB Ma5 and Nobilis® IB 4/91 (both vaccines are live, freeze-dried, attenuated vaccines against IB Massachusetts and 4/91 serotypes, respectively, Intervet International B.V., Boxmeer, Holland). Birds and evaluation efficacy of vaccinesTwo hundred and five healthy 1-day-old commercial Cobb chickens were obtained from Dakahlia Poultry Company, Egypt. The study was performed in experimental units under strict sanitary conditions, besides feed and water were provided ad libitum. Five birds were selected and euthanized for blood and tissue specimen collection. The remaining birds were randomly divided into 2 groups (A and B), 100 birds for each. This followed negative results for anti-IBV antibodies and IBV detection in blood and tissue specimens collected from five chicks. Chickens in group B were individually vaccinated with both Nobilis® IB MA5 and Nobilis® IB 4/91, separately and simultaneously, at 1 and 14 days of age via the oculo-nasal route. The chickens in group A (as a control group) were inoculated with phosphate buffer saline (PBS). All chicken groups were routinely vaccinated against NDV at the age of 6 and 16 days and IBDV at 9 and 18 days. Eighteen days after the second immunization for IBV, the experimental chicken groups A and B were split into two subgroups: A1, A2, and B1, B2, respectively. At 32 days of age, chickens in subgroups A2 and B2 were oculo-nasally exposed to the IBV field strain “IBV-EGY-ZU/CK/Dak-127/2021” at a dose of 0.1 ml of 104.0 EID50 (Fig. 1). Clinical observation and samplingAll chickens were monitored twice daily during the experiment period to record any clinical signs and mortality rates, besides the detection of gross pathologic lesions in the euthanized and dead birds post challenge (PC). Chickens were euthanized at 2, 3, 4, and 8 days of age (1, 2, 3, and 7 days post-first IBV vaccination; dpv), then at 15, 16, 17, and 21 days of age (1, 2, 3, and 7 days post-second IBV vaccination; dpv), and also at 33, 34, 35, 37, and 39 days of age [1, 2, 3, 5, and 7 days post challenge; (dpc)]. Then, these birds were submitted for necropsy; the organs of the trachea and kidneys collected at 3 and 7 dpv and dpc were sampled for histopathology as well as cytokine expression was detected in the kidney samples collected at 1 and 2 dpv and dpc. Blood samples were collected weekly from 0-day-olds until the end of the experiment for screening anti-IBV antibodies post vaccination (PV). Oropharyngeal and cloacal swabs were collected from chicken groups at zero, 1, 3, 5, and 7 dpc for virus shedding detection. Five live birds from each group were weighed at 39 and 46 days of age (7 and 14 dpc) to determine the live body weight (BW) and body weight gain (BWG). ELISA analyses of IB antibody levelsThe specific IBV antibodies in the collected sera were analyzed using a commercially available IBV antibody ELISA test kit, IDEXX IBV (IDEXX Laboratories, Inc., Westbrook, ME), following the manufacturer’s instructions. Serum samples were tested in triplicate. Optical density values were read at a wave length of 650 nm using an ELISA Microplate reader (CLINDIAG, Clindiag Systems B.V.B.A., Belgium).
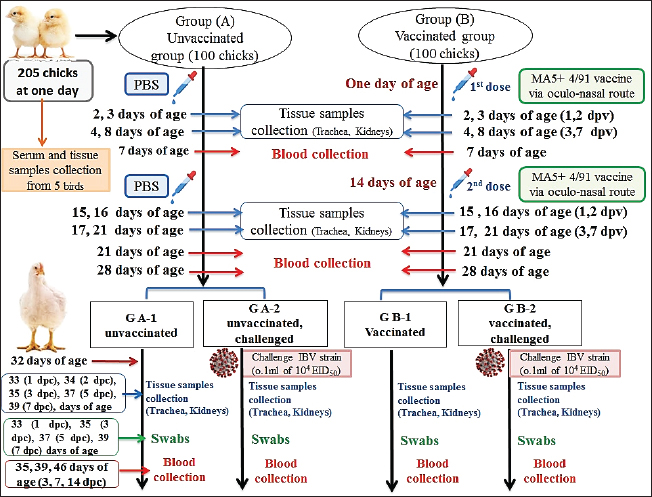
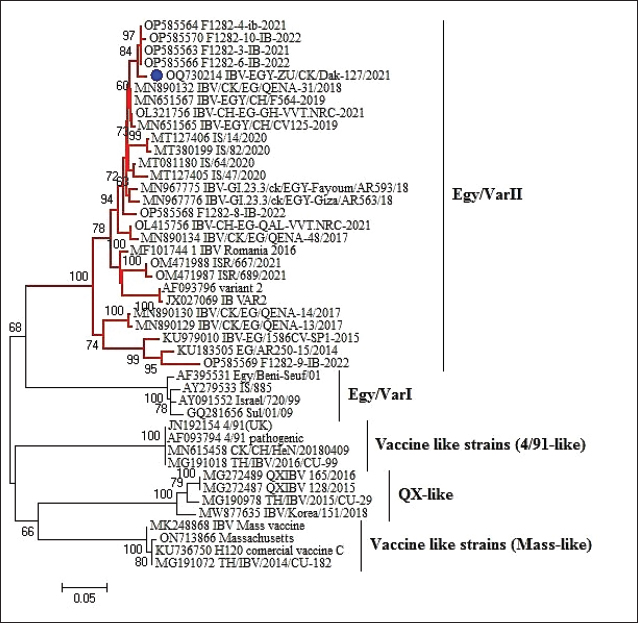
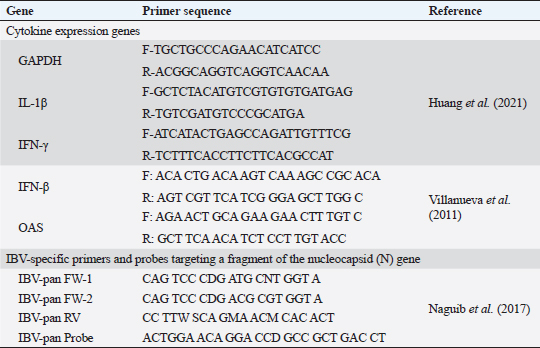
Fig. 1. A summarized experimental design for studying the evaluation efficacy of vaccines against challenge with the IBV strain with an illustration of the timeline of vaccines, challenge with the IBV strain, and collected tissue, blood, and swabs from each group all over the experiment. Detection of cytokine expressions by quantitative real-time PCR (RT-qPCR)Extraction of total RNA was carried out from the collected kidney samples on the first and second days after each vaccination and challenge using a total RNA extraction kit (Applied Biotechnology Co. Ltd., Egypt) in accordance with the manufacturer’s instructions. At that time, the purity and concentration of extracted RNA were detected using a NanoDrop 1,000 spectrophotometer at a wavelength of 260–280 nm. The cDNA was synthesized with the ABT H-minus cDNA synthesis kit (Applied Biotechnology Co. Ltd., Egypt), following the manufacturer’s instructions. Then, cDNAs were stored at −20℃ for RT-qPCR. The primer sequences for the amplification of cytokine genes are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control of the mRNA for normalization. IL-1β, IFN-β, IFN-γ, and oligoadenylate synthetases (OAS) expression levels were analyzed by RT-qPCR in the Applied Biosystems™ StepOne™ Real-Time PCR System (Thermo Fisher Scientific Inc.) using the WizPureTM qPCR Master (SYBR) kit (Wizbiosolutions Inc., Korea) according to the manufacturer’s instructions. The RT-qPCR cycling condition was set as previously described (Huang et al., 2020; Ma et al., 2019). Finally, the method of 2−ΔΔCt was applied to calculate the relative expression levels of the target genes. Measurement of IB virus shedding in the collected swabsThe RNA was extracted from the collected swabs using a total RNA extraction kit (Applied Biotechnology Co. Ltd., Egypt) in accordance with the manufacturer’s instructions. The quantitative real-time reverse transcriptase PCR targeting of the IBV-nucleocapsid (N) gene was performed using the WizPureTM qPCR Master (PROBE) kit (Wizbiosolutions Inc., Korea). The specific primers and probe (Table 1) were used in a 25 µl as a total reaction volume, and the amplification was performed in an Applied Biosystems™ StepOne™ Real-Time PCR System (Thermo Fisher Scientific Inc.) with cycling conditions as previously described by Naguib et al. (2017). The standard curve used for absolute quantification was based on extracted RNA from a tenfold dilution of 4/91 vaccinal strains (Nobilis® IB 4/91). Data of swabs are expressed as the number of viral RNA copies (log10) per ml. Subsequently, the positive IBV swabs were inoculated in specific pathogenic free embryonated chicken eggs (SPF-ECEs) to distinguish between the vaccinal (egg-adapted) and challenge IBV strains, especially in the vaccinated challenged group. Histopathological examinationThe collected tissue specimens from the trachea and kidneys were immediately fixed for 48 hours in neutral buffered formalin 10%. After that, the washing of specimens was performed using distilled water, and then dehydrated in ascending-graded ethanol, cleared in two changes of xylene, impregnated, and embedded in paraffin. They were sectioned at 5 μm thick, stained with hematoxylin and eosin (Suvarna et al., 2018), and examined microscopically for pathological alterations. Statistical analysesThe statistical data analyses of cytokine mRNA expressions and ELISA were carried out using GraphPad Prism Software (GraphPad Prism 9 Software, San Diego, CA). Data are shown as the mean ± standard error of the mean (SEM). Statistical significance was considered at p < 0.05. Data analyses of BW, BWG, and virus shedding were conducted using SPSS version 21.0, and data are shown as the mean ± SEM. Ethical approvalAccording to institutional and national policies for the use and care of laboratory animals, all study procedures and animal care activities were carried out. The Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Zagazig University, Egypt, gave their approval to the experimental techniques used in this study under the approval number: ZU-IACUC/2/F/72/2021. ResultsGenetic analyses of the field IBV strainAlignment and phylogenetic analyses of the partial S1 gene sequence showed that the field IBV “IBV-EGY-ZU/CK/Dak-127/2021” strain was analogous with Egy/VarII and closely related to Egyptian strains; F1282-IB-2021 and 2022 (Fig. 2). Table 2. ABW and BWG of experimental groups after being challenged with variant II IBV strain.
