| Research Article | ||
Open Vet. J.. 2024; 14(8): 1779-1788 Open Veterinary Journal, (2024), Vol. 14(8): 1779–1788 Research Article Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donorsMartina Quagliardi*, Giacomo Rossi, Matteo Cerquetella, Alessandra Roncarati, Livio Galosi, Sara Mangiaterra and Alessandra GavazzaSchool of Biosciences and Veterinary Medicine, University of Camerino, Matelica, Italy *Corresponding Author: Martina Quagliardi. School of Biosciences and Veterinary Medicine, University of Camerino, Matelica, Italy. Email: martina.quagliardi [at] unicam.it Submitted: 09/02/2024 Accepted: 09/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
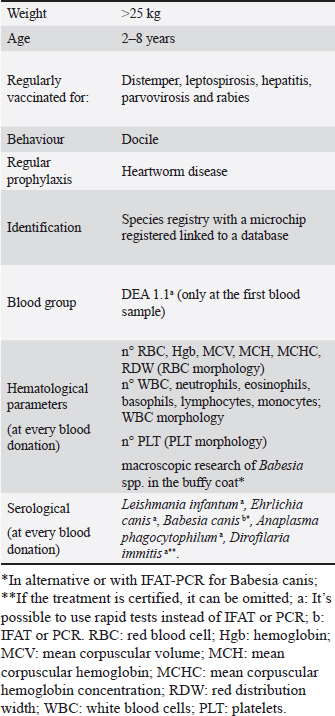
ABSTRACTBackground: In recent years, the field of transfusion medicine for dogs has advanced significantly, becoming a crucial aspect of veterinary clinical practice. Nowadays, blood still remains a fundamental biological source and the welfare and health status of eligible species-specific blood donors are essential for veterinary transfusion medicine. Aim: This study focused on evaluating two shelters in Central Italy (Marche region), located in Tolentino (TS) and in Camerino (CS), in order to assess the potential of the shelter dogs as canine blood donors. Methods: We evaluated a total of 45 dogs from these shelters based on physical (age and size), clinical, behavioural, and blood analysis criteria described in the Italian Ministerial Guideline for Veterinary Transfusion Medicine (2016). Results: At the TS shelter, out of 206 resident dogs, 125 met the donation criteria (60.68%), with 28 (13.59%) selected for the study due to the impossibility to collect the samplings or other exclusion causes. In the CS shelter, of the 149 dogs, 17 (11.41%) were identified as potential blood donors and included in the study. Among these, seven dogs (25%) from TS and five dogs (29.41%) from CS were found to have DEA1 negative blood group. High percentages (TS=25.24%, CS=40.27%) of dogs were excluded for seniority and 29.53% in CS for behavioural issues. Notable findings included reduced erythrocyte mean corpuscular volume [mean TS=63.93 fl; CS=64.00 fl] and Reticulocyte Hemoglobin [mean TS=22.39 pg; CS=21.38 pg]. Additionally, in both shelters’ dogs showed a modest increase in eosinophils levels [mean TS=1.59 K/μl; CS=1.02 K/μl]. Conclusion: Shelter dogs can fulfill the blood donation requirements set by the Italian Ministerial Guideline on Veterinary Transfusion Medicine. They are generally in good health and present a low risk of transmitting parasitic diseases; however, many are ineligible for donation due to behavioural pathologies or temperament issues and seniority. Keywords: Dog, Transfusion medicine, Guideline, Blood donor, Shelter. IntroductionTransfusion medicine in veterinary medicine is an expanding field, offering new opportunities for development, research, and applications. Its global adoption in recent years has led various countries to establish diverse regulatory frameworks. For instance, the American College of Veterinary Internal Medicine (ACVIM) in the United States issued the consensus statement in 2005 (Wardrop et al., 2005), then updated in 2016 (Wardrop et al., 2016), addressing the testing for infectious diseases in canine and feline blood donors. These laboratory tests, including blood type identification, crossmatching tests, and pathogen screening are essential to increase the safety of blood donation. They help monitor the donor’s health, reduce post-transfusion adverse reactions in recipients, and minimize the risk of vector-borne diseases (Reine, 2004; Wardrop et al, 2023). In Italy, the 2016 Ministerial Guideline on Veterinary Transfusion Medicine sets out detailed criteria for selecting donor animals (dogs, cats, and horses). It covers eligibility, exclusion standards, required blood tests, and procedures for collecting and storing blood. For example, donor dogs should weigh over 25 kg, be aged between 2 and 8 years, be docile, clinically healthy, regularly vaccinated, and undergo regular prophylaxis against heartworm, ectoparasite, and endoparasite. Blood donor animals must be healthy and constantly monitored. This approach, not only supports preventive medicine, but also allows