| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 407-415 Original Research Impact of ultrastructural and molecular identified Babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findingsAbdelmoneim A. Ali1, Nahla A. Refat1, Rehab E. Mowafy2*, Safaa A. Gaheen2 and Manar A. AbdelMageed11Pathology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Pathology Department, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC) (Zagazig Provincial Lab.), Zagazig, Egypt *Corresponding Author: Rehab E. Mowafy. Pathology Department, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC) (Zagazig Provincial Lab.), Zagazig, Egypt. Email: mowafyrehab [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
AbstractBackground: Babesiosomes are apicomplexan parasites of both marine and freshwater fish species. Aim: In this study, we recorded the prevalence of Babesiosoma spp in two Egyptian freshwater fish species; the common carp and the African catfish with full pathological evaluation of the diseased condition, hematological and biochemical analysis of some parameters with exact recognition of the parasite with different methods. Methods: Two hundred and forty fish blood samples from Al-Sharqiya and Al-Ismailia governorates from August 2022 to January 2023 followed by blood film examinations, performing electron microscopy and molecular detection of the parasite via polymerase chain reaction. Results: The total infection prevalence was 63.75% with a higher prevalence observed among African catfish (42.5%) than Common carp (21.25%). Regarding hematologic parameters, the obtained results showed a significant decrease in the hematocrit values and a significant increase in the total leukocyte and lymphocyte values in both infected fish species. The serum ferritin, superoxide dismutase, and glutathione peroxidase were also significantly increased. However, the total iron binding capacity was significantly decreased. There was also a significant increase in the total serum bilirubin in the examined fish, all at (p < 0.001). Histopathologically, the lesions were more intense in the African catfish than the common carp but generally, the infected fish showed many changes with the gills being severely affected with pronounced hyperplasia of secondary lamellae with fusion and telangiectasis. The spleen, heart, and kidney are also affected. Conclusion: Serious adverse effects on the health status of previously examined fishes infected with Babesia spp. were observed and detected by several diagnostic and descriptive tools. Histopathological, hematological, and biochemical studies give an idea of the extent of these changes which are largely fatal affecting the economic system depending on the fish industry. Keywords: Babesiosoma, Egypt, Common carp, African catfish, Blood parasites. IntroductionThe importance of fish as a relatively cheap source of protein underscores the significance of maintaining fish farming conditions as healthy as possible (Soliman and Yacout, 2016). Parasitic infections can adversely affect high productivity in aquaculture, and they are considered bioindicators of water pollution. Among the possible parasitic infections in fish are blood parasites. The significance of these parasites lies in their detrimental pathological effects on their hosting fish (Davies and Johnston, 2000). Babesiosomes are intraerythrocytic parasites of marine and freshwater fish (Bruno et al., 2006). They belong to the family Dactylosomatidae. This family has two known genera: Dactylosoma and Babesiosoma. Members of the genus Babesiosoma are Heteroxenous coccidians that cycle between two hosts; an invertebrate, which is the main host, and a vertebrate which is an intermediate host (Barta, 1991). There are five known species in the Babesiosoma genus; Babesiosoma Bettencourt, Babesiosoma mariae, and Babesiosoma tetragonis which are recognized in fish hosts in addition to Babesiosoma jahni and Babesiosoma stableri which are described in amphibian hosts (Netherlands et al., 2019). The life cycles of only two babesiosomes have been investigated. These are B. mariae recognized in fish hosts in which a leech vector has been identified and B. stableri from a frog host in which also a leech vector has been identified (Barta, 1991; Negm-Eldin, 1998). The common feature in all described babesiosomes is that during merogony in the vertebrate host, they produce four merozoites in a cruciform or a rosette-shaped arrangement. One oocyst enclosed eight sporozoites during sporogony in their invertebrate host (Shahi et al., 2013). Much attention was drawn to the validation of the taxonomic affinities of these parasites which resulted in a scarcity of data regarding its prevalence and specific pathological effect in fish hosts. In this study, we are focusing light on the prevalence of babesiosomes in two species of Egyptian fresh-water fish; African catfish and Common carp, collected from Al-Sharqiya and Al-Ismailia governorates in the period between August 2022 and January 2023 and describing the hematological, biochemical, and histopathological changes in the hosting fish. Materials and MethodsStudy area and fish samplingTwo hundred and forty live fish, 120 Cyprinus carpio (common carp) and 120 Clarias gariepinus (African catfish) had blood samples taken from their caudal peduncles. Local fishermen caught these fish for 6 months, from August 