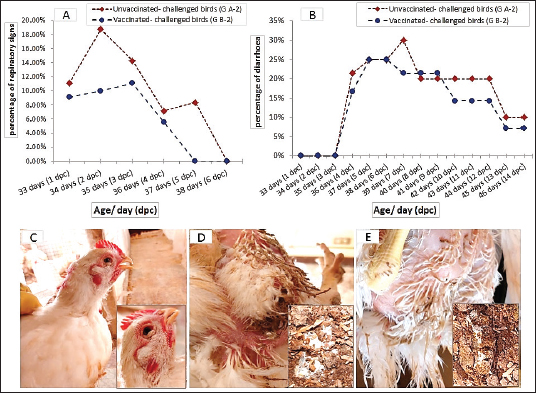
Clinical signs observationNo obvious clinical signs were observed in the chickens of the control groups; vaccinated-unchallenged (group B-1) and unvaccinated-unchallenged (group A-1) during the experiment. One day after the challenge, the birds in the unvaccinated-challenged A-2 group (11.11%) showed respiratory signs in the form of watery eyes, conjunctivitis, sneezing, and rales. In the vaccinated-challenged B-2 group (9.09%) noticed only watery eyes and sneezing. The peak of respiratory signs occurred at 2 dpc in unvaccinated-challenged birds with a percentage of 18.75%, and then gradually declined. Whereas the signs in vaccinated-challenged birds were low in occurrence and severity, and were unnoticeable at 5 dpc, while they continued in the unvaccinated-challenged birds with noticeable gasping. White diarrhea was recorded at 4 dpc in 21.43% of the unvaccinated-challenged birds that were huddled together, which was intense and aggravated until 7 dpc. A milder degree of diarrhea appeared in 16.67% of the vaccinated-challenged groups (Fig. 3).
Fig. 2. Partial S1 gene sequence of the field IBV strain in relation to the available sequences on GenBank, the IBV strain is marked with a blue circle. Phylogenetic relationships through a bootstrap trial of 1,000 were determined with MEGA6.
Fig. 3. The occurrence of clinical signs in unvaccinated and vaccinated chickens after challenge with variant II IBV strain; (A) Percentage of birds manifesting respiratory signs, (B) Percentage of birds suffering from diarrhea, (C) Conjunctivitis and gasping showed in an unvaccinated-challenged bird at 5 dpc, (D) Massive white diarrhea in unvaccinated-challenged birds, (E) Mild white diarrhea in vaccinated-challenged birds. Table 1. The primer sequences were used in the detection of cytokine expression and IBV using real-time machine.
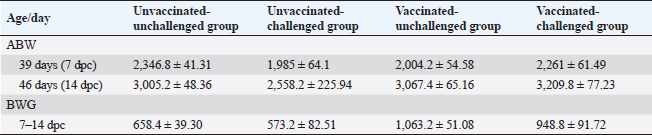
Average body weight (ABW) and BWG of chickens post exposure to infection with the variant II IBVBW in all groups was recorded post-inoculation with the variant II IBV strain at 7 and 14 dpc (Table 2). The average weight of vaccinated-challenged chickens was high in relation to unvaccinated-challenged ones. In addition, the BWG of vaccinated-unchallenged and vaccinated-challenged chickens was improved compared with the control groups, unvaccinated-unchallenged and unvaccinated-challenged. Macroscopic lesionsAt necropsy, the chickens of the control groups, unvaccinated-unchallenged (group A-1) and vaccinated-unchallenged (group B-1) showed no lesions (Fig. 4A and B). The unvaccinated-challenged (group A-2) and vaccinated-challenged birds (group B-2) that were euthanized at 1, 2, 3, 5, and 7 dpc were examined for the macroscopic lesions. The gross lesions in unvaccinated-challenged birds were recorded as early as 1 dpc in the form of tracheitis, but no lesions were recorded in vaccinated-challenged birds. On the second and third dpc, severe tracheitis with mucous accumulation and congested lungs was noticed in unvaccinated-challenged birds, while mild respiratory lesions were seen in vaccinated-challenged birds. Tracheal mucous and flakes with congestion of the lungs increased on day 5 PC in unvaccinated birds and reduced on day 7. The vaccinated-challenged birds had slight tracheal inflammation and congestion in the lungs on day 5, with no lesions at 7 dpc.