for ongoing health monitoring of the animals and tracking of epidemiological infectious disease trends (Morganti et al., 2022). This is particularly pertinent in shelter environments, where managing individual health and controlling infectious diseases can be more challenging, from the perspective of preventive medicine (Mutinelli et al., 2013). Given this context, our study aimed to explore the feasibility of using shelter dogs in central Italy as blood donors. Historically, this has never been pursued due to unknown clinical and transfusion histories of shelter dogs and potential ethical concerns (Yagi and Bean, 2016). Currently, specific blood donation programs for dog shelters are absent, yet they could be viable candidates for transfusion medicine practices, provided their physical well-being is maintained and ethical considerations are respected (DeLuca et al, 2006; Mutinelli et al., 2013). Additionally, canine blood donation could foster awareness of transfusion practices and encourage blood donation among dog owners and, by extension, in humans (Ashall et al, 2017; Wilder and Humm, 2019). In 2012, the Marche region implemented a surveillance plan for Leishmaniasis control, as it is an endemic area for this protozoan infection (Surveillance Plan, 2012). Blood samples for this study were collected as part of this regional plan (“Regional law”), with authorization from the respective shelter managers. Marche Region is a territory of Central Italy fronting on the Adriatic Sea and comprises five provinces; in the eastern part is bathed by the Adriatic Sea, the Umbrian-Marchigian section of the Apennines serves as its mountain backbone and it is mainly characterized by hills [Website Britannica.com]. This preliminary study is the first of its kind in the region, aiming to assess the suitability of shelter dogs as blood donors, considering their health status and the potential to contribute to transfusion medicine. Materials and MethodsBlood samples were collected during the 2020–2021 years from two shelters in Central Italy-Marche Region: “Monti Azzurri” in Tolentino and “Colle Altino” in Camerino, located in the mountain area of the region. A manager and an official veterinarian are present in both shelters. In these shelters, dogs are divided by fences, single or with more individuals, with indoor kennels and material for heating, bowls and a stool in front. At least twice a day food, water and cleaning by the operators are guaranteed. As previously stated, this collection of blood samples was conducted as part of a regional initiative (“regional law”), aimed at evaluating the welfare status of dogs in shelters. The President of the Regional Council of Marche region has promulgated the Regional Law 20 April 2015, n. 18 concerning the amendments to the Regional Law 20 January 1997, n. 10 “norms in matter of pets and prevention of stray animals.” The presence of a large fenced green area, and communicating with the boxes, for daily walks and socialization are institutionalized to guarantee animal welfare in the shelters; rules on health checks and proper management of the animals are also present. Dog populations of shelters and recruitmentFor the study, we used detailed shelter registers from both the Tolentino Shelter (TS) and the camerino shelter (CS). The TS register, provided by the shelter staff, included records from December 2020 and an update from December 2021. Meanwhile, the CS register, maintained by the animal manager, was complete and up to date as of October 2021. These registers contained extensive information about each dog, such as the animal’s name, microchip number, estimated birth year, size (small, medium, and large), breed, sex, coat colour, date of entry into the shelter, and other relevant notes (e.g., if the dog was seized, previously owned, aggressive, and so on). Dogs that did not meet the criteria set in the Ministerial Guideline on Veterinary Transfusion Medicine were excluded from both shelters as potential blood donors evaluated in this study (Table 1). Selected dogs were between 2 and 8 years old, weighed more than 25 kg, displayed docile behaviour and were regularly vaccinated against distemper, leptospirosis, hepatitis, parvovirosis, and rabies. Each dog was