2022 to January 2023, from the Nile River branches in the Al-Sharqiya governorate and the Al-Ismailia lakes. The prevalence of the parasitic infection depending on the result of the blood film was calculated according to the formula given by Agbabiaka et al. (2017). Prevalence (%)=no. of infected fish/total no. of fish samples × 100. Detection of Babesiosoma spp in blood filmsThin blood smears spread on grease-free glass slides were air-dried, fixed with 100% methanol for 2 minutes, and stained in a 1:20 dilution of Giemsa stain (10–20 minutes) (Kjemtrup and Conrad, 2000). Dried stained blood films were examined microscopically for the identification of intra-erythrocytic protozoa with its variable stages. Molecular detection of Babesiosoma spp via polymerase chain reaction (PCR)DNA extraction from blood samples (n=30) was performed using the QIA amp DNA Blood Mini Kit (Qiagen, Germany, GmbH, Catalogue no. 51106) following the manufacturer’s instructions. The primers used for the detection of part of the 18SrRNA gene of Babesia spp., were: forward primer 5′-GTTTCTGMCCCATCAGCTTGAC-3′ and reverse primer 5′-CAAGACAAAAGTCTGCTTGAAAC-3′ and the amplicon size was 422 bp. The annealing temperature for the PCR run was 61°C and the reaction was repeated for 45 cycles according to Hilpertshauser et al. (2006). The PCR product was run on an agarose gel electrophoresis according to Sambrook et al. (1989) and the gel was photographed by a gel documentation system. Electron microscopy detection of Babesiosoma sppTwo pelleted red blood cell samples from morphologically confirmed positive fishes of both species were prepared for transmission electron microscopy (TEM) by immersing and fixing them in a modified Karnovsky (1965) solution, which was made up of 2.5% paraformaldehyde and 2.5% buffered glutaraldehyde in 0.1 M sodium phosphate buffer pH 7.4, and storing them overnight at 4°C. A three 15-minute wash in 0.1 M sodium phosphate buffer and 0.1 M sucrose was then performed on the pellet. 90 minutes were spent postfixing in 2% sodium phosphate-buffered osmium tetroxide (pH 7.4), followed by three 15-minute washes in 0.1 M sodium phosphate. After being dehydrated in increasing ethanol grades, the samples were mixed with acetone: Epon (2:1, followed by 1:1 and 1:2 for 30 minutes each). Finally, the pellet was left in an epon pure solution at 4°C for a whole night. The epon was reconstituted the following day and incubated for around 48 hours at 70°C to facilitate polymerization. The pellets were cut using an ultramicrotome set to a section thickness of 50–100 nm. The sections were then rinsed to copper grids and post-converted, as per Reynolds's 1963 instructions: 10% uranyl acetate for 10 minutes, then 1% lead citrate for 5 minutes. A TEM was used to examine the sections after they had dried for around 15 minutes. The EM Unit at Mansoura University in Egypt used a JEOL JEM-2100 to observe ultrathin sections at 160 kV. Hematological and serum biochemical analysesFor hematological investigation, 25 blood samples were drawn from the fish that were being investigated and placed in tubes containing EDTA. The clear supernatant serum was carefully aspirated into dry, sterile, labeled vials after the serum was extracted by centrifugation at 3,000 rpm for 10 minutes. This method was employed for serum analysis in all labeled collected samples. Manual methods were used to measure the total leukocytic count, packed cell volume, hemoglobin concentration, and erythrocytic count (Blaxhall and Daisley, 1973). Differential leukocytic counts were calculated according to Cole (1986). The iron (Fe) and total iron binding capacity (TIBC/transferrin test), total bilirubin (direct and indirect) were measured in the sera according to Lobetti et al. (2000), superoxide dismutase (SOD), and Glutathione peroxidase (GPx) were assayed in sera according to Miller et al. (1993). Histopathological analysisFreshly necropsied fish were used to obtain tissue samples from the gills, heart, spleen, and kidneys. The tissue samples were then preserved in 10% buffered neutral formalin, dried using increasing concentrations of ethyl alcohol (70%–100%), cleaned in xylene, and embedded in paraffin wax. Hematoxylin and eosin (H&E) were used to cut and stain paraffin sections that were approximately 4–5 μm thick (Suvarna et al., 2019). A light microscope was used to examine the stained sections, and an AmscopeTM digital camera that was attached was used to take photomicrographs of the sections. Statistical analysisOur obtained data were analyzed by using a T-test as described by Petrie and Waston (1999). Ethical approvalThe study received the ethical approval number (ZU-IACUC/2/F/2023) from the Faculty of Veterinary Medicine, Zagazig University, Egypt. ResultsPrevalence of Babesiosoma spp infectionOne hundred fifty-three of 240 examined blood samples were positive for the blood parasite Babesiosoma spp out of 240 from both infected fish species with prevalence of 63.75%. Among 120 C. carpio (Common carp) fish, only 51 blood samples were positive with a prevalence of 21.25%. Among 120 C. gariepinus (African catfish), only 102 blood samples had a prevalence of 42.5%. Detailed prevalence data are shown in Table 1. Table 1. Showing the prevalence of Babsiosoma spp., in C. gariepinus and C. carpio among different localities in Al-Sharqiya and Al-Ismailia governorate.