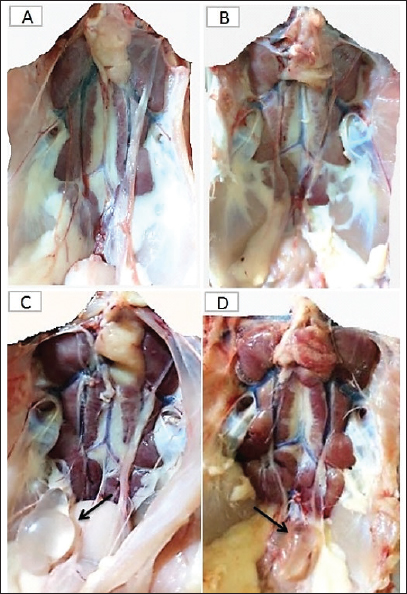
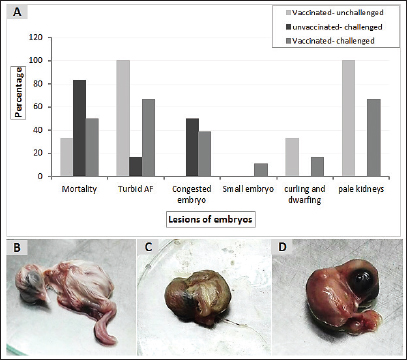
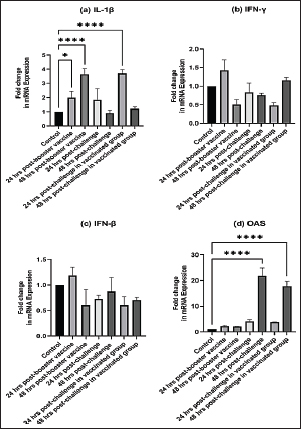
Fig. 4. The macroscopic lesions in the unvaccinated and vaccinated chickens after challenge with the variant II IBV strain. (A) Normal kidney tissue from an unvaccinated/unchallenged chicken, (B) Normal kidney tissue from a vaccinated/ unchallenged chicken, (C) Nephritis and nephrosis in the kidney and fluid accumulation in vestigial right oviduct (arrow) in unvaccinated/challenged chicken, and (D) Nephritis and nephrosis in the kidney with fluid accumulation in vestigial right oviduct (arrow) in vaccinated/challenged chicken. Swollen and inflamed kidneys were observed on day 3 PC. Pale plugged and inflamed kidneys were detected with a visible tubule structure and/or ureters distended with urates in unvaccinated-challenged birds at the fifth and seventh dpc. The vaccinated-challenged ones revealed milder kidney lesions with a low frequency. Furthermore, during the examination, fluid accumulation in the vestigial right oviduct was noticed in both unvaccinated-challenged and vaccinated-challenged chickens (Fig. 4C and D). Also, the intestinal tracts were distended and filled with yellowish content. Microscopic changesFirst, the microscopic examination of the trachea and kidneys collected from birds at 3 and 7 days after each vaccination revealed mild histopathological changes, including mucosal mononuclear cell infiltration in the trachea at 3 and 7 days post-first vaccination, as well as kidney tissue showing mononuclear cell aggregation with a few vacuolations at the seventh day. After the challenge, the histopathology of the trachea and kidney sections of different experimental groups was examined at 3 and 7 dpc. In the trachea, at 3 dpc, the tracheal sections of unvaccinated-unchallenged and vaccinated-unchallenged birds had a normal histologic structure. The detected histological changes in the unvaccinated-challenged group were severe and included mucosal congestion, epithelial desquamation, and inflammatory cell infiltration with the presence of luminal necrotic epithelium mixed with mucous and erythrocytes. While in the trachea of the vaccinated-challenged group, the lesions were less severe and included hyperplasia in the mucosal glands, notable inflammatory cell infiltration, and intermediate mucosal congestion (Fig. 5A–D). At 7 dpc, the tracheal examination in the unvaccinated-challenged group showed severe necrosis in the lamina epithelial with the presence of epithelial desquamation, vascular congestion, and notable mucosal infiltration with inflammatory cells. Whereas mucosal inflammatory cell infiltration was only seen in the trachea of the vaccinated-challenged group compared to the unvaccinated-unchallenged group. However, the trachea of the vaccinated-unchallenged group noticed mild vascular congestion and mucosal inflammatory cell infiltration (Fig. 5I–L). In the kidneys, the microscopic examination of the unvaccinated-unchallenged and vaccinated chickens showed a normal histological structure throughout the period of the experiment (Fig. 5E, F, M, and N). At 3 dpc, the kidney of the vaccinated-challenged group had vascular congestion in comparison to the unvaccinated-challenged group, which displayed higher vascular congestion, interstitial minute hemorrhages, and vacuolation and necrosis of some renal tubules (Fig. 5G and H). On the other hand, at 7 dpc, the examination of the kidneys revealed necrotic tubular epithelium and vascular congestion, which were severe in the unvaccinated-challenged and mild in the vaccinated-challenged group; in addition, interstitial hemorrhage was seen in the unvaccinated-challenged group (Fig. 5O and P). Measurement of shed IBV in the collected swabs and detection in SPF ECEsThe virus shedding was detected and measured in the collected tracheal and cloacal swabs at zero, 1, 3, 5, and 7 dpc with variant II IBV strain by quantitative real-time RT-PCR using IBV-specific primers and probes of the N gene. The cloacal swabs collected from the vaccinated group before the challenge (zero day) revealed virus detection with 102.66 ± 0.06, but there was no virus detection in the unvaccinated group. After the challenge, there was no virus detection in any of the collected tracheal swabs of vaccinated challenged birds, while IBV was detected at 3 dpc with 102.8 ± 0.23 in unvaccinated-challenged birds. In the case of cloacal swabs, the virus was detected at 3, 5, and 7 dpc with 103.01 ± 0.27, 103.48 ± 0.28, and 102.64 ± 0.23, respectively, in the unvaccinated-challenged birds. The detection of IBV shedding in the vaccinated-challenged birds was from one dpc until 5 dpc and ranged from 102.1 ± 0.67 to 102.9 ± 0.08, with no virus detection at 7 dpc. The positive cloacal swabs using real-time qRT-PCR were submitted for inoculation in SPF-ECEs. The inoculated chicken embryos revealed a mortality of up to 83% in the unvaccinated-challenged group, and 50% and 33.3% in the vaccinated-challenged and vaccinated-unchallenged swabs, respectively. The detection of turbid allantoic fluid resulting in urate accumulation was 100% in vaccinated-unchallenged swabs, followed by 50% in vaccinated-challenged swabs and 16.7% in unvaccinated-challenged swabs. There was no evidence of curled and dwarfed embryos in the unvaccinated-challenged swabs, while it was detected in 16.7% and 33.3% of inoculated embryos with vaccinated-challenged and vaccinated-unchallenged swabs, respectively (Fig. 6). Cytokine mRNA expressions in kidneysAs early as 24 and 48 hours post-inoculation of vaccines and/or the IB variant II strain, the mRNA expression of IL-1, IFN- γ, IFN- β, and OAS in renal tissue was measured relative to the housekeeping gene, GAPDH. Based on the mRNA expression of control tissues at each time point, the fold changes in cytokine mRNA expression in tissues of the vaccinated and infected groups were determined. No upregulation of cytokine expression was detected in groups after the first vaccination. There was an initial upregulation in the IL-1β and OAS mRNA expression with an observed significant increase in fold change of IL-1β expression (Fig. 7a) at 24 hours ( p= 0.0305) and 48 hours ( p < 0.0001) PV and 24 hours PC in the vaccinated group ( p < 0.0001). Also, a significant increase in OAS mRNA expression (Fig. 7d) was seen at 48 hours PC in unvaccinated and vaccinated birds ( p < 0.0001). However, no significant changes ( p values ranged from 0.1468 to 0.9913) were seen in IFN-γ and IFN- β mRNA gene expression (Fig. 7b and c), post-inoculation of vaccines, and variant II IBV strain.
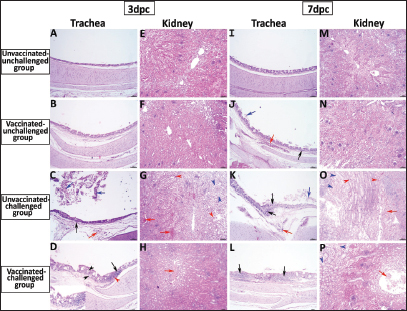
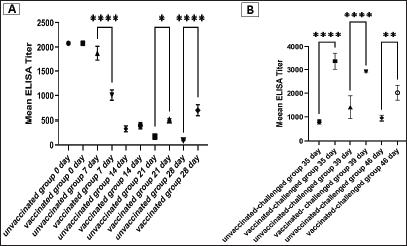
Fig. 5. Photomicrographs of H&E stained tracheal and kidney sections from unvaccinated-unchallenged, vaccinated-unchallenged, unvaccinated-challenged, and vaccinated-challenged chickens at 3 and 7 dpc with variant II IBV strain. (A) Normal tracheal structure. (B) Normal tracheal structure. (C) Trachea showing mucosal congestion (red arrow), inflammatory cell infiltration (black arrow) the presence of luminal necrotic epithelium mixed with mucous and erythrocytes, and desquamation of epithelial lining (blue arrows). (D) Trachea showing hyperplasia of the mucosal glands (black arrowheads), mucosal hemorrhage (red arrowhead), and notable inflammatory cell infiltration (black arrow). (E) Normal structure of the kidney. (F) Normal structure of kidney. (G) Kidney showing vascular congestion (red arrows), interstitial minute hemorrhages (red arrowheads), and vacuolation and necrosis of renal tubules (blue arrowheads). (H) Kidney showing vascular congestion (red arrow). (I) Normal tracheal structure. (J) Trachea showing mild vascular congestion (red arrow), mucosal inflammatory cell infiltration (black arrow), and desquamation of epithelial lining (blue arrow). (K) Trachea showing necrotic lamina epithelia with epithelial desquamation (blue arrow), vascular congestion (red arrow), and notable mucosal infiltration with inflammatory cells (black arrows). (L) Trachea showing notable mucosal inflammatory cell infiltration (black arrows). (M) Normal structure of kidney. (N) Normal structure of kidney. (O) Kidney showing necrotic tubular epithelium (blue arrowheads), vascular congestion (red arrow), and interstitial hemorrhage (red arrowheads). (P) Kidney showing necrotic tubular epithelium (blue arrowheads), and vascular congestion (red arrow).. ELISA IBV antibody responseTo assess the vaccine regimen’s effectiveness on the immune system’s reaction, the antibody titer against IBV was measured by using a commercial ELISA kit. The mean of the maternally derived anti-IBV antibody titer was 2,082.49 ± 40.75 withstand until the seventh day of age. Chickens vaccinated with both Nobilis® IB MA5 and Nobilis® IB 4/91, separately and simultaneously at 1 and 14 days of age via the oculo-nasal route, developed an increased antibody response. The antibody level was significantly increased at 14 days PV and was 704.0 ± 111.98 compared with the unvaccinated group, which was 101.27 ± 10.61. After the challenge, ELISA recorded significant antibody titers reached 3,376.33 ± 201.99 ( p < 0.0001) in vaccinated chickens, while they were 811.28 ± 50.39 in unvaccinated ones at 35 days of age (3 dpc). In addition, at 7 and 14 dpc in vaccinated birds, the antibody titers were 2,927.74 ± 14.00 and 2,031.92 ± 183.47, respectively, as shown in Figure 8.
