| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 324–334 Original Research The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parametersAhmed A.M. Abdel Aziz1, El-Sayed A. Abdel Aziz1, Mohamed H. Khairy1, Charbel Fadel2, Mario Giorgi3 and Ahmed S. Abdelaziz1*1Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Veterinary Medicine, University of Sassari, Sassari, Italy 3Department of Veterinary Sciences, University of Pisa, Pisa, Italy *Corresponding Author: Ahmed S. Abdelaziz. Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: asabdelaziz [at] vet.zu.edu.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
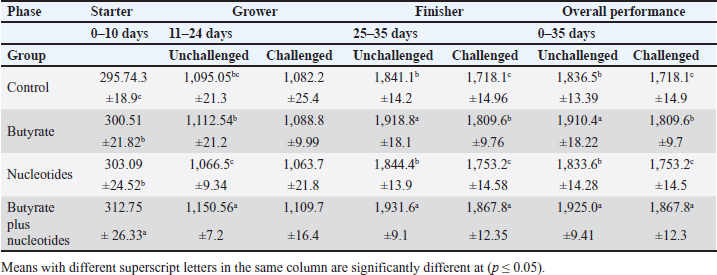
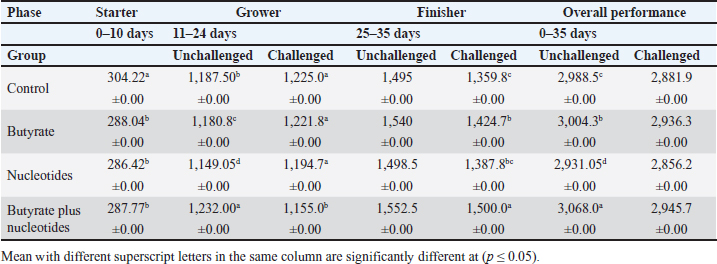
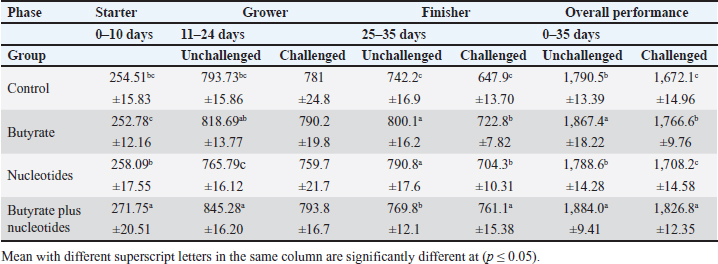
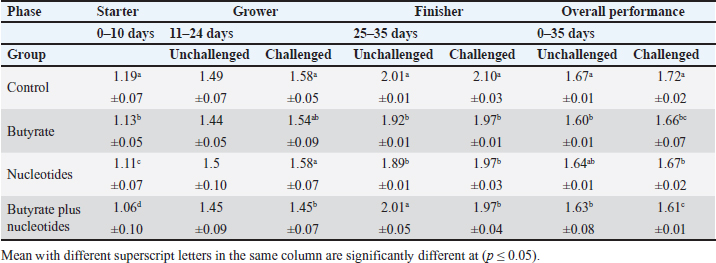
ABSTRACTBackground: Butyric acid and its derivatives support the immune system, lessen inflammation, and lessen oxidative stress in broilers in addition to preserving gut homeostasis and epithelial integrity. Broiler performance has also been demonstrated to rise with the addition of nucleotides to the diet. Aim: The purpose of the study was to ascertain the effects of butyric acid and nucleotides added to feed on the overall performance, immunity, oxidant/antioxidant enzyme levels, intestinal histology, and hepatic functions of broilers. Methods: Four experimental groups of thirty chickens, each were used in the present study. The groups were assigned as a control group that received normal diet without additives, butyrate (B) group received the diet supplemented with butyric acid (250 g/ton feed), nucleotides (N) group received the diet supplemented with nucleotides (200 g/ton feed), and the fourth group received the diet supplemented with a combination of butyrate and nucleotide (BN) (250 g/ton B feed, and 200 g/ton N feed, respectively). Necrotic enteritis was produced in ten birds from each group to assess the immune-modulatory effect of these supplements, antioxidant status, intestinal histology, and liver functions were measured in all experimental groups. Results: The addition of butyric acid and nucleotides to feed enhanced body weight, growth performance, hepatic functions, and antioxidant capabilities. Histological sections of the gut from challenged or unchallenged (with necrotic enteritis) groups in the BN group showed considerable improvement, as shown by strong proliferation in intestinal crypts and villus enterocytes. Conclusion: Nucleotides and butyric acid can be added to broiler feeding regimens to enhance growth and health. Keywords: Nucleotides, Growth performance, Immunity, Butyric acid, Broiler nutrition. IntroductionAnimal welfare and health must be considered to effectively rear commercial poultry in today’s environments. A comprehensive plan that shields the bird from dangerous infections, particularly foodborne ones, must then be put into place. One part of a complex and well-coordinated strategy to create a healthy bird that is also a safe and wholesome product for consumers is immunomodulation (Swaggerty et al., 2019). Over the past 10 years, Egyptian poultry farming has changed from a traditional agricultural practice to an intensive industrial one. Egypt is producing more broiler chicken meat as a result of rising customer demand for reasonably priced animal protein. Simultaneously, a dearth of well-defined approaches (zootechnical, biosecurity, and so on) for agricultural development has led to a host of problems, including outbreaks of avian illness and ineffective production methods (Shatokhin et al., 2017). The greatest substitutes for antibiotic growth promoters (AGPs) may be probiotics and organic acids (Castanon, 2007; Carvalho and Santos, 2016). The benefits of probiotics include altered host metabolism, immunostimulant properties, pathogen exclusion inhibition, improved nutrient absorption, and a reduction in the risk to human health. Because they can create short-chain fatty acids inside the gastrointestinal tract, probiotics and prebiotics may provide an additional mechanism of action that could boost poultry production. Herbs, organic acids, vitamins, and minerals may be more helpful than AGPs in reducing pathogen load and preventing heat stress. Nucleotides, ractopamine, betaine, and carnitine may be utilized to produce meat of higher quality (Kuldeep et al., 2014). In poultry, organic acids are used to reduce the pH of the intestinal tract, which supports beneficial bacteria and suppresses pathogenic bacteria, eliminating the need for antibiotics (Hassan et al., 2010). They are added to drinking water and chicken meals to stimulate a favorable growth response that enhances avian immunity, performance, and nutrient digestibility. About 3,000 parts per million of butyrate can be used to make butyrate glycerides, which can improve intestinal