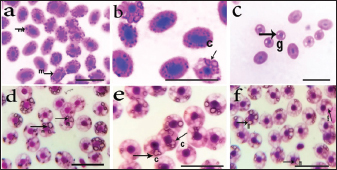
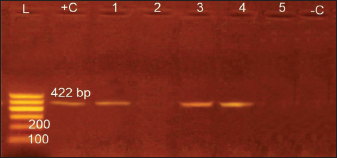
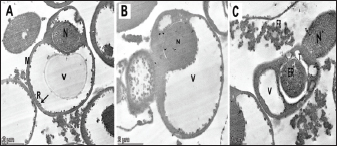
Identification of Babesiosoma spp in blood filmsThree different intra-erythrocytic morphological forms of Babesiosoma spp. in Giemsa-stained blood films were seen in both C. carpio (common carp) and Clarias gariepinus (catfish) blood, where cruciform meront (c), undivided meronts (m), and elongated gamonts (g) were demonstrated in Figure 1. Initial phases of primary merogony are thought to be characterized by undivided meronts with circular margins that appear uneven. The center of the organism was often unstained, but the cytoplasm and nucleus, which were only arranged peripherally, were stained—though the nucleus was only slightly stained. The trophozoite underwent its initial nuclear division with a little enlargement. A tetra nucleate organism is created by two nuclear divisions. Each pole has a fissure with distinct nuclei, resulting in "bow-tie." The meront appeared as a typical cruciform shape which is more diagnostic for the Babesiosoma spp. Cruciform meront (divided meronts) was characterized by a rosette or cross shape. Gamonts were detected after the secondary merogonic stages appearance with large oval weakly stained organisms and also showed diffuse nuclei with paler cytoplasm. Molecular detection of Babesiosoma spp via PCRPCR findings were the most accurate diagnostic tool that exhibited an identification of intra erythrocytic protozoan DNA in a representative sample of examined fish blood demonstrated in Figure 2 with three positive blood samples out of five (60%) with specific bands visualized at 422 bp. 18 samples out of 30 examined were positive. Transmission electron microscopySome intra-erythrocytic stages as dividing form (merogony) and trophozoites stages of Babesiosoma spp. were observed by TEM within the cytoplasm of the host fish erythrocytes. The dividing form was characterized by pale-staining cytoplasm with numerous cytoplasmic organelles as numerous polyribosomes, mitochondria, rough endoplasmic reticulum, and a roughly spherical nucleus dark in color as in C. carpio (common carp) fish Figure 3A. This dividing form could be also observed in C. garcinia (African catfish) with the same constituents and even architecture in Figure 3B while trophozoites could be distinguished by their irregular appearance, cytoplasm contained more densely packed ribosomes and their nucleoplasm was more densely stained with the unique presence of the tubular food vacuole around endoplasmic reticulum (Fig. 3C).
Fig. 1. Photomicrograph of Babesiosoma spp. in a Giemsastained blood film from both common carp (a, b, and c) and African catfish (d, e, and f) at 1,000×; showing undivided meronts (m), cruciform meront (c) and elongated gamonts (g)) scale bar=10 γm).
Fig. 2. Gel electrophoretic pattern of Babesiosoma spp. L: 200 bp DNA ladder. + C: Positive control – C: Negative control. Lanes (1, 3, 4): positive amplifications of Babesiosoma spp at 422 bp.