Fig. 6. Pathologic changes induced in the inoculated chicken embryos with the positive IBV swab suspensions. (A) Percentage of embryo gross lesions, (B) Normal embryo of the control group, (C) Curled and dwarfed embryo inoculated with vaccinated-unchallenged cloacal swab, (D) Curled and dwarfed embryo inoculated with vaccinated-challenged cloacal swab. DiscussionIBV causes financial losses in the chicken sector worldwide (De Wit et al., 2011; Jackwood, 2012). Good biosecurity plays a major role in containing IBV infections and minimizing the spread of the virus, but it is generally accepted that effective control in commercial operations is not possible without the use of vaccination. Various live attenuated and inactivated IBV vaccines are usually used to control IBV infections in broilers. Attenuated IBV vaccines, including classical and/or variant serotypes, are utilized in many countries according to their need to protect birds against wild circulating IBVs within their permitted regulations. IBV is characterised by high genetic, serotype, and pathotypic diversity. In spite of comprehensive immunoprophylaxis strategies, the emergence of new genetic lineages is recurrently seen in the field, causing disease control to be more challenging (Cavanagh et al., 1997; Jackwood, 2012). Similar to other countries, Egypt’s poultry industry loses profit due to viral diseases, including IB (Eid, 2004; Abozeid and Naguib, 2020). A trial to investigate a different approach to a prophylactic vaccination strategy stemming from heterologous protectotype theory was applied in the current study. For this purpose, 205 healthy 1-day-old commercial Cobb chickens were used to evaluate the vaccine program through the simultaneous application of both Ma5 and 4/91 strains at days 1 and 14 of age and then challenging them with a field variant II IBV strain at day 32 of age. According to the manufacturer’s instructions, there is no evidence of interference between Ma5 and 4/91 when given together in simultaneous vaccination (Intervet International, 2003). Also, Jackwood et al. (2020) showed that the simultaneous administration of up to four different types of live attenuated vaccines can respond properly without interference and lead to adequate protection against infection with IBV. Clinically, the vaccinated birds showed mild findings in comparison to the unvaccinated ones (control positive group), which had typical IB signs and lesions. The clinical signs and lesions in the trachea and kidneys of unvaccinated birds were consistent with the results of previous studies (Abdel-Moneim et al., 2012; Abd El Rahman et al., 2015; Sultan et al., 2015; Zanaty et al., 2016; Mahmoud et al., 2021). Similarly, the variant strains produce lesions ranging from mild to severe (Abou El-Fetouh et al., 2016; Ismail et al., 2020), according to many factors such as host immunity, breed, husbandry, co-infections, and adverse environmental conditions (Najimudeen et al., 2022). Regarding vaccine protection, many authors documented that when the chickens received the Mass-type and variant strains of the IB vaccine, fewer clinical symptoms and lesions were observed in the vaccinated birds after the challenge (Shirzad et al., 2012; Sultan et al., 2019). Also, using a combined vaccination program incorporating different vaccine strains such as Mass-type and 4/91 could provide sufficient protection against the heterologous field IBV variant strain (Sasipreeyajan et al., 2012; Shirzad et al., 2012). Similar to the lesions commonly seen in infection with variant II IBV as a nephropathogenic strain that has an effect on the respiratory and renal systems (El-Mahdy et al., 2012; Mahmoud et al., 2019; 2021), we recorded fluid accumulation in the vestigial right oviduct in the unvaccinated-challenged and vaccinated-challenged chickens with differing severity. It is probable, as the IBV is continually evolving, especially in chicken flocks. Since variations and variants of different genotypes sometimes lead to changes in their pathogenicity and tropism (Amarasinghe et al., 2018a). Finally, Moharam et al. (2020) recognized based on whole genome sequencing that the Egyptian variant strain (EGY/NR725/2016) had inter-genotypic recombination (gene 6b) with a viral strain/genotype that was closely related to the QX-like genotype (GI-19 lineage) that was characterized by infection of the kidneys, respiratory tract, and reproductive tract (Wibowo et al., 2019; Najimudeen et al., 2020). However, their analysis of the partial S1 sequences of the same isolate indicates that it is a prototype of currently circulating viruses (G1–23). Therefore, we aspire to more investigation on the pathogenesis and pathogenicity of the IBV strain in this study, in addition to isolating more strains from the field and linking them to studying whole genome sequencing to help in clarifying the explanations of the variations that occur with the characteristics of some IBV strains in the Egyptian field.
Fig. 7. Fold changes in mRNA expressions of IL-1β, IFN-γ, IFN-β, and OAS in kidneys as early as 24 and 48 hours post inoculation of vaccines and/or infection with variant II IBV strain “IBV-EGY-ZU/CK/Dak-127/2021” are illustrated in Figure 7a–d. The figures display the mean ± SEM (bar), *p < 0.05, ****p < 0.0001, which represent a significant difference compared to the control group.