health and alter an animal’s immunological response and energy expenditure. The food intervention changed the composition of the gut microbiota but had no effect on the alpha diversity. The dietary treatment had a particularly large impact on Bifidobacterium, which showed an increase in both species diversity and abundance (Yang et al., 2018). The current study’s goal was to assess how well butyric acid and nucleotides may enhance the immune system, intestinal histology, liver function, and general performance of chickens. Materials and MethodsOrganic compounds as feed additivesIn this investigation, butyric acid was supplied as a commercial powder product (Prophorce® SR 130; Perstorp, Sweden). It is made up of 38% silicic acid and 62% butyric acid triglycerides. In accordance with the manufacturer’s recommendations, 250 g of it was added to each ton of broiler feed. Regarding the nucleotides, this study used a cream-colored powder called Nucleoforce® (Bioiberica, Spain). In accordance with the manufacturer’s recommendations, 200 g of it was added to each ton of broiler feed. The supplements were completely mixed in with the feed using a professional micro-mixer feed mill. Experimental designOne hundred twenty unsexed (as hatched) broiler chicks (Ross 388), aged one day that appeared to be in good health were acquired from Al-Abrar Company in Cairo, Egypt. They were given commercial diets that were balanced and devoid of chemicals and prescription drugs. They had unlimited access to feed and water. The chicks were housed in sanitary circumstances. The birds were split into four groups of thirty birds each: a control group that received a normal diet without additives, a group supplemented with butyrate (B) received the diet supplemented with butyric acid (250 g/ton feed), a group supplemented with nucleotides (N) received the diet supplemented with nucleotides (200 g/ton feed), and a group supplemented with both butyrate and nucleotides (BN) (250 g/ton B feed, and 200 g/ton N feed, respectively). Within the trial housing, each group was separated into distinct groups of hens; this also applied to the ensuing subgroups for challenged and unchallenged birds. ImmunizationSeven-day-old chicks were immunized with the Newcastle disease virus vaccine (Merck & Co. Inc., Netherlands) using eye drops. Day 9 saw the administration of the infectious bursal disease vaccine (Merck & Co. Inc., Netherlands). Weighing and assessing the growth rateEvery chick was weighed before the experiment started. After that, the chicks were weighed at 8 a.m. every week until the trial was over. Individual live body weights were added together and divided by the total number of birds in each group to determine the average live body weight per week. The body weight difference between two consecutive weights was subtracted to get the body weight gain per meal phase. In addition, the number of birds in each group was divided by the sum of the individual body weight gains to get the average body weight gain per week. The daily average body weight was calculated by dividing the weekly average weight gain by seven. Feeding consumptionAt the beginning of each day, a set amount of feed was given to each group. To find the overall amount of feed consumed, the leftover feed was weighed at the end of the day and subtracted from the applied amount. The total daily food intake was divided by the number of birds in each group to determine the average feed consumption per bird per group. Finally, the average daily feed consumption was added to determine the average weekly feed consumption per bird. In accordance with Wanger et al. (1983), the feed conversion ratio (FCR) was computed Process for challenging necrotic enteritisDuring the growth phase (11–24 days), necrotic enteritis was created, which is why each treatment was split into two sub-groups at that time: the challenged group (n=10) and the unchallenged group (n=20). As stated by Shojadoost et al. (2012), there were three steps involved in introducing the necrotic enteritis challenge: A. On day 11 of life, administer the coccidial vaccine (Coccivav B; Merck & Co., Inc., Netherlands) via eye drops at 10 times the indicated dose. B. On day 14 of life, the crop is inoculated with 3 ml of Clostridium perfringens broth (NetB toxin), which is received from the Animal Health Research Institute in Cairo, Egypt, twice a day for 3 days in a row. There were 108 colony-forming units per milliliter (ml). C: According to Keyburn et al. (2006), a necropsy was performed on three birds from each treatment group on the 18th day of life. The small intestine was examined for gross pathological lesions using a six-point scoring system. Analysis of blood and assessment of biochemical and antioxidant/oxidant parametersThe broiler chicks were intraperitoneally given 1.5 