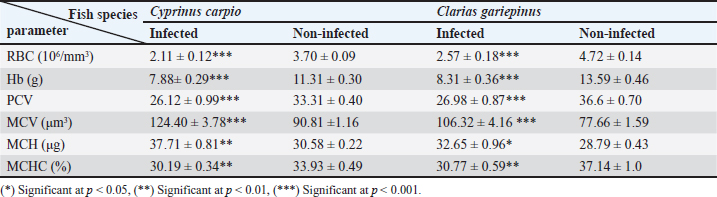
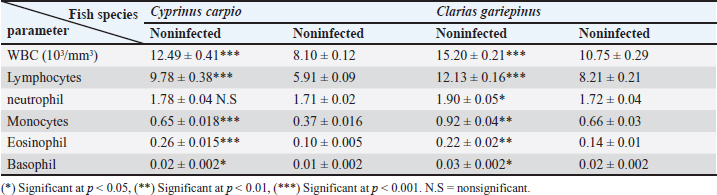
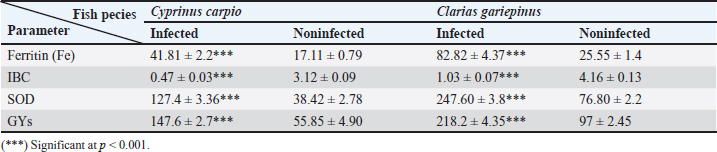
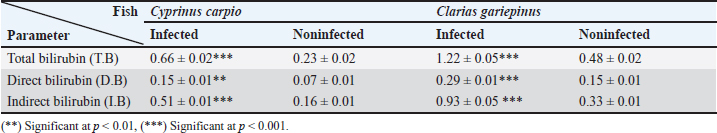
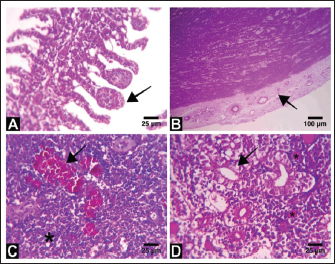
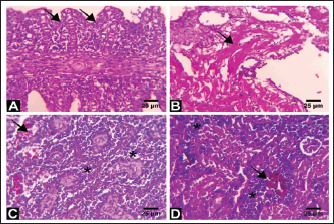
Fig. 3. Electron micrographs (TEM) of A) C. carpio (common carp) showing the dividing form of Babesiosoma spp. (N) nucleus, (R) ribosomes, and (V) vacuole (MB) membrane. B and C) Clarias garpinius (catfish) showing dividing form and trophozoite subsequently. (T) Tubular food vacuole and (ER) endoplasmic reticulum. Hematological picture and serum biochemical findingsBabesiosoma infection induced serious effects on the hematological findings of the infected examined fishes as demonstrated in Table 2. Our results declared significant decreases in values of RBCs, Hb, PCV, and MCV at p < 0.001 in infected fishes than that of un-infected ones. Significant decrease of MCH and MCHC in infected C. carpio at p < 0.01 and of MCHC in Clarias garpinius while a significant decrease in MCH value in C. garpinius at p < 0.05 was detected. The leucocytic picture (leukogram) demonstrated in Table 3 had a significant increase in total leucocyte and lymphocyte values in both infected fish species at p < 0.001 when compared with noninfected fish. Neutrophils had a nonsignificant increase in infected C. carpio and a significant increase in C. garpinius at p < 0.05 while a significant increase of monocytes and eosinophil of infected C. carpio at p < 0.001 and p < 0.01 in C. garpinius. Basophil had a significant increase in both fish species at p < 0.05. Biochemical findings included different parameters related to anemia as a result of erythrocytic damage at which significant increases of iron or Fe (ferritin), SOD, and GPx in both infected fish species at p < 0.001while significant decrease in TIBC at p < 0.001 in both infected fishes species was detected and are shown in Table 4. Total bilirubin in both direct and indirect forms exhibited a significant increase in fishes at p < 0.001 except direct bilirubin in C. carpio revealed a significant increase in fishes at p < 0.01. These results are shown in Table 5. Histopathological findingsThe Babesiosoma-infected fish showed a myriad of pathological changes. The pathological changes did not change much between the two investigated species although it was more intense in the infected Common carp fishes. In Figures 4 and 5, we show representative photomicrographs of the most commonly observed histopathological changes in the infected common carp and the African catfish, respectively. The gills showed moderate to severe hyperplasia in the secondary lamellar epithelium with lamellar fusions and prominent telangiectasis. The hearts showed a notable thickening of the pericardium with serofibrinous exudate, dilated blood vessels, and leukocytic infiltration, whereas the myocardium exhibited intermuscular edema and partial muscular hyalinization. The spleen showed pronounced activation of the melanomacrophage centers with marked expansion of the erythroid compartment and depletion of the lymphoid elements. The posterior kidneys showed vacuolation of the renal tubular epithelium, marked renal hematopoietic tissue hyperplasia, and mild activation of the melanomacrophage centers with a notable presence of hyaline casts inside some renal tubules. Table 2. Erythrogram (Mean ± S.E) of the infected fishes compared with control (n=5).
Table 3. Leukogram (Mean ± S.E) of the infected fishes compared with control (n=5).
Table 4. Some serum biochemical and antioxidant parameters (Mean ± S.E) of the infected fish compared with control (n=5).