Fig. 8. Specific IBV antibody titers of the unvaccinated and vaccinated groups before (A) and after (B) challenge with variant II IBV strain “IBV-EGY-ZU/CK/Dak-127/2021” at 32 days old. The figures display the mean ± SEM (bar), *p < 0.05, **p < 0.01, ****p < 0.0001, which represents a significant difference compared to the unvaccinated/ unvaccinated-challenged group. Moreover, the BWG was improved in vaccinated birds after infection compared to the unvaccinated-challenged (control-positive) birds. The BWG of unvaccinated birds was affected following infection, and this may be attributed to decreased food intake and loss of water due to watery droppings as a result of kidney damage induced by the virus infection (Najimudeen et al., 2022). Among the tissues examined by histopathology are the trachea and kidneys, which are considered the main target organs of IBV (Zhao et al., 2015). Marked histopathological changes occurred at 3 and 7 days after infection in the unvaccinated group (control-positive). While the lesions shown in the trachea and kidneys of vaccinated birds were mild to intermediate. This revealed that the vaccine protocol could minimize virus replication and reduce the severity of lesions. However, mild tracheal and kidney microscopic lesions were seen after the administration of vaccines (Ma5 and 4/91) in the absence of IBV infection. Despite the vaccine strain’s lack of virulence, the live vaccine was still able to replicate and caused minimal histological lesions after vaccination (Lee et al., 2010). On the other hand, Pohuang et al. (2016) detected that the IBV vaccine and booster vaccination, especially with the 4/91 strain may cause more tracheal epithelial damage than the other vaccinated groups. The results obtained from detection and measurements of the virus shedding in the collected swabs after infection with variant II IBV strain in unvaccinated and vaccinated birds revealed that the vaccination protocol used in this study succeeded in preventing virus shedding from the trachea of vaccinated birds compared to unvaccinated birds. It is probable that a live vaccine produced the high mucosal immunity that prohibits invasion and propagation of the virus in the tracheal mucosa (Pohuang et al., 2016). Disparately, Sultan et al. (2019), when applying IB-Ma5: IB-793B on day-1 and day-14, respectively, reduced the IBV shedding titers in the trachea of the vaccinated birds. Wherever there is a mismatch in the vaccine application method in this study with other studies. In addition, the virus shed in the cloacal swabs of vaccinated birds was minimized and not prolonged when compared with that in unvaccinated ones. In general, the applied vaccine protocol could prevent and diminish the challenged IBV replication in the trachea and gastro-renal tract, respectively. As a consequence, the lesions induced by the virus in the trachea and kidneys of vaccinated birds were milder. The main outcome of these results is that the current vaccine regimen could decrease the wild IBV load in the environment, which in turn could hinder the recombination and evolution of IBVs. However, there was detection of the virus in the cloacal swabs collected from the vaccinated birds before the challenge. Comparably, Matthijs et al. (2008) demonstrated that the IBV H120 vaccine is able to spread extensively among broilers. Accordingly, the inoculation of suspensions of cloacal swabs from vaccinated-challenged birds in SPF-ECEs revealed evidence of curling and dwarfing in 16.7% of embryos, which was 33.3% in vaccinated-unchallenged swabs, with no evidence in infected swabs. On the other hand, the SPF-chicken embryos inoculated with infected cloacal swabs appeared congested with mortality. For IBV embryos lethal strains generally result in embryos with cutaneous hemorrhages; nonlethal strains result in stunting, curling, or clubbing of down or urates depositing in the mesonephron of the kidney (Cavanagh, 2008; OIE, 2018). The detection of viral strains of vaccines (egg-adapted) in cloacal swabs, parallel with the detected embryo pathological lesions, gave a probability that the virus shed from the vaccinated-challenged birds not only returned to the challenge variant II IBV strain but also to the Ma5 and/or 4/91 vaccine strains. ELISA has been shown to be a trustworthy and accurate approach for detecting an initial rise in IBV antibody levels (Chen et al., 2011). In the current study, there was considerable maternal-derived antibody (MDA) (2,082.49 ± 40.75) which was variable in modifying the possible side effects of simultaneous live IBV vaccination at day one of age. Thus, the MDA is reduced at 1 week PV compared to unvaccinated birds and this is attributed to its neutralisation by the vaccine virus. However, the vaccinated birds showed a high significant increase in the antibody level at 2 weeks PV. This result is compatible with Chhabra et al. (2015), who confirmed that the partial neutralisation of the vaccine virus occurred by the maternal antibodies in the broilers, with a subsequent decrease in replication of the vaccine virus and poor stimulation of the humoral response (Raggi and Lee, 1965; Davelaar and Kouwenhoven, 1977; Mondal and Naqi, 2001). The antibody titer of the vaccinated chickens was magnified significantly at 3 and 7 dpc with the variant II IBV strain, and this was symmetric with the actual low and mild signs, macro and microscopic lesions showed in the trachea and kidney of vaccinated birds at the same time compared to the unvaccinated ones. Besides, the clearance and minimized virus load in the trachea and cloacal swabs of vaccinated birds, respectively. In turn, these support the role of humoral immunity in disease recovery and virus clearance (Toro and Fernandez, 1994; Thompson et al., 1997). In addition, the broadening of protection produced by the combination of different IBV vaccine strains could be explained by increasing the cellular and local immune responses (Smialek et al., 2017). From the abovementioned results, there was an obvious decrease in the occurrence and severity of clinical pathological findings, reduced tissue viral replication rates, and viral shedding in the vaccinated-infected chickens. These indicate that the vaccine program could achieve protection against the challenge with the field variant II IBV strain (IBV-EGY-ZU/CK/Dak-127/2021). Pohuang et al. (2016) indicated that the birds vaccinated with one dose of Ma5 and 4/91 at 1 day old achieved protection from the clinical signs (tracheal rales=0/20), had a low histopathological lesion score (1.00 ± 0.9), and produced an ELISA antibody titer (2,002 ± 1,179.3) against IBV at 7 dpc with QX-like IBV. Many studies have shown that using IB booster vaccinations can produce better field IBV protection against heterologous IBV strains than using a single dose of vaccination (Cook et al., 1999; Jackwood et al., 2015; Ismail et al., 2020). Terregino et al. (2008) found improvement in protection against heterologous IB serotypes (QX-like IBV variant strain) in commercial chicks after applying a vaccination protocol with the Ma5 and 4/91 (793B strain). Awad et al. (2015) showed that the combined vaccination of H120 and CR88 (a 793B strain) at 1-day-old chicks, followed by CR88 at 14 days old, achieved greater protection against the Middle East isolates (IS/885/00-like and IS/1494/06-like); also, Chhabra et al. (2015) suggested great protection for the Q1 IBV strain when using the same vaccine protocol. In addition to the adaptive host response that contributes to the control of IBV in infected chickens, the innate host response is also important and considered the first line of defense against infection (Akira, 2001; Chhabra et al., 2015; Amarasinghe et al., 2018b). As a part of the innate host response, the cytokines are considered critical components, as shown in other host-virus infection models (Amarasinghe et al., 2018b; Zhang et al., 2021). The information related to the significance of innate immunity in the early time of vaccination and/or infection with IBV in the kidneys of chickens is inadequate. Consequently, our interest was to determine the role and level of IL-1β, IFN-γ, IFN-β, and OAS in the kidney as early as PV and/or IBV infection. In the present study, the initial upregulation was recorded in the IL-1β and OAS mRNA expression, with a significant increase in IL-1β mRNA expression detected at 24 and 48 hours PVs and 24 hours PC in the vaccinated group. This indicated that