g/kg of urethane to gently put them to sleep. Following that, on days 3, 7, 14, and 28, blood and liver samples were taken from randomly chosen birds in different pens within each treatment group. Following that, blood samples were quickly centrifuged at 4,000 g for 15 minutes. Until testing, the serum was stored frozen at −20°C. Alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were measured using the colorimetric method to evaluate the liver function enzymes (Thomas, 1998). The same methodology as described by Doumas et al. (1971) was also used to measure the levels of total proteins (TPs) and albumin. Serum globulins were computed by deducting the measured levels of albumin from the levels of TPs. In addition, the levels of uric acid and creatinine were measured (in the supplementary material). We assessed the serum levels of IgM and IgY in accordance with Morrill et al. (2012). Regarding the serum levels of oxidant/antioxidant parameters, the method outlined by Sotoh (1978) was used to estimate the levels of malondialdehyde (MDA), and the method outlined by Paglia and Valentine (1967) was used to estimate the levels of glutathione (GSH), glutathione peroxidase (GPX), and catalase (CAT). A histopathological analysisBefore being routinely processed in paraffin wax, small intestines were collected and preserved for 24 hours in 10% neutral formalin (Al-Nasr, Cairo, Egypt). Samples were divided into pieces using a thickness of roughly five microns using the Banchroft et al. (1996) method. As a result, materials were examined under a microscope after being stained with hematoxylin and eosin. Statistics and data analysisThe computerized SPSS Statistics (21.0) was used to examine the data. The findings were presented as mean ± standard error of the mean. ANOVA or one-way analysis of variance was used to assess the overall variation. The significance was verified using the Duncan test. Significant values of probability were defined as p < 0.05. Ethical approvalZagazig University Ethical Committee accepted the procedure that the study’s animals were to follow (Approval number: ZU-IACUC/2/F/106/2022). ResultsEffects of butyric acid and nucleotides on broilers performanceThe broiler performance was evaluated using four factors: live weight, feed intake, weight gain, and feed conversion rate. The evaluation of measurements was based on three factors: overall performance, the necrotic challenge procedure, and the production stages (starter, grower, and finisher). According to Tables 1–4, the BN group outperformed the other groups significantly in terms of live weight, weight gain, and FCR. In terms of feed efficiency, group N was ranked second best. In comparison to the control group, group B’s live weight and FCR were much higher, but lower than the N and BN groups (p < 0.05). Table 1. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on live body weight of broiler chickens given daily for 5 weeks.
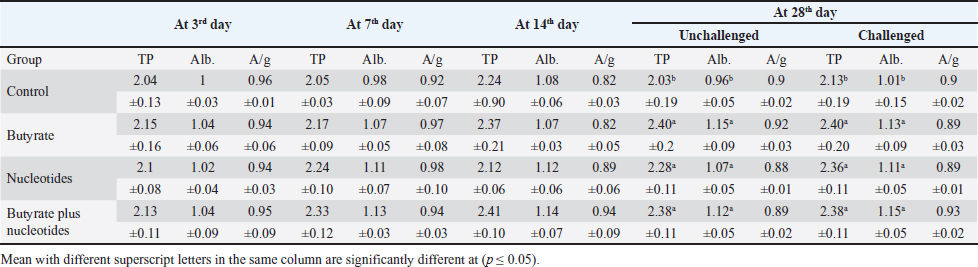
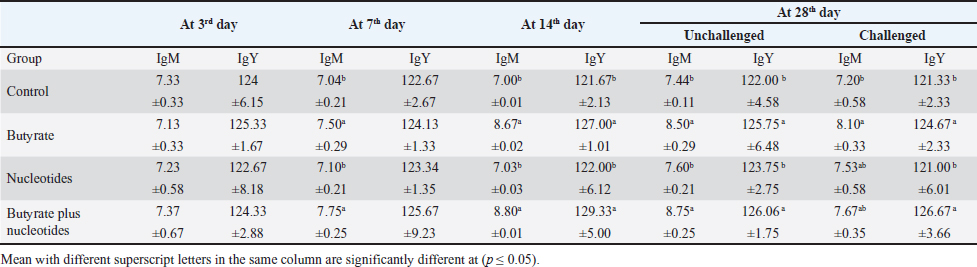
Butyric acid and nucleotides effects on broiler serum parametersAt 3, 7, 14, and 28 days, there were no appreciable differences in the serum activity of ALP, ALT, and AST between the different groups (Data are not shown). When comparing the groups at 3 days of age, there was no discernible difference in TP, albumin, albumin/globulin ratio (Table 5), IgM, or IgY (Table 6). It was evident at 7 days old, groups B and BN had significantly higher levels of IgM. Compared to the control and N groups, the B and BN groups had significantly higher levels of IgM and IgY at 14 days of life. Table 5 demonstrates that at 28 days of age, the B, N, and BN groups’ TP and albumin concentrations significantly increased in comparison to the control group, whether or not they were challenged. However, Table 6 shows that only the B and BN groups showed a statistically significant rise in IgM, and IgY concentrations when compared to the control group. Table 2. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on feed intake of broiler chickens given daily for 5 weeks.
Table 3. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on weight gain of broiler chickens given daily for 5 weeks.
Table 4. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on FCR of broiler chickens given daily for 5 weeks.
Table 5. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on TP (g/dl), albumin (Alb.) (g/dl) and albumin/globulin ratio A/g of broiler chickens given daily for 5 weeks.
Table 6. Effect of butyric acid 250 g/ton, nucleotides 200 g/ton, and their combination on IgM (mg/dl) and IgY (mg/dl) of broiler chickens given daily for 5 weeks.
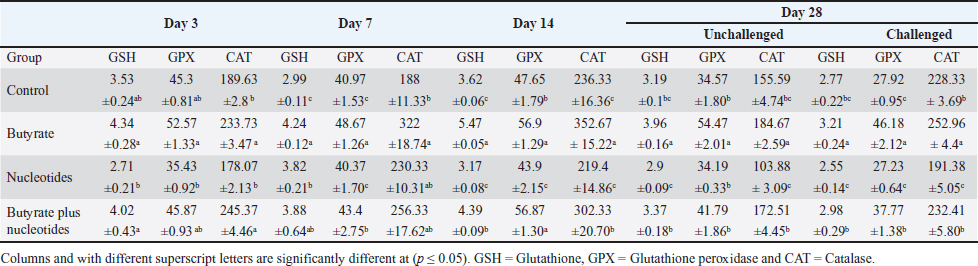
Table 7. Effect of butyric acid at 250 g/ton, nucleotides at 200 g/ton in feed on GSH (mmol/ml), GPX (U/l), and CAT (U/l) in broiler chickens.