DiscussionBabesiosis is considered one of the most important diseases in Egypt specifically in its acute form causing seriously recognized clinical manifestations and lowering productive performance (El-diasty et al., 2017). The identification of Babesia spp. depend on microscopical examination of blood films which varies with the examiner’s proficiency and experience but is considered a cheap and easy technique applied in all standard laboratories and even in the field for confirmation of acute cases (Lempereur et al., 2017). Our study documented the occurrence of Babesiosoma spp. in C. carpio (common carp) and C. garpinius (catfish) with a total prevalence of 63.75% which is considered a high prevalence compared with other international studies. This prevalence could not be nationally compared accurately because of unknown published local studies sharing the same geographical locations, fish species, and season of sample collection. Detection of most of the intraerythrocytic developmental stages of Babesiosoma spp. under the microscope was similar to those previously described by many authors (Barta, 1991; Lom and Dykova, 1992; Zulfiqar et al., 2012; Shahi et al., 2013). Hematological components have been developed for the evaluation of fish health conditions. Blood is considered the most reliable and easier indicator to demonstrate the general condition of the animal body. Therefore, in the presence of blood infection, blood parameters can serve as a standard biomarker (bioindicator) to determine diseased conditions and metabolic disturbances in fish (Celik, 2004). Table 5. Bilirubin parameters (Mean ± S.E) of the infected fish compared with control (n=5)
Fig. 4. Representative photomicrographs of H&E stained tissue sections of Babesiosoma spp infected common carp fish. A) Tissue section from the gills showing mild hyperplasia of the secondary lamellar epithelium and prominent telangiectasis (arrow). B) Heart section showing marked pericarditis where the epicardium is diffusely thickened by eosinophilic serofibrinous exudate with dilated blood vessels and leukocytic infiltration (arrow). C) Splenic tissue section showing pronounced activation of the melanomacrophage centers (arrow) and notable depletion of the lymphoid compartment with relative expansion of the erythroid compartment (asterisk). D) Tissue section from the posterior kidney showing vacuolation and degeneration in some renal tubules (arrow) with the presence of hyaline casts in others (asterisk). Scale Bar: 25 μm in A, C, and D, 100 μm in B. Both light and electron microscopic examinations corroborated the acquired results, which showed that the primary merogonic cycle produces merozoites that either enter a subsequent primary cycle of development or start a secondary merogony. The progeny of secondary merogony, known as Barta and Desser (1986), either replicate secondary merogony or form gamonts. According to Guimarães et al. (2003), who reported that the trophozoites' plasma membrane invasions enclosed trace amounts of cell hemoglobin, trophozoites are distinguished by their tubular feeding structure, which is formed by the simultaneous invasion of the erythrocyte and parasite plasma membranes. PCR testing is a quick and very accurate method of identifying certain genetic alterations and parasite infectious illnesses. The examination tests work by finding the DNA of Babesia in a sample with higher sensitivity, specificity, and accuracy values than microscopic examination as mixed infection may represent false results, particularly with microscopy (Souza et al., 2016). Thus, molecular diagnosis should be utilized to routinely diagnose blood-borne diseases because of the insufficiency of clinical, light microscopy, or hematological findings alone which cannot provide a reliable diagnosis (Gad et al., 2023). It has been demonstrated that the PCR is an extremely sensitive and specific method, especially for identifying carriers of babesiosis (Zulfiqar et al., 2012). There are numerous established and real-time PCR tests that can be used to identify Babesia species in tick and vertebrate hosts. According to Wang et al. (2015), these methods are typically more sensitive than microscopy. Depending on the size and target gene, they may allow phylogenetic analysis and/or identification to the genus or species level. Babesia species, even those of similar size and infecting the same animal host, exhibit far greater diversity, according to more recent molecular studies that use gene sequencing and phylogenetic analyses. These studies have reinforced the significance of using molecular techniques for species identification by demonstrating that Babesia spp. actual groups into several discrete clades that separate what appear to be identical parasites by light microscopy (Criado-Fornelio et al., 2004; Schnittger et al., 2012; Yabsley and Shock, 2013). Among other blood indices, our hematological data for fish with babesiosis showed a significant decrease in the erythrocytic count, hemoglobin concentration, and packed cell volume. All of this might be caused by the parasites' direct destruction of the erythrocytes in infected fish as opposed to healthy fish. These outcomes were consistent with those of Mahmoud et al. (2015). Moreover, the parasites merely serve as a stressor; during the early stages of stress, catecholamine is released, changing the packed cell volume and possibly causing red blood cells to be released from the spleen (Wells and Weber, 1990) or swell as a result of fluid entering the intracellular compartment (Chiocchia and Motais, 1989). Due to their correlation with anemia, a few biochemical indicators including bilirubin, iron, and TIBC (measured by the Transferrin test) were examined. Our findings for these parameters matched those of El-diasty et al. (2017).