the viral vaccine had been able to motivate the production of IL-1β which in turn has the ability to potentiate the adaptive host responses characterised by the IgM response and the recruitment of CD4+ cells to the site of infection (Schmitz et al., 2005). Although Najimudeen et al. (2022) detected no upregulation of IL1-β in kidneys at 3 days post-infection (dpi), they found a significantly increased expression at 7 dpi with a variant IBV strain. The significant difference in OAS expression was at 48 hours PC in unvaccinated and vaccinated birds. The inoculation with the IBV strain induced significant host responses in the kidneys, characterised by immune cell recruitment and expression of immune mediators such as IL-1β, and OAS. The rapid initiation of such cytokines in kidneys early following IBV strain inoculation might be explained by the IBV strain-induced significant host responses in the kidneys characterized by immune cell recruitment and expression of immune mediators such as IL-1β and OAS. This may be adequate for the control of viral replication and associated with the severity of the pathological lesions (Kameka et al., 2014). In addition, it is possible that these immune cells and mediators may have contributed to the clearance of IBV, as has been seen previously (Seo and Collisson, 1997; Okino et al., 2014). Where IL-1β plays an important role in stimulating the cellular response and recruiting cells, such as macrophages, which in turn potentiates the adaptive host responses (Schmitz et al., 2005; Babcock et al., 2008), and it is capable of reducing viral infections (Watashi et al., 2013). In addition, the OAS is a critical part of the interferon-dependent host defence system against viruses, and its activation leads to the inactivation of viral mRNA (Rogozin et al., 2003; Itsui et al., 2006). As for the IFN-γ and IFN- β mRNA gene expression, there were no significant changes as early as 24 and 48 hours after the vaccination and PC in unvaccinated and vaccinated birds. Suppression of the host IFN innate immune response following IBV infection has been previously reported (Kint et al., 2016). Najimudeen et al. (2022) detected no upregulation of IFN-γ mRNA expression in kidneys at 3 dpi with a variant IBV strain; however, they recorded a significant increase at 7 and 10 dpi. Moreover, Zhang et al. (2017) found that IBV infection increased the expression level of IFN-β in the kidney beginning at 4 dpi. Thus, there is the possibility that the expression of interferon may increase at later time points (not included in the present study). Where IFNs, including type I (IFN-β) and type II (IFN-γ), play important roles in antiviral defense, inhibit viral replication, and work as immune effectors or modulators (Echebli et al., 2018; Ivashkiv, 2018). ConclusionIn fact, complete protection against IBV infection usually requires the use of the homologous vaccine, but it is not usually applicable due to the continuous genetic evolution of IBVs. However, fortunately, vaccination with a heterologous vaccine provides acceptable protection. In this study, it could be concluded that the use of simultaneous successive classic and variant IBV vaccines confers broad cross-protection against a challenge with a variant IBV strain with regards to viral load, body performance, and induction of immune response, as well as protection from severe clinical signs and lesions. More trials are needed on a large scale to evaluate long-term shedding, discriminate the field virus from vaccine one in shedding, and study rolling and reverse pathogenicity. AcknowledgmentThe authors would like to thank Prof. Dr. Shimaa M. G. Mansour, Virology Department, Faculty of Veterinary Medicine, Zagazig University, for her contribution with advice and instructions in the practical work of molecular and ELISA. Conflict of interestNo potential conflict of interest was reported by the author(s). FundingThis study was not supported by any funding source, and all this was contributed by the authors. Author contributionsAll authors cooperated in the purpose and design of the study; Amal A.M. Eid planned the concept and design of the study. Material preparation, data collection, analysis, and investigation were performed by Amal A.M. Eid, Reham M.M. ElBakrey, Ali M. Mahmoud, Esraa E. Hamouda, Mohamed Metwally, and Rasha M.M. Ezz-Eldin. The manuscript’s original draft was written by Amal A.M. Eid, Reham M.M. ElBakrey, and Mohamed Metwally. Each author reviewed and edited the previous versions of the manuscript. Amal A.M. Eid revised the final manuscript. The final manuscript was read and approved by all authors. Data availabilityThe article contains all the information necessary to understand this investigation. Upon a reasonable request, the corresponding author will provide any further information needed. ReferencesAbdel-Moneim, A.S., Afifi, M.A. and El-Kady, M.F. 2012. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch. Virol. 157 , 2453–2457. Abdel-Moneim, A.S., El-Kady, M.F., Ladman, B.S. and Gelb, J. 2006. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 3, 1–9. Abdel-Moneim, A.S., Madbouly, H.M., Gelb, J. and Ladman, B.S. 2002. Isolation and identification of Egypt/Beni-Seuf/01 a novel genotype of infectious bronchitis virus. VMJ-G 50(4), 1065–1078. Abd El Rahman, S., Hoffmann, M., Lueschow, D., Eladl, A. and Hafez, H.M. 2015. Isolation and characterization of new variant strains of infectious bronchitis virus in Northern Egypt. Adv. Anim. Vet. Sci. 3, 362–371. Abdel-Sabour, M.A., Al-Ebshahy, E.M., Khaliel, S.A., Abdel-Wanis, N.A. and Yanai, T. 2017. Isolation and molecular characterization of novel infectious bronchitis virus variants from vaccinated broiler flocks in Egypt. Avian. Dis. 61(3), 307–310. Abou El-Fetouh, A., Mohamed, M.H., Refat, N., Ahmed, M. and El-Zanaty, A.E. 2016. Pathological studies on infectious bronchitis disease in chickens. Zagazig. Vet. J. 44, 248–259. Abozeid, H.H. and Naguib, M.M. 2020. Infectious bronchitis virus in Egypt: genetic diversity and vaccination strategies. Vet. Sci. 7(4), 204. Ahmed, H.N. 1954. Incidence and treatment of some infectious viral respiratory diseases of poultry in Egypt. Ph.D. thesis, Cairo University, Giza, Egypt. Akira, S. 2001. Toll-like receptors and innate immunity. In Advances in immunology. Ed., Dixon, F.J. Cambridge, MA: Academic Press, vol. 78, pp: 1–56. Amarasinghe, A., Abdul-Cader, M.S., Almatrouk, Z., van der Meer, F., Cork, S.C., Gomis, S. and Abdul-Careem, M.F. 2018b. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV). Vet. Microbiol. 215, 1–10. Amarasinghe, A., De Silva Senapathi, U., Abdul-Cader, M.S., Popowich, S., Marshall, F., Cork, S.C., der Meer, F., Gomis, S. and Abdul-Careem, M.F. 2018a. Comparative features of infections of two Massachusetts (Mass) infectious bronchitis virus (IBV) variants isolated from Western Canadian layer flocks. BMC. Vet. Res. 14(1), 1–12. Awad, F., Forrester, A., Baylis, M., Lemiere, S., Ganapathy, K., Hussien, H.A. and Capua, I. 2015. Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Vet. Rec. Open. 2(2), e000111. Awad, F., Hutton, S., Forrester, A., Baylis, M. and Ganapathy, K. 2016. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian. Path. 45(2), 169–177. Babcock, A.A., Toft-Hansen, H. and Owens, T. 2008. Signaling through MyD88 regulates leukocyte recruitment after brain injury. J. Immunol. 181(9), 6481–6490. Bande, F., Arshad, S.S., Omar, A.R., Hair-Bejo, M., Mahmuda, A. and Nair, V. 2017. Global distributions and strain diversity of avian infectious bronchitis virus: a review. Anim. Health. Res. Rev. 18(1), 70–83. Cavanagh, D. 2007. Coronavirus avian infectious bronchitis virus. Vet. Res. 38(2), 281–297. Cavanagh, D (ed). 2008. SARS-and other coronaviruses: laboratory protocols. Berlin, Germany: Springer Science and Business Media, p: 454. Cavanagh, D., Elus, M.M. and Cook, J.K.A. 1997. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian. Path. 26(1), 63–74. Chen, H.W., Wang, C.H. and Cheng, I.C. 2011. A type-specific blocking ELISA for the detection of infectious bronchitis virus antibody. J. Virol. Methods. 173(1), 7–12. Chhabra, R., Forrester, A., Lemiere, S., Awad, F., Chantrey, J. and Ganapathy, K. 2015. Mucosal, cellular, and humoral immune responses induced by different live infectious bronchitis virus vaccination regimes and protection conferred against infectious bronchitis virus Q1 strain. Clin. Vaccine. Immunol. 