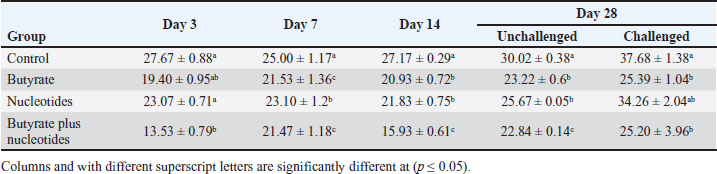
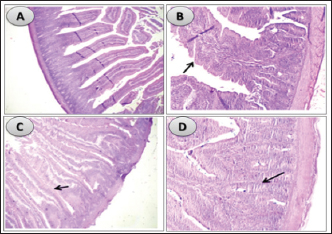
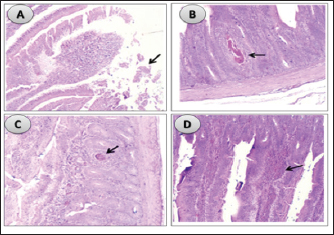
Influence of nucleotides and butyric acid on broiler serum antioxidant parametersTable 7 illustrates how the current research revealed that, at 3 days of age, the CAT level was considerably higher in the B and BN groups than in the control group. When it came to MDA in Table 8, the BN group outperformed the control and N groups by a significant margin. Furthermore, it seems that at that age, nucleotides had no effect on these characteristics. Table 7 shows that after 7 days, the BN and N groups performed better in antioxidant measures than both the control and N groups (p < 0.05). Regarding the 14-day broilers, the findings indicated that the B group had the highest quantities of GSH, GPX, and CAT. In terms of MDA activity, the BN group fared the best (lowest value, p < 0.05, Table 8). When compared to the control group, adding nucleotides or butyrate alone to the meal resulted in a statistically significant decrease in MDA after 28 days in the unchallenged groups. However, compared to the other groups, the BN group’s MDA concentrations were significantly decreased. Butyric acid and nucleotides’ histopathological effects in broiler intestinesAs can be seen in Figures 1 and 2, all of the intestinal sections in 1A showed normal morphology in the control, unchallenged group. The majority of the intestinal villi in 1C and 1B, the unchallenged B and N groups, respectively, displayed minor groups of proliferation in their epithelium, whereas 1D, the BN group, showed intense proliferation. In comparison to the challenged groups, necrotic enterocytes were seen in the control group. Hemorrhage within the intestinal crypts was observed in the group B (Fig. 2B). The gut of the challenged N group is depicted in 2C, where there is severe infiltration of inflammatory cells in the mucosa and submucosa, along with mixed bleeding. The colon had wide villi surrounded by proliferative enterocytes and leukocytic infiltration in the lamina propria and submucosa in the 2D group, BN challenged group. DiscussionThe stomach and intestine are regarded as key organs that digestive health and host defense depend on. The dynamic balance of the gut environment is necessary for the preservation of a functional and healthy gut. Numerous factors related to diets, infectious disease agents, and stocking density may have an adverse effect on this balance, endangering the health and growth of broiler chickens (Gomes et al., 2014). Since AGP use in chicken has been reduced or completely stopped, the prevalence of intestinal illnesses such as C. difficile-caused necrotic enteritis has increased. The poultry industry is looking for alternate ways to improve bird health as a result of the rise in enteric diseases (Teirlynck et al., 2011). Table 8. Effect of butyric acid at 250 g/ton, nucleotides at 200 g/ton in feed and necrotic enteritis challenge on MDA (nmol/l) in broiler chickens.
Fig. 1. Histological sections of the intestine segments in unchallenged birds, ×100. (A) in unchallenged control group showing normal intestinal coats. (B) in unchallenged butyrate group showing mixed hyperplastic villus epithelium (arrow). (C) in unchallenged nucleotides group showing thickened villi from proliferative enterocytes and intestinal crypts (arrow). (D) in unchallenged BNs group showing intensive proliferation in intestinal crypts and villus enterocytes (arrow).