also registered with a microchip in the species registry, linked to a database and identified at the species registry. Furthermore, all dogs were regularly treated both for endoparasites and ectoparasites. Every year, all dogs are treated with pesticide repellents spot on (Frontline Tri-Act®-Boehringer Ingelheim) or collar (Seresto®-Bayer) to avoid punctures of phlebotomies and mosquitoes and the transmission of ectoparasites. Prophylaxis with ivermectin tablets (Cardotek 30®-Boehringer Ingelheim) against heartworm disease is carried out on all dogs in both shelters. Also, dogs are treated twice a year for gastrointestinal parasites with febantel, pyrantel and praziquantel (Drontal®-Vetoquinol). During the shelters’ dog evaluation, whenever a dog was chosen for examination after it was selected for the study, the facility’s operators and the veterinary doctor provided the animal’s health record, which included clinical and medical history. This was followed by a comprehensive physical evaluation, assessing various health indicators such as body condition score, skeletal and muscular development, sensory state, behavioural signs, weight, mucous membranes condition, and lifting of the skin to assess the state of hydration, and capillary refill time. Each dog in both shelters was carefully evaluated through a behavioral visit by specialist veterinarians and experienced dog educators; a report on the behavioural evaluation is present for each dog in a personal data sheet. Blood sampling and laboratory testsFor this study, in certain instances, dogs that met the criteria outlined in the Ministerial guideline were tested multiple times over the course of the study. Whole blood samples, each 5 ml, were drawn from the cephalic vein of dogs and immediately stored in two types of tubes: one containing K3-EDTA and one with granules and a clot activator. These samples were then processed at the Veterinary Teaching Hospital’s Laboratory at the University of Camerino. The analysis included complete blood count (CBC), blood group typing, a biochemical profile and tests for hemoparasitosis. Electrophoresis was employed in ambiguous cases of Leishmaniasis diagnosis. Occasionally, challenging field conditions or the dogs’ restlessness post-venipuncture hindered the collection of adequate blood quantities for all tests stipulated in the Ministerial Guideline on Veterinary Transfusion Medicine. In such instances, only a subset of the panels could be conducted. For each blood sample, an aliquot of serum was frozen for further study. Table 1. Criteria and laboratory tests required by the Italian Ministerial Guideline on Veterinary Transfusion Medicine (2016) to evaluate a potential dog blood donor.
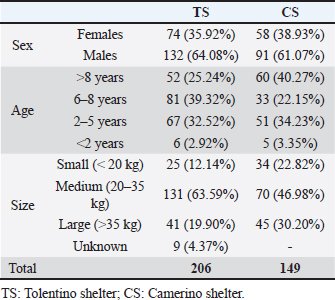
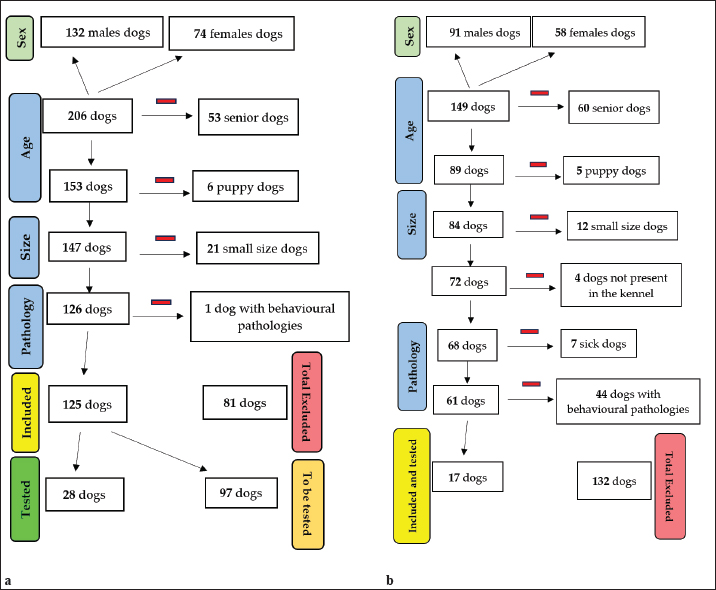
Blood group typing was carried out on blood with EDTA by rapid immunochromatographic test (Lab Test BT DEA1®-Alvedia®). CBC evaluation were performed on samples with K3 EDTA anticoagulant with the Procyte® IDEXX analyzer and a blood smear test was carried out. Evaluated parameters included: red blood cell (RBC); hematocrit (HCT); hemoglobin (Hb); mean corpuscular volume (MCV); mean corpuscular Hb (MCH); MCH concentration (MCHC); red distribution width (RDW); % reticulocytes; reticulocyte Hb (Retic-Hb); Leukocytes; % segmented neutrophils; % lymphocytes; % monocytes; % eosinophils; % basophils; absolute value of segmented neutrophils, lymphocytes, monocytes, eosinophils, and basophils; platelets (PLT); PLT distribution width (PDW); mean PLT volume (MPV); Plateletcrit. Serum was obtained using the Rotina 46R of Hettich Zentrifugen® centrifuge and then analyzed using the machine BT3500 Vet of Biotecnica Instruments®. The biochemical profile took into account the following parameters: Calcium (Ca; Arsenazio III); Blood urea nitrogen (ultra violet-U.V. method); Gamma-glutamide transferase (GGT; IFCC-International Federation of Clinical Chemistry method); Alkaline phosphatase (ALP; DEA-diethanolamine method); Glucose (Glu; Trinder method); Creatinine (Jaffè method); glutamic-oxalacetic transaminase (GOT; IFCC method); Alanine amino-transferase (GPT; IFCC method); cholesterol (Trinder method); Triglycerides (Trinder method); albumin (Alb; VBC-Bromocresol Green method); Phosphorus (U.V.