22(9), 1050–1059. Cook, J.K., Jackwood, M. and Jones, R.C. 2012. The long view: 40 years of infectious bronchitis research. Avian. Path. 41(3), 239–250. Cook, J.K., Orbell, S.J., Woods, M.A. and Huggins, M.B. 1999. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian. Path. 28(5), 477–485. Davelaar, F.G. and Kouwenhoven, B. 1977. Influence of maternal antibodies on vaccination of chicks of different ages against infectious bronchitis. Avian. Path. 6(1), 41–50. Davelaar, F.G., Kouwenhoven, B. and Burger, A.G. 1984. Occurrence and significance of infectious bronchitis virus variant strains in egg and broiler production in the Netherlands. Vet. Quart. 6(3), 114–120. De Wit, J.J., Cook, J.K. and Van der Heijden, H.M. 2011. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian. Path. 40(3), 223–235. Echebli, N., Tchitchek, N., Dupuy, S., Bruel, T., Peireira Bittencourt Passaes, C., Bosquet, N., Le Grand, R., Bourgeois, C., Favier, B., Cheynier, R., Lambotte, O. and Vaslin, B. 2018. Stage-specifc IFN-induced and IFN gene expression reveal convergence of type I and type II IFN and highlight their role in both acute and chronic stage of pathogenic SIV infection. PLoS One 13, e0190334. Eid, A.A.M. 1998. Infectious bronchitis virus infection in Egypt. In The Proceedings of the International symposium on infectious bronchitis and pneumovirus infections in poultry. Eds., Kaleta, E.F. and Heffels-Redmann, U. Rauischholzhausen, Germany, pp: 145–156. Eid, A.A.M. 2004. The recent situation of IB disease in chickens in Egypt. In IV. International symposium of the 20-23 June 2004 on avian corona and pneumovirus infections, Rauischholzhausen. Gießen, Germany: VVB Laufersweiler Verlag, pp: 68–74. El-Mahdy, S.S., Salama, E. and Ahmed, A. 2012. Efficacy of some living classical and variant infectious bronchitis vaccines against local variant isolated from Egypt. Nat. Sci. 10, 292–299. Franzo, G., Legnardi, M., Tucciarone, C.M., Drigo, M., Martini, M. and Cecchinato, M. 2019. Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Vet. Res. 50(1), 1–9. Ghetas, A.M., Kutkat, M.A., Amer, M.M. and Awaad, M.H. 2016. Isolation and molecular identification of IBV isolates in different governorates in Egypt. J. Egypt. Soc. Parasitol. 240(3825), 1–6. Habibi, M., Karimi, V., Langeroudi, A.G., Ghafouri, S.A., Hashemzadeh, M., Farahani, R.K., Maghsoudloo, H., Abdollahi, H. and Seifouri, P. 2017. Combination of H120 and 1/96 avian infectious bronchitis virus vaccine strains protect chickens against challenge with IS/1494/06 (variant 2)-like infectious bronchitis virus. Acta. Virol. 61(2), 150–160. Huang, X., Liu, W., Zhang, J., Liu, Z., Wang, M., Wang, L., Zhou, H., Jiang, Y., Cui, W., Qiao, X., Xu, Y., Li, Y. and Tang, L. 2021. Very virulent infectious bursal disease virus-induced immune injury is involved in inflammation, apoptosis, and inflammatory cytokines imbalance in the bursa of fabricius. Dev. Comp. Immunol. 114, 103839. Huang, X., Xu, Y., Lin, Q., Guo, W., Zhao, D., Wang, C., Wang, L., Zhou, H., Jiang, Y., Cui, W., Qiao, X., Li, Y., Ma, G. and Tang, L. 2020. Determination of antiviral action of long non-coding RNA loc107051710 during infectious bursal disease virus infection due to enhancement of interferon production. Virulence 11(1), 68–79. Intervet International, B.V. 2003. Scientific discussion, live attenuated avian infectious bronchitis virus variant strain 4-91. Boxmeer, The Netherlands: EMEA, pp: 1–9. Available via https://www.ema.europa.eu/en/documents/scientific-discussion/nobilis-ib-4-91-epar-scientific-discussion_en.pdf Ismail, M.I., Tan, S.W., Hair-Bejo, M. and Omar, A.R. 2020. Evaluation of the antigen relatedness and efficacy of a single vaccination with different infectious bronchitis virus strains against a challenge with Malaysian variant and QX-like IBV strains. J. Vet. Sci. 21(6), e76. Itsui, Y., Sakamoto, N., Kurosaki, M., Kanazawa, N., Tanabe, Y., Koyama, T., Takeda, Y., Nakagawa, M., Kakinuma, S., Sekine, Y., Maekawa, S., Enomoto, N. and Watanabe, M. 2006. Expressional screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J. Viral. Hepat. 13(10), 690–700. Ivashkiv, L.B. 2018. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18(9), 545–558. Jackwood, M.W. 2012. Review of infectious bronchitis virus around the world. Avian. Dis. 56(4), 634–641. Jackwood, M.W., Clark, R., Cheng, S. and Jordan, B.J. 2020. Protection following simultaneous vaccination with three or four different attenuated live vaccine types against infectious bronchitis virus. Avian. Path. 49(4), 335–341. Jackwood, M.W. and De Wit, S. 2013. Infectious bronchitis. Diseases of poultry, pp: 139–159. Jackwood, M.W., Jordan, B.J., Roh, H.J., Hilt, D.A. and Williams, S.M. 2015. Evaluating protection against infectious bronchitis virus by clinical signs, ciliostasis, challenge virus detection, and histopathology. Avian. Dis. 59(3), 368–374. Jungherr, E.L., Chomiak, T.W. and Luginbuhl, R.E. 1956. Immunologic differences in strains of infectious bronchitis virus. In The Proceedings of the 60th Annual Meeting of the United States Livestock Sanitary Association, Chicago, IL, 1956, pp 203–209. Kameka, A.M., Haddadi, S., Kim, D.S., Cork, S.C. and Abdul-Careem, M.F. 2014. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology 450, 114–121. King, D.J. and Cavanagh, D. 1991. Infectious bronchitis. Diseases of poultry, vol. 9, pp: 471–484. Kint, J., Langereis, M.A., Maier, H.J., Britton, P., van Kuppeveld, F.J., Koumans, J., Wiegertjes, G.F. and Forlenza, M. 2016. Infectious bronchitis coronavirus limits interferon production by inducing a host shutoff that requires accessory protein 5b. Virol. J. 90(16), 7519–7528. Lee, H.J., Youn, H.N., Kwon, J.S., Lee, Y.J., Kim, J.H., Lee, J.B., Park, S.Y., Choi, I.S. and Song, C.S. 2010. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine 28(16), 2887–2894. Lin, S.Y. and Chen, H.W. 2017. Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. Int. J. Mol. Sci. 18(10), 2030. Ma, S., Qiao, X., Xu, Y., Wang, L., Zhou, H., Jiang, Y., Cui, W., Huang, X., Wang, X.,Tang, L. and Li, Y. 2019. Screening and identification of a chicken dendritic cell binding peptide by using a phage display library. Front. Immunol. 10, 1853. Madbouly, H., Abdel-Moneim, A.S., Gelb, J., Ladman, B.S. and Nancy, B.R. 2002. Molecular characterization of three Egyptian infectious bronchitis virus isolates. VMJ-G 50, 1053–1064. Mahmoud, A.M., ElBakrey, R.M., Mansour, S.M. and Eid, A.A. 2021. Avian Coronavirus in Egypt: epidemiological acuities and pathogenicity in commercial broiler chickens. Slov. Vet. Res. 58, 109–120. Mahmoud, A., Shahin, A. and Eid, A. 2019. The role of infectious bronchitis virus in respiratory and renal problems in broiler chickens. Zagazig. Vet. J. 47(1), 32–44. Matthijs, M.G.R., Bouma, A., Velkers, F.C., Van Eck, J.H.H. and Stegeman, J.A. 2008. Transmissibility of infectious bronchitis virus H120 vaccine strain among broilers under experimental conditions. Avian. Dis. 52(3), 461–466. Miłek, J. and Blicharz-Domańska, K. 2018. Coronaviruses in avian species–review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 62(3), 249. Moharam, I., Sultan, H., Hassan, K., Ibrahim, M., Shany, S., Shehata, A.A., Abo-ElKhair, M., Pfaff, F., Höper, D., ELKady, M., Beer, M., Harder, T., Hafez, H. and Grund, C. 2020. Emerging infectious bronchitis virus (IBV) in Egypt: evidence for an evolutionary advantage of a new S1 variant with a unique gene 3ab constellation. Infect. Genet. Evol. 85, 104433. Mondal, S.P. and Naqi, S.A. 2001. Maternal antibody to infectious bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Vet. Immunol. Immunopathol. 79, 31–40. Moreno, A., Franzo, G., Massi, P., Tosi, G., Blanco, A., Antilles, N., Biarnes, M., Majó, N., Nofrarías, M., Dolz, R., Lelli, D., Sozzi, E., Lavazza, A. and Cecchinato, M. 2017. A novel variant of the infectious bronchitis virus resulting from recombination events in Italy and Spain. Avian. Path. 46(1), 28–35. Naguib, M.M., El-Kady, M.F., Lüschow, D., Hassan, K.E., Arafa, A.S., El-Zanaty, A., Hassanb, M.K., Hafez, H.M., Grund, C. and Harder, T.C. 2017. New real time and conventional RT-PCRs for updated molecular diagnosis of infectious bronchitis virus infection (IBV) in chickens in Egypt associated with frequent co-infections with avian influenza and Newcastle Disease viruses. J. Virol. Methods. 