Fig. 2. Histological sections of the intestine segments in challenged birds, ×100. (A) in the control group, showing necrotic villus tips, duodenal villi (arrow) beside coccidial stages of vaccine (arrow) within necrotic enterocytes. (B) in challenged butyrate group showing hemorrhage within the intestinal crypts (arrow). (C) in challenged nucleotides group showing mixed hemorrhage (arrow) and intense inflammatory cells in sub-mucosa and mucosa. (D) in challenged BNs group showing broad villi lined by proliferative enterocytes and leukocytic infiltration (arrow) in lamina propria and submucosa. It has previously been determined that butyric acid is important for the development of the intestinal epithelium in fowl. The intestinal villi epithelium has demonstrated modest proliferation in the current investigation, which is consistent with earlier research. In addition, it works well against bacteria that produce acid, such as Salmonella spp., C. perfringens, and Escherichia coli, as gastrointestinal tract pH has been observed to be lowered by 0.6% butyric acid (Panda et al., 2009). Because of these factors, butyric acid might be a good substitute for preserving the health of the gastrointestinal system and raising chicken productivity. In fact, the immunity appeared to be much improved compared with the control group. This was shown by the presence of proliferative enterocytes with extravasated erythrocytes and lymphocyte infiltration in the mucosa, in addition to the bleeding and necrotic debris in some segments. In Japanese quails, Sikandar et al. (2017) showed that on day 21, the sodium butyrate groups had more goblet cells with acidic mucins and larger villus lengths and diameters than the control groups. By day 35, there was a noticeable rise in the villus surface area and height in the jejunum and duodenum. When butyric acid was used for the whole production cycle in Salmanzadeh (2013), the crypts appeared to be very deep. Similar to the results of the current investigation, supplemented butyrate had no effect on chicken liver enzymes, specifically AST and ALT, according to Adil et al. (2010). Likely, Elnesr et al. (2019) found that butyrate had no effect on AST or ALT in Japanese quails at 21 days of age, with a decrease in their values by day 24. These organic acids support liver function by taking part in redox activities, improving vessel wall flexibility and decreasing permeability, and forming readily soluble compounds with cholesterol for simpler removal (Kvan et al., 2019). For example, compared to the control group, Lan et al. (2020) found a significant decrease in AST and ALT levels in heat-stressed birds following butyrate administration. The results of the present study showed that butyrate supplementation significantly increased the antioxidant indices MDA, GHS, GPX, and CAT in 3-day-old birds. On day 14, it performed better than the other groups in terms of metrics, especially for MDA, the lipid peroxidation indicator, which is higher in all age groups. It is in fact known that butyric acid regulates a number of crucial regulatory enzyme functions that are engaged in numerous metabolic pathways (Jacobbson et al., 1985; Janet et al., 1998). Thus, butyric acid is crucial for the control of antioxidant enzymes (Kumar et al., 2010). Liu et al. (2021) provided an explanation for the butyrate effect on GPX activity, stating that it does so via raising glutamate levels (glutamate is thought to be the precursor for GSH synthesis in the anti-oxidation system). According to Ali et al. (2010), strong antioxidant enzyme activity lowers lipid peroxidation, which in turn lowers MDA levels. According to Deepa et al. (2018), including various forms of butyric acid in the diet raised superoxide dismutase (SOD) activity and lowered MDAs, suggesting improved free radical scavenging ability and lessened tissue or cell damage. One of the most important defensive mechanisms against oxidative stress is CAT, which is also observed to be enhanced as a result of the presence of different types of butyric acid. When supplementing with sodium butyrate and gamma-amino butyric acid, respectively, Zhang et al. (2011) (after heat stress) and Al Wakeel et al. (2017) (healthy circumstances) similarly confirm similar effects. Furthermore, a number of investigations have demonstrated that sodium butyrate might mitigate the adverse consequences linked to oxidative stress generated by corticosterone (Zhang et al., 2011; Chen et al., 2013). In terms of overall performance, both group B and group N outperformed the control group significantly. That finding was most apparent during the initial stages of the experiment, but it progressively diminished as the production cycle came to an end and after the necrotic enteritis challenge. In fact, it is believed that providing nucleotides to young birds has more benefits (Chiofalo et al., 2011). As demonstrated, the enlarged villi (proliferative enterocytes, and hence potentially a better absorption rate) can be translated into or partially justify this increased performance. In fact, Rutz et al. (2007) found that dietary pyrimidine is necessary for the synthesis of rRNA in the jejunum’s crypts. The study’s results were consistent with those of earlier investigations. According to Daneshmand et al. (2017), live body weight and average daily increase were two performance metrics that were enhanced by adenosine, uridine, and cytidine together. Wu et al.’s (2018) research supported the improved intestinal morphological picture provided by dietary nucleotide fortification. Specifically, pathogen-free chickens fed the base diet supplemented with 0.1% yeast nucleotides demonstrated significantly increased ileal villus height and villus height to crypt depth ratio. Furthermore, broiler hens receiving dietary nucleotide supplementation at 0.05% and 0.1% exhibited a significant decrease in crypt depth and an increase in duodenal villus height. Dietary nucleotide supplementation was found to increase mucosal