Fig. 5. Representative photomicrographs of H&E stained tissue sections of a Babesiosoma spp-infected African catfish. A) Tissue section from the gills showing extensive hyperplasia of the secondary lamellar epithelium and adhesion of the secondary lamellae (arrows). B) Myocardium section showing intermuscular edema and partial muscular hyalinization (arrow). C) Tissue section from the spleen showing mild activation of the melanomacrophage centers (arrow) and marked depletion of the lymphoid elements (asterisks). D) Section from the posterior kidney showing renal hematopoietic tissue hyperplasia (asterisks) with the presence of pigmented macrophage aggregates (arrow) and mild degeneration of the renal tubules. Scale Bar: 25 μm. Important antioxidant markers like GPx and SOD were also impacted by blood parasite infection as a reaction to stressful situations and oxidative stress brought on by excessive reactive oxygen species (ROS) production (Naiel et al., 2021). The initial line of defense is represented by SOD, which breaks down superoxide into hydrogen peroxide and oxygen (Li et al., 2015), whereas CAT catalyzes the breakdown of hydrogen peroxide (Wang et al., 2017). Excessive ROS can cause lipid peroxidation, which can result in membrane damage and cell death, in addition to its detrimental effects on cellular proteins and DNA (Jiao et al., 2019). The histopathological changes described in Babesiosoma spp., infected fish corroborates the histological changes reported in other Babesia-infected species where the kidneys showed vacuolation and degeneration of renal epithelium and hyaline cylinders in the tubular lumen, the spleen exhibited the presence of aggregates melanomacrophages and depletion of the lymphoid elements (Habela et al., 1991; El-diasty et al., 2017; Jasik et al., 2023). Pericarditis was described (Habela et al., 1991) in Merino sheep experimentally infected by Babesia ovis and myocardial pathology is frequently reported in canine babesiosis (Lobetti, 2005; Petra et al., 2018). Moreover, changes in gills reflect hypoxia associated with anemia due to parasitism (Gilmour and Perry, 2018). AcknowledgmentThe authors thank Prof. Dr. Nashwa Abdelrazik Abbasa, Central lab. For Fish Research, for her help in sample collection and first recognition via blood. Authors' contributionAAA and REM contributed to the idea, design of the research project, and supervision. SAG and REM contributed to practical application as well as the data collection, MAA, and REM writing the paper after the result preparation and supervision. AAA and NAR critical revision of the essential intellectual content with supervision. The submitted article has been reviewed and approved by all authors. Competing interestsThe authors declare no competing interests. FundingThe authors did not receive any financial support from anywhere Data availability All data used were available and have been included in the manuscript. ReferencesAgbabiaka, L.A., Akande, T.T. and Ekeocha, C. 2017. Assessment of parasites associated with African catfish farmed at Owerri Federal Constituency, Imo State Nigeria. Agric. Biol. J. North. Am. 8, 168–172. Barta, J.R. 1991. The dactylosomatidae. Adv. Parasitol. 30, 1–37. Barta, J.R. and Desser, S.S. 1986. Light and electron microscopic observations on the intraerythrocytic development of Babesiosoma stableri (Apicomplexa, Dactylosomatidae) in frogs from Algonquin Park, Ontario. Protozool. J. 33, 359–368. Blaxhall, P.C. and Daisley, K.W. 1973. Routine haematological methods for use with fish blood. J. Fish. Biol. 5, 771–781. Bruno, D.W., Nowak, B. and Elliott, D.G. 2006. Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Dis. Aquat. Organ. 70, 1–36. Celik, E.S. 2004. Blood chemistry (Electrolytes, lipoproteins and enzymes) values of black scorpionfish ( Scorpaena porcus Linneaus, 1758) in the Dardannelles. Turkey. J. Biol. Sci. 4, 716–719. Chiocchia, G. and Motais, R. 1989. Effect of catecholamines on deformability of red cells from trout: relative roles of cyclic AMP and cell volume. J. Physiol. 