245, 19–27. Najimudeen, S.M., Barboza-Solis, C., Ali, A., Buharideen, S.M., Isham, I.M., Hassan, M.S., Ojkic, D., Marle, G.V., Cork, S.C., der Meer, F.V., Boulianne, M. and Abdul-Careem, M.F. 2022. Pathogenesis and host responses in lungs and kidneys following Canadian 4/91 infectious bronchitis virus (IBV) infection in chickens. Virology 566, 75–88. Najimudeen, S.M., Hassan, M.S.H., Cork, S.C. and Abdul-Careem, M.F. 2020. Infectious bronchitis coronavirus infection in chickens: multiple system disease with immune suppression. Pathogens 9(10), 779. OIE (Terrestrial Manual). 2018. Avian infectious bronchitis (Chapter 2.3.2). In Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: OIE. Available via http://www.oie.int/standard-setting/ter-restrial-manual/access-online/ (Accessed 7 August 2021). Okino, C.H., Santos, I.L.D., Fernando, F.S., Alessi, A.C., Wang, X. and Montassier, H.J. (2014). Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral. Immunol. 27(8), 383–391. Pohuang, T., Tanasatian, S. and Sasipreeyajan, J. 2016. Efficacy of different vaccination programs of live 4/91 strain against Thai QX-like infectious bronchitis virus in broiler chickens. Thai. J. Vet. Med. 46(3), 419–425. Raggi, L.G. and Lee, G.G. 1965. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis vaccine. J. Immunol. 94(4), 538–543. Rogozin, I.B., Aravind, L. and Koonin, E.V. 2003. Differential action of natural selection on the N and C-terminal domains of 2′-5′ oligoadenylate synthetases and the potential nuclease function of the C-terminal domain. J. Mol. Biol. 326(5), 1449–1461. Sabra, M., Abdellatif, W., Ahmed, A.I. and Osman, N. 2020. Molecular characterization and phylogenetic analysis of full-length S1 gene of GI-16 and GI-23 infectious bronchitis virus in Qena, Egypt. J. World’s. Poult. Res. 10(1), 71–80. Sasipreeyajan, J., Pohuang, T. and Sirikobkul, N. 2012. Efficacy of different vaccination programs against Thai QX-like infectious bronchitis virus. Thai. J. Vet. Med. 42(1), 73–79. Schalk, A.F. and Hawn, M.C. 1931. An apparent new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 78, 413–423. Schmitz, N., Kurrer, M., Bachmann, M.F. and Kopf, M. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. Virol. J. 79(10), 6441–6448. Seo, S.H. and Collisson, E.W. 1997. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. Virol. J. 71(7), 5173–5177. Shirzad, M.R., Asasi, K. and Mohammadi, A. 2012. Efficacy of vaccination programmes using two commercial live infectious bronchitis vaccines against a field IRFIB 32 strain. Bulg. J. Vet. Med. 15(4), 260–272. Smialek, M., Tykalowski, B., Dziewulska, D., Stenzel, T. and Koncicki, A. 2017. Immunological aspects of the efficiency of protectotype vaccination strategy against chicken infectious bronchitis. BMC. Vet. Res. 13, 1–7. Sultan, H., Abdel-Razik, A.G., Shehata, A.A., Ibrahim, M. and Talaat, S. 2015. Characterization of infectious bronchitis viruses circulating in Egyptian chickens during 2012 and 2013. J. Vet. Sci. Med. Diagn. 4, 5. Sultan, H.A., Ali, A., El Feil, W.K., Bazid, A.H.I., Zain El-Abideen, M.A. and Kilany, W.H. 2019. Protective efficacy of different live attenuated infectious bronchitis virus vaccination regimes against challenge with IBV variant-2 circulating in the Middle East. Front. Vet. Sci. 6, 341. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques, 8th ed. Elsevier Health Sciences. Taha, M., Moustafa, M.M., Mohamed, S., Attia, S.A. and Hatem, M.E. 1991. Effect of both mycoplasma galliseplicum and infectious bronchitis virus classical and variant strains on the egg production of broiler breeder flocks. Vet. Med. 39, 169–179. Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. Terregino, C., Toffan, A., Serena Beato, M., De Nardi, R., Vascellari, M., Meini, A., Ortali, G., Mancin, M. and Capua, I. 2008. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian. Path. 37(5), 487–493. Thompson, G., Mohammed, H., Bauman, B. and Naqi, S. 1997. Systemic and local antibody responses to infectious bronchitis virus in chickens inoculated with infectious bursal disease virus and control chickens. Avian. Dis. 41, 519–527. Toro, H. and Fernandez, I. 1994. Avian infectious bronchitis: specific lachrymal IgA level and resistance against challenge. J. Vet. Med. 41, 467–472. Valastro, V., Holmes, E.C., Britton, P., Fusaro, A., Jackwood, M.W., Cattoli, G. and Monne, I. 2016. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect. Genet. Evol. 39, 349–364. Villanueva, A.I., Kulkarni, R.R. and Sharif, S. 2011. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev. Comp. Immunol. 35(1), 28–34. Watashi, K., Liang, G., Iwamoto, M., Marusawa, H., Uchida, N., Daito, T., Kitamura, K., Muramatsu, M., Ohashi, H., Kiyohara, T., Suzuki, R., Li, J., Tong, S., Tanaka, Y., Murata, K., Aizaki, H. and Wakita, T. 2013. Interleukin-1 and tumor necrosis factor-α trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J. Biol. Chem. 288(44), 31715–31727. Wibowo, M.H., Ginting, T.E. and Asmara, W. 2019. Molecular characterization of pathogenic 4/91-like and QX-like infectious bronchitis virus infecting commercial poultry farms in Indonesia. Vet. World. 12(2), 277. Xu, C., Zhao, J., Hu, X. and Zhang, G. 2007. Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene. Vet. Microbiol. 122, 61–71. Yan, S., Liu, X., Zhao, J., Xu, G., Zhao, Y. and Zhang, G. 2017. Analysis of antigenicity and pathogenicity reveals major differences among QX-like infectious bronchitis viruses and other serotypes. Vet. Microbiol. 203, 167–173. Yang, C.Y., Peng, P., Liu, X., Cao, Y. and Zhang, Y. 2023. Effect of monovalent and bivalent live attenuated vaccines against QX-like IBV infection in young chickens. Poult. Sci. 102(4), 102501. Yu, L., Jiang, Y., Low, S., Wang, Z., Nam, S.J., Liu, W. and Kwang, J. 2001. Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian. Dis. 45, 416–424. Zanaty, A., Arafa, A.S., Hagag, N. and El-Kady, M. 2016. Genotyping and pathotyping of diversified strains of infectious bronchitis viruses circulating in Egypt. World. J. Virol. 5(3), 125. Zhang, T., Li, D., Jia, Z., Chang, J. and Hou, X. 2017. Cellular immune response in chickens infected with avian infectious bronchitis virus (IBV). Eur. J. Inflamm. 15(1), 35–41. Zhang, Y., Xu, Z. and Cao, Y. 2021. Host antiviral responses against avian infectious bronchitis virus (IBV): focus on innate immunity. Viruses 13(9), 1698. Zhao, Y., Cheng, J.L., Liu, X.Y., Zhao, J., Hu, Y.X. and Zhang, G.Z. 2015. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 180, 49–58. | ||
| How to Cite this Article |
| Pubmed Style Eid AA, Mahmoud AM, Hamouda EE, Metwally MA, Ezz-eldin RM, Elbakrey RM. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 90-107. doi:10.5455/OVJ.2024.v14.i1.9 Web Style Eid AA, Mahmoud AM, Hamouda EE, Metwally MA, Ezz-eldin RM, Elbakrey RM. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. https://www.openveterinaryjournal.com/?mno=175112 [Access: January 11, 2026]. doi:10.5455/OVJ.2024.v14.i1.9 AMA (American Medical Association) Style Eid AA, Mahmoud AM, Hamouda EE, Metwally MA, Ezz-eldin RM, Elbakrey RM. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 90-107. doi:10.5455/OVJ.2024.v14.i1.9 Vancouver/ICMJE Style Eid AA, Mahmoud AM, Hamouda EE, Metwally MA, Ezz-eldin RM, Elbakrey RM. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J.. (2024), [cited January 11, 2026]; 14((1) (Zagazig Veterinary Conference)): 90-107. doi:10.5455/OVJ.2024.v14.i1.9 Harvard Style Eid, A. A., Mahmoud, . A. M., Hamouda, . E. E., Metwally, . M. A., Ezz-eldin, . R. M. & Elbakrey, . R. M. (2024) The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 90-107. doi:10.5455/OVJ.2024.v14.i1.9 Turabian Style Eid, Amal A.m., Ali M. Mahmoud, Esraa E. Hamouda, Mohamed A. Metwally, Rasha M.m. Ezz-eldin, and Reham M. Elbakrey. 2024. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 90-107. doi:10.5455/OVJ.2024.v14.i1.9 Chicago Style Eid, Amal A.m., Ali M. Mahmoud, Esraa E. Hamouda, Mohamed A. Metwally, Rasha M.m. Ezz-eldin, and Reham M. Elbakrey. "The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus." Open Veterinary Journal 14 (2024), 90-107. doi:10.5455/OVJ.2024.v14.i1.9 MLA (The Modern Language Association) Style Eid, Amal A.m., Ali M. Mahmoud, Esraa E. Hamouda, Mohamed A. Metwally, Rasha M.m. Ezz-eldin, and Reham M. Elbakrey. "The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 90-107. Print. doi:10.5455/OVJ.2024.v14.i1.9 APA (American Psychological Association) Style Eid, A. A., Mahmoud, . A. M., Hamouda, . E. E., Metwally, . M. A., Ezz-eldin, . R. M. & Elbakrey, . R. M. (2024) The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 90-107. doi:10.5455/OVJ.2024.v14.i1.9 |