-Ultra Violet method); Direct bilirubin (Sulfanilic method); Total Bilirubin (sulfanilic method); indirect bilirubin; total proteins (TP; biuret method); Albumin/globulin ratio; corrected calcium; Calcium/phosphorus ratio; globulins. The separated serum samples were employed for hemoparasitoses testing, as required by the Ministerial Guideline on Veterinary Transfusion Medicine. The SNAP® Test 4Dx® Plus (IDEXX) and the SNAP® Leishmania Tests were used for the control of Ehrlichiosis, Anaplasmosis, Borreliosis and Heartworm Disease, and Leishmaniasis. For the latter, dogs had already been tested with indirect immunofluorence according to the Marche region surveillance plan for the control of Leishmaniasis. In the case of Babesiosis and Brucellosis, testing was sporadically carried out on a random selection of the specimens. The smear test was used to exclude the presence of Babesia canis, while Canine Brucellosis Antibody Test Kit D-TEC® CB (Zoetis) for Brucella canis. Stool examination by flotation test was performed twice a year on random samples. Data processingMicrosoft® Excel® program for Microsoft 365 MSO (Version 2201 Build 16.0.14827.20180) was used to make the statistical part. The functions used were: sum, mean, median, standard deviation, and percentage. Ethical approvalNot needed for this study. ResultsDogs’ populationsIn 2021, the TS accommodated 206 dogs and CS hosted 149 dogs. Table 2 illustrates the demographic composition of these populations, focusing on the distribution and percentage of sex, age, and size across both shelters. Notably, both shelters exhibited a higher percentage of male dogs [TS=64.08%; CS=61.07%] compared to females [TS=35.92%; CS=38.93%]. A significant majority (approximately 80%) of the dogs were of medium-large size (weighing over 25 kg). The sum of the percentages of medium-large breed dogs in TS was 83.49% while in CS 77.18%. While specific breeds were not identifiable, there was a notable prevalence of mixed breeds. These mixed breeds predominantly displayed characteristics akin to Maremmano shepherds and hound breeds. Figure 1 presents two flow diagrams that describe the criteria for the progressive exclusion of dogs, as outlined in the Ministerial Guideline on Veterinary Transfusion Medicine. Each diagram begins with the total population of the respective shelter, starting with the total consistency of the populations, categorized by sex. On the left side of the diagram, selection criteria (age, size, and pathologies) are delineated. Some dogs considered suitable for collection in the preliminary phase, once physically contained outside or inside the box, proved not to be able to sustain too close contact with the operators, not maintaining their well-being and tranquillity, which is why they did not return to the chosen population. Concerning CS, most of the dogs with behavioural pathologies resulted to be phobic, unpredictable or unruly. The pathologies found in the preliminary phase of the dogs’ clinical history were leishmaniasis, heart disease, paraplegia, and hypothyroidism. The selection criteria are used to identify and exclude unsuitable candidates, which are shown on the right side of the diagram. The process concludes with the final counts: the total number of dogs included in and excluded in the study, along with the count of specimens that were effectively tested. Out of the TS population, one hundred and twenty-five dogs (60.68%) were potential candidates for this study based on the Ministerial guideline on Veterinary Transfusion Medicine criteria, yet only twenty-eight underwent blood testing due to the COVID-19 pandemic situation and, later, the continuous changing of the population. In the case of CS, all one hundred and forty-nine dogs in the population were evaluated based on the anamnestic and/or clinical records, with only seventeen selected for the study because they met the criteria to become possible blood donors. Most of the excluded dogs exhibited behavioural pathologies or temperament issues, making them ineligible for donation, as opposed to clinical and organic diseases. Table 2. Description of total shelter populations and their main characteristics.
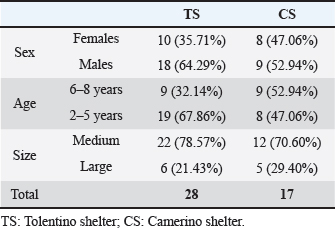