412, 321–332. Coles, E.H. 1986. Veterinary Clinical pathology.4th ed. Textbook. WB Sanders Company, Philadelphia, London, Toronto. Criado-Fornelio, A., Gónzalez-del-Río, M.A., Buling, S.A. and Barba, J.C. 2004. The ‘Expanding Universe’ of piroplasms. Vet. Parasitol. 119, 337–345. Davies, A.J. and Johnston, M.R.L. 2000. The biology of some intraerythrocytic parasites of fishes, Amphibia and Reptiles. Adv. Parasitol. 45, 1–107. El-diasty, M.M., Nesma, M.R., Zaglol, A.K. and Rehab, R.A. 2017. Biochemical, pathological and molecular studies on babesiosis in calves. Egypt. J. Agric. Res. 95, 1269–1283. Gad, S.A., Azza, S.E., Marwa, M.K. and Moataz, M.M. 2023. Hematological and molecular profiling of some blood pathogens in dog breeding farm in Egypt. J. Adv. Vet. Res. 13, 344–351. Gilmour, M.K. and Perry, F.S. 2018. Conflict and compromise: using reversible remodeling to manage competing physiological demands at the fish gill. Physiology 33, 412–422. Guimarães, A.M., Lima, J.D. and Ribeiro, F.B. 2003. Ultrastructure of Babesia equi trophozoites isolated in Minas Gerais, Brazil. Pesq. Vet. Bars. 23, 101–105. Habela, M.A., Reina, D.I., Navarrete, E., Redondo, II. and Hernández, S. 1991. Histopathological changes in sheep experimentally infected with Babesia ovis. Vet. Parasiotol. 38, 1–12. Hilpertshauser, H., Peter, D., Manuela, S., Lise, G. and Alexander, M. 2006. Babesia spp. Identified by PCR in ticks collected from domestic and wild ruminants in Southern Switzerland. Appl. Environ. Microbiol. 72, 6503–6507. Jasik, K., Kleczka, A., Filipowska, S., Anna, K. and Sandra, F. 2023. Histopathological analysis of selected organs of rats with congenital babesiosis caused by Babesia microti. Vet. Sci. 10, 291. Jiao, W., Qi, H., Yanmin, X., Huijie, J., Houjuan, X. and Xiaohua, T. 2019. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: through oxidative stress and apoptosis. Fish. Shellfish. Immuol. 86, 239–245. Karnovsky, M.J. 1965. Karnovsky fixative, developed by Karnovsky, is fixative for electron microscopy. https://en.wikipedia.org/wiki/Karnovsky_fixative Kjemtrup, A.M. and Conrad, P.A. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30, 1323–1337. Lempereur, L., Relja, B., Isabel, F., Cátia, M., Ana, D., Marcos, S. and Sara, Z. 2017. Guidelines for the detection of Babesia and Theileria parasites. Vector-Borne Zoon. Dis. 17, 51–65. Li, J., Yongping, X., Liji, J. and Xiaoyu, L. 2015. Effects of a probiotic mixture (Bacillus subtilis YB-1 and Bacillus cereus YB-2) on disease resistance and non-specific immunity of sea cucumber, Apostichopus japonicus (Selenka). Aqua. Res. 46, 3008–3019. Lobetti, R.G., 2005. Cardiac involvement in canine babesiosis. J. S. Afr. Vet. Assoc. 76, 4–8. Lobetti, R.G., Möhr, A.J., Dippenaar, T. and Myburgh, E. 2000. A preliminary study on the serum protein response in canine babesiosis. J. S. Afr. Vet. Assoc. 71, 38–42. Lom, J. and Dykova, I. 1992. Protozoans parasites of fishes. Developments in aquaculture and fisheries science. Amsterdam, The Netherlands: Elsevier, pp: 1–315. Mahmoud, M.S., Omnia, M.K., Soad, M.N., Seham, H.M.H., Salwa, M.H., Dalia, M.M., Marta, G.S. and Carlos, E.S. 2015. Serological and molecular diagnostic surveys combined with examining hematological profiles suggests increased levels of infection and hematological response of cattle to babesiosis infections compared to native buffaloes in Egypt. Parasitol. Vect. 8, 319. Miller, J.K., Brzezinska, S.E. and Madsen, F.C. 1993. Oxidative stress, antioxidants, and animal function. J. Dairy. Sci. 76, 2812–2823. Naiel, M.A.E., Khames, M.K., El Razek, N.A., Gharib, A.A. and El-Tarabily, K.A. 2021. The dietary administration of miswak leaf powder promotes performance, antioxidant, immune activity, and resistance against infectious diseases on Nile tilapia (Oreochromis niloticus). Aqua. Rep. 20, 100707. Negm-Eldin, M.M. 1998. Life cycle, host restriction and longevity of Babesiosoma mariae HOARE, 1930 (Apicomplexa: Dactylosomatidae). Deut. Tiera. Wochen. 105, 367–374. Netherlands, E.C., Cook, C.A., Du Preez, L.H., Vanhove, M.P., Brendonck, L. and Smit, N.J. 2019. An overview of the dactylosomatidae (Apicomplexa: Adeleorina: Dactylosomatidae), with the description of dactylosoma Kermiti n. Sp. Parasitising Ptychadena anchietae and Sclerophrys gutturalis from South. Int. J. Parasitol. Parasites. Wildlife. 