weight and trigger enterocyte differentiation in both the Carver and Walker (1995) and Sanderson and He (1994) experiments. Furthermore, Groenewegen (2010) and Jung and Batal (2012) noted that supplementing young broiler chicks’ meals with nucleotides resulted in improvements in intestinal architecture, as seen by the extended villi in the small intestine. In addition, broilers with supplements at 0.03% (Navneet et al., 2017), 0.025% (Adil et al., 2010), and 1.5% (Trairatapiwan et al., 2017) showed improvements in growth performance. The effects of the different nucleotide supplementation concentrations on the result, however, were not compared. Daneshmand et al. (2017) observed that nucleotide supplementation raised IgA concentrations in birds at 11 and 21 days of age but had no effect on IgG concentrations in jejunal samples. Our results regarding the effect of nucleotides on immunoglobulins also aligned with their findings. In fact, it was seen that the intestinal mucosa and submucosa had an infiltration of inflammatory cells in contrast to the challenged control group. Furthermore, our findings corroborated those of Sauer et al. (2012), who found that freshly weaned supplemented piglets had plasma IgA values that were considerably greater than those of a control group, with no treatment changes in plasma IgG and IgM. Increased plasma IgA concentrations suggested that humoral immunity was supported by including nucleotides in the weaning diet (Trairatapiwan et al., 2017). MDA levels in group N in the present study dramatically decreased. On the other hand, as shown by Frankič et al. (2006), nucleotide supplementation at a dose of 10 mg/kg in poultry did not affect MDA or GPX levels. In seabream (Sparus aurata) fingerlings, nucleotides given at 250 or 500 mg/kg of fish meal significantly elevated SOD and CAT, according to El-Nokrashy et al. (2021). However, a significant increase in GSH and a decrease in MDA concentrations were observed with a mere 500 mg/kg of nucleotide supplementation. Reda et al. (2018) found that whereas GPX significantly increased in all dietary nucleotide-supplemented groups, the addition of 0.25% nucleotides significantly boosted SOD and MDA activities in the serum. Although it is uncertain if supplements containing nucleotides have anti-oxidative characteristics per se, prior research has shown that added nucleotides may have an antioxidant impact at higher dosages than those previously utilized (Bacha et al., 2013). Since, as was previously indicated, there was no thorough comparison of the various added concentrations of nucleotides on the effect, more research is needed to support this. Nucleotides might be working by promoting the synthesis of mRNA, which is necessary later on for the production of the enzymes that combat oxidative stress. TP, albumin, and albumin/globulin ratio values in all three supplemented groups were considerably greater than in the control group. Butyric acid regulates mitochondrial gene expression and affects the body’s metabolic activity by blocking histone deacetylase or activating G-protein-coupled receptors 41 and 43 (Dangond and Gullans, 1998). This enhances the liver’s capacity to anabolize plasma proteins, such as albumin and globulins (Mollica et al., 2017). Furthermore, nucleotides will naturally increase the liver’s metabolic activity because they are co-enzymes, vital participants in energy-transfer activities, and precursors for the production of nucleic acids (Sauer et al., 2009). As far as we know, no previous research has combined butyric acid with nucleotides in chickens. That combination would enhance the bird’s function by working in concert to affect the liver, enterocyte proliferation, gut, and systemic immune response. In support of this assumption, the challenged BN group’s villi, which were lined by proliferative enterocytes and leukocytic infiltration in the lamina propria and submucosa, did not exhibit any hemorrhage, necrotic debris, edema, or partial hyaline degeneration (in the muscular coat), in contrast to the challenged groups B and N. Rather than representing further necrotic enteritis damage, this is an indication of an enhanced host immune response. Moreover, an increased humoral immune response was observed, with significantly greater immunoglobulin values compared to the control group, albeit primarily attributable to butyric acid. Second, during all growth periods and with or without challenges, the poultry’s overall performance was the best for this group. Furthermore, MDA values were considerably lower in the BN group, despite the fact that the serum antioxidant parameters were equal in the B and BN groups. The results show that supplementing with butyric acid and nucleotides together seems to be more advantageous than supplementing with either one of them alone, with butyrate having the largest impact. According to Lee et al. (2007), feeding organic acids and nucleotides as dietary supplements to weaned pigs exhibited an immune-modulatory effect as well as a synergistic effect on the proliferation of mesenteric lymph node lymphocytes and Peyer’s patches. Peyer’s patches are the principal immunological sensors of the intestine because of their ability to create particular types of immunoglobulins and convey luminal antigens and germs, among many other things. While we did assess the immune-modulatory effect, we regrettably did not assess these tissues in our study. Nevertheless, more research is always preferred to give more justifications. ConclusionGrowth performance, antioxidant activity, and intestinal villi proliferation were all improved by adding butyric acid and nucleotides to the meal. It also had an immune-modulatory effect, as seen by the increased immunoglobulin levels and the immune cells’ infiltration to the inflamed site without causing additional damage to the intestinal lining. This combination may be proposed for broiler feeding regimens to enhance health and growth performance. AcknowledgmentThe authors would like to thank all people who supported the experimental work. Conflict of interestNo conflict of interest is to be declared. FundingNot applicable. Data availabilityAll data will be made available upon reasonable request. Authors’ contributionsAhmed Abdel Aziz and Ahmed S. Abdelaziz did the experimental work, data analysis, drafted the manuscript and supported the publishing of the manuscript. El-Sayed Abdel Aziz, and Mohamed Khairy proposed the research theme, supervised the work, and revised the final manuscript. Charbel Fadel, Mario Giorgi performed data analysis and critically revised the manuscript. ReferencesAdil, S., Banday, T., Bhat, G.A., Mir, M.S. and Rehman, M. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 479485. Ali, M., Qota, E. and Hassan, R. 2010. Recovery from adverse effects of heat stress on slow-growing chicks using natural antioxidants without or with sulphate. Int. J. Poult. Sci. 9(2), 109–117. Al Wakeel, R.A., Shukry, M., Abdel Azeez, A., Mahmoud, S. and Saad, M.F. 2017. Alleviation by gamma amino butyric acid supplementation of chronic heat stress-induced degenerative changes in jejunum in commercial broiler chickens. Stress 20(6), 562–572. Bacha, U., Nasir, M., Ali, M.A., Muhammad, J. and Sheikh, A.A. 2013. Nucleotides supplementation improves various function of the body. J. Anim. Health. Prod. 1(1), 1–5. Banchroft, J., Stevens, A. and Turner, D. 1996. Theory and practice of histological techniques. London, UK: Churchil Livingstone, pp: 129–134. Carvalho, I.T. and Santos, L. 2016. Antibiotics in the aquatic environments: a review of the European scenario. Environ. Int. 94, 736–757. Carver, J.D. and Walker, V.A. 1995. The role of nucleotides in human nutrition. J. Nutr. Biochem. 6, 58–72. Castanon, J.I.R. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86, 2466–2471. Chen, Z., Tang, J., Sun, Y. and Xie, J. 2013. Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J. Anim. Plant. Sci. 23(23), 1634–1641. Cherrington, C.A., Hinton, M. and Mead, G.C. 1991. Organic acids: chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 32, 87–108. Doumas, B.T., Watson, W.A. and Biggs, H.G. 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31(1), 87–96. Elnesr, S., Ropy, A. and Abdel-Razik, A. 2019. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Animal 13(6), 1234–1244. El-Nokrashy, A., El-Banna, R., Edrise, B., Abdel-Rahim, M., Jover-Cerdá, M. and Goda, A.S. 2021. Impact of nucleotide enriched diets on the production of gilthead sea bream, Sparus aurata fingerlings by modulation of liver mitochondrial enzyme activity, antioxidant status, immune gene expression, and gut microbial ecology. Aquaculture 535, 736398. Frankič, T., Pajk, T., Rezar, V., Levart, A. and Salobir, J. 2006. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food. Chem. Toxicol. 44(11), 1838–1844. Gomes, A., Quinteiro-Filho, W., Ribeiro, A., Ferraz-de-Paula, V., Pinheiro, M. and Palermo-Neto, J. 2014. Overcrowding stress decreases macrophage activity and increases Salmonella enteritidis invasion in broiler chickens. Avian. Pathol. 43, 82–90. Groenewegen, P. 2010. Seven is the magic number. Poult. World. 25(8), 18–20. Available via https://www.poultryworld.net/poultry/seven-is-the-magic-number/ (Accessed 1 October 2023). Hassan, H.M.A., Mohamed, M.A., Youssef, A.W. and Hassan, E.R. 2010. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal micro-flora of broilers. Asian-Austral. J. Anim. Sci. 23, 1348–1353. Jacobbson, K.G., Riesenfeld, J. and Lindaw, V. 1985. Biosynthesis of heparin: effects of n-butyrate on cultured mast cells. J. Biol. Chem. 260, 12154–12159. Janet, G.S., Wallace, H.Y. and Bruce, G.J. 1998. Butyric acid from the diet: actions at the level of gene expression. Crit. Rev. Food. Sci. Nutr. 38, 259–297. Jung, B. and Batal, A.B. 2012. Effect of dietary nucleotide supplementation on performance and development of the gastrointestinal tract of broilers. Br. Poult. Sci. 53, 98–105. Keyburn, A.L., Sheedy, S.A., Ford, M.E., Williamson, M.M., Awad, M.M., Rood, J.I. and Moore, R.J. 2006. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74(11), 6496. Kuldeep, D., Ruchi, T., Khan, R., Sandip, C., Marappan, G., Kumaragurubaran, K., Mani, S., Desingu, P. and Sunkara, L. 2014. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: the trends and advances-a review. Int. J. Pharmacol. 10, 129–159. Kumar, A.P., Chougala, M., Nandini, C.D. and Salimath, P.V. 2010. Effect of butyric acid supplementation on serum and renal antioxidant enzyme activities in streptozotocin-induced diabetic rats. J. Food. Biochem. 34, 15–30. Kvan, O., Duskaev, G., Rakhmatullin, S. and Kosyan, D. 2019. Changes in the content of chemical elements in the muscle tissue of broilers on the background of plant extract and tetracyclines. Int. J. Environ. Sci. Dev. 10(12), 419–423. Lan, R., Zhao, Z., Li, S. and An, L. 2020. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions. Poult. Sci. 99, 5491–5500. Lee, D.N., Liu, S.R., Chen, Y.T., Wang, R.C., Lin, S.Y. and Weng, C.F. 2007. Effects of diets supplemented with organic acids and nucleotides on growth, immune responses and digestive tract development in weaned pigs. J. Anim. Physiol. Anim. Nutr. 91(11–12), 508–518. Liu, W., La, A.L.T.Z., Evans, A., Gao, S., Yu, Z., Bu, D. and Ma, L. 2021. Supplementation with sodium butyrate improves growth and antioxidant function in dairy calves before weaning. J. Anim. Sci. Biotechnol. 12(1), 1–9. Mollica, M.P., Mattace Raso, G., Cavaliere, G., Trinchese, G., De Filippo, C., Aceto, S. and Crispino, M. 2017. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 66(5), 1405–1418. Morrill, K., Conrad, E., Polo, J., Lago, A., Campbell, J., Quigley, J. and Tyler, H. 2012. Estimate of colostral immunoglobulin G concentration using refractometry without or with caprylic acid fractionation. J. Dairy. Sci. 95(7), 3987–3996. Navneet, F., Singh, C., Singh, D., Anil, K. and Singh, N.K. 2017. Effect of nucleotides supplementation on broiler performance. Ind. J. Poult. Sci. 52(1), 114–116. Paglia, D.E. and Valentine, W.N. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70(1), 158–169. Panda, A.K., Rao, S.V., Raju, M.V.L.N. and Sunder, G.S. 2009. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Austral. J. Anim. Sci. 22(7), 1026–1031. Reda, R.M., Selim, K.M., Mahmoud, R. and El-Araby, I.E. 2018. Effect of dietary yeast nucleotide on antioxidant activity, non-specific immunity, intestinal cytokines, and disease resistance in Nile tilapia. Fish. Shellfish. Immunol. 80, 281–290. Rutz, F., Xavier, E.G., Anciuti, M.A., Roll, V.F.B. and Rossi, P. 2007. The role of nucleotides in improving broiler prestarter diets: the Brazilian experience. Nutritional biotechnology in the feed and food industries: proceedings of Alltech’s 23rd Annual Symposium. The new energy crisis: food, feed or fuel? Stamford, UK: Alltech UK, pp: 175–181. Salmanzadeh, M. 2013. Evaluation of dietary butyric acid supplementation on small intestinal morphology, performance and carcass traits of Japanese quails. Rev. Méd. Vét. 164(10), 481–485. Sanderson, I.R. and He, J. 1994. Nucleotide uptake and metabolism by intestinal epithelial cells. J. Nutr. 124, 131–137. Sauer, N., Bauer, E. and Mosenthin, R. Dietary nucleotides: potential contenders as feed additive for monogastrics? In Proceedings of the 18th International Scientific Symposium on Nutrition of Domestic Aanimals, 2009, Radenci, Slovenia. Sauer, N., Eklund, M., Bauer, E., Gänzle, M.G., Field, C.J., Zijlstra, R.T. and Mosenthin, R. 2012. The effects of pure nucleotides on performance, humoral immunity, gut structure and numbers of intestinal bacteria of newly weaned pigs. J. Anim. Sci. 90, 3126–3134. Shatokhin, Y., El-Gammal, M. and Dmitry, P. 2017. Arab republic of Egypt broiler poultry industry: investment challenges and opportunities. Rome, Italy: Food and Agriculture Organization United Nations. Shojadoost, B., Vince, A.R. and Prescott, J.F. 2012. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 43(1), 1–12. Sikandar, A., Zaneb, H., Younus, M., Masood, S., Aslam, A., Khattak, F., Ashraf, S., Yousaf, M.S. and Rehman, H. 2017. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Austral. J. Anim. Sci. 30(5), 690. Sotoh, K. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 90, 37–43. Swaggerty, C.L., Callaway, T.R., Kogut, M.H., Piva, A. and Grilli, E. 2019. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms 7(3), 65. Teirlynck, E., Haesebrouck, F., Gussem, M.D.E., Dewulf, J., Van Immerseel, F. and Ducatelle, R. 2011. Morphometric evaluation of “dysbacteriosis” in broilers. Avian. Pathol. 40(2), 139–144. Thomas, L. 1998. Clinical laboratory diagnostics use and assessment of clinical laboratory results, 1st ed. Frankfurt, Germany: TH Books, Verlagsgesellschaft. Trairatapiwan, T., Lertpatarakomol, R., Jaipeng, P. and Paditporn, K. 2017. Effect of nucleotides supplementation on growth performance, humoral immunity, and intestinal morphological structure of broiler chickens. J. Mahan. Vet. Med. 12(1), 1–10. Wanger, D., Furrow, R. and Bradley, B. 1983. Subchronic toxicity of growth promoters in broiler chickens. Vet. Pathol. 20, 253–359. Wu, C., Yang, Z., Song, C., Liang, C., Li, H., Chen, W., Lin, W. and Xie, Q. 2018. Effects of dietary yeast nucleotides supplementation on intestinal barrier function, intestinal microbiota, and humoral immunity in specific pathogen-free chickens. Poult. Sci. 97, 3837–3846. Yang, X., Yin, F., Yang, Y., Lepp, D., Yu, H., Ruan, Z., Yang, C., Yin, Y., Hou, Y. and Leeson, S. 2018. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 8, 4940. Zhang, W., Gao, F., Zhu, Q., Li, C., Jiang, Y., Dai, S. and Zhou, G. 2011. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 90(11), 2592–2599. | ||
| How to Cite this Article |
| Pubmed Style Abdel-aziz AA, Abdel-aziz EA, Khairy MH, Fadel C, Giorgi M, Abdelaziz AS. The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 324-334. doi:10.5455/OVJ.2024.v14.i1.29 Web Style Abdel-aziz AA, Abdel-aziz EA, Khairy MH, Fadel C, Giorgi M, Abdelaziz AS. The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. https://www.openveterinaryjournal.com/?mno=178050 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.29 AMA (American Medical Association) Style Abdel-aziz AA, Abdel-aziz EA, Khairy MH, Fadel C, Giorgi M, Abdelaziz AS. The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 324-334. doi:10.5455/OVJ.2024.v14.i1.29 Vancouver/ICMJE Style Abdel-aziz AA, Abdel-aziz EA, Khairy MH, Fadel C, Giorgi M, Abdelaziz AS. The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 324-334. doi:10.5455/OVJ.2024.v14.i1.29 Harvard Style Abdel-aziz, A. A., Abdel-aziz, . E. A., Khairy, . M. H., Fadel, . C., Giorgi, . M. & Abdelaziz, . A. S. (2024) The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 324-334. doi:10.5455/OVJ.2024.v14.i1.29 Turabian Style Abdel-aziz, Ahmed A.m., El-sayed A. Abdel-aziz, Mohamed H. Khairy, Charbel Fadel, Mario Giorgi, and Ahmed S. Abdelaziz. 2024. The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 324-334. doi:10.5455/OVJ.2024.v14.i1.29 Chicago Style Abdel-aziz, Ahmed A.m., El-sayed A. Abdel-aziz, Mohamed H. Khairy, Charbel Fadel, Mario Giorgi, and Ahmed S. Abdelaziz. "The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters." Open Veterinary Journal 14 (2024), 324-334. doi:10.5455/OVJ.2024.v14.i1.29 MLA (The Modern Language Association) Style Abdel-aziz, Ahmed A.m., El-sayed A. Abdel-aziz, Mohamed H. Khairy, Charbel Fadel, Mario Giorgi, and Ahmed S. Abdelaziz. "The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 324-334. Print. doi:10.5455/OVJ.2024.v14.i1.29 APA (American Psychological Association) Style Abdel-aziz, A. A., Abdel-aziz, . E. A., Khairy, . M. H., Fadel, . C., Giorgi, . M. & Abdelaziz, . A. S. (2024) The effect of butyric acid and nucleotides supplementation on broiler (Gallus gallus domesticus) growth performance, immune status, intestinal histology, and serum parameters. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 324-334. doi:10.5455/OVJ.2024.v14.i1.29 |