11, 246–260. Petra, B., Kuleš, J., Barić, R.R. and Mrljak, V. 2018. Canine babesiosis: where do we stand? Acta. Vet. 68, 127–160. Sambrook, J., Fritsch, E.F. and Tom, M. 1989. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory. University of Michigan, Ann Arbor, USA. Schnittger, L., Anabel, E.R., Monica, F.C. and David, A.M. 2012. Babesia: a world emerging. Infection, genetics and evolution. Infect. Genet. Dis. 12, 1788–1809. Shahi, N., Yousuf, M.A. and Yaseen, F. 2013. First report of blood parasites in fishes from Kashmir and their effect on the haematological profile. Open. Vet. J. 5, 89–95. Soliman, N.F. and Dalia, M.M.Y. 2016. Aquaculture in Egypt: status, constraints and potentials. Aquacult. Int. 24, 1201–1227. Souza, S.S., Henry, S.B., Patrick, S. and Yvonne, Q. 2016. Comparison of Babesia microti real-time polymerase chain reaction assays for confirmatory diagnosis of babesiosis. Am. J. Trop. Medi. Hyg. 95, 1413–1416. Suvarna, S.K., Christopher, L., Bancroft, J.D. and Paley, B.W. 2019. Bancroft’s theory and practice of histological techniques, 8th ed. Oxford, UK: Churchill Livingstone Elsevier. Wang, L., Chenxia, G., Jianchao, W., Jing, D., Peijun, Z. and Yuehong, L. 2017. Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aqua. Int. J. 25, 2091–2099. Wang, G., Patrick, V., Jian, Z., Paul, V. and Gary, P.W. 2015. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn. Microbiol. Infect. Dis. 82, 109–113. Wells, R.M.G. and Weber, R.E., 1990. The spleen in hypoxic and exercised rainbow trout. J. Exp. Biol. 150, 461–466. Yabsley, M.J. and Barbara, C.S. 2013. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int. J. Parasitol. Parasites. Wildlife. 2, 18–31. Zulfiqar, S., Sadia, S., Muhammad, A., Arif, M., Bhutta, S.I., Sikandar, H. and Shazia, Q. 2012. Detection of Babesia bovis in blood samples and its effect on the hematological and serum biochemical profile in large ruminants from southern Punjab. Asian. Pac. J. Trop. Biol. Med. 2, 104–108. | ||
| How to Cite this Article |
| Pubmed Style Ali AA, Refat NA, Mowafy RE, Gaheen SA, Abdelmageed MA. Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 407-415. doi:10.5455/OVJ.2024.v14.i1.37 Web Style Ali AA, Refat NA, Mowafy RE, Gaheen SA, Abdelmageed MA. Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. https://www.openveterinaryjournal.com/?mno=179268 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.37 AMA (American Medical Association) Style Ali AA, Refat NA, Mowafy RE, Gaheen SA, Abdelmageed MA. Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 407-415. doi:10.5455/OVJ.2024.v14.i1.37 Vancouver/ICMJE Style Ali AA, Refat NA, Mowafy RE, Gaheen SA, Abdelmageed MA. Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 407-415. doi:10.5455/OVJ.2024.v14.i1.37 Harvard Style Ali, A. A., Refat, . N. A., Mowafy, . R. E., Gaheen, . S. A. & Abdelmageed, . M. A. (2024) Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 407-415. doi:10.5455/OVJ.2024.v14.i1.37 Turabian Style Ali, Abdelmoneim A., Nahla A. Refat, Rehab E. Mowafy, Safaa A. Gaheen, and Manar A. Abdelmageed. 2024. Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 407-415. doi:10.5455/OVJ.2024.v14.i1.37 Chicago Style Ali, Abdelmoneim A., Nahla A. Refat, Rehab E. Mowafy, Safaa A. Gaheen, and Manar A. Abdelmageed. "Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings." Open Veterinary Journal 14 (2024), 407-415. doi:10.5455/OVJ.2024.v14.i1.37 MLA (The Modern Language Association) Style Ali, Abdelmoneim A., Nahla A. Refat, Rehab E. Mowafy, Safaa A. Gaheen, and Manar A. Abdelmageed. "Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 407-415. Print. doi:10.5455/OVJ.2024.v14.i1.37 APA (American Psychological Association) Style Ali, A. A., Refat, . N. A., Mowafy, . R. E., Gaheen, . S. A. & Abdelmageed, . M. A. (2024) Impact of ultrastructural and molecular identified babesiosoma spp. in both Egyptian freshwater fishes (common carp and African catfish): Hematological, biochemical, and histopathological findings. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 407-415. doi:10.5455/OVJ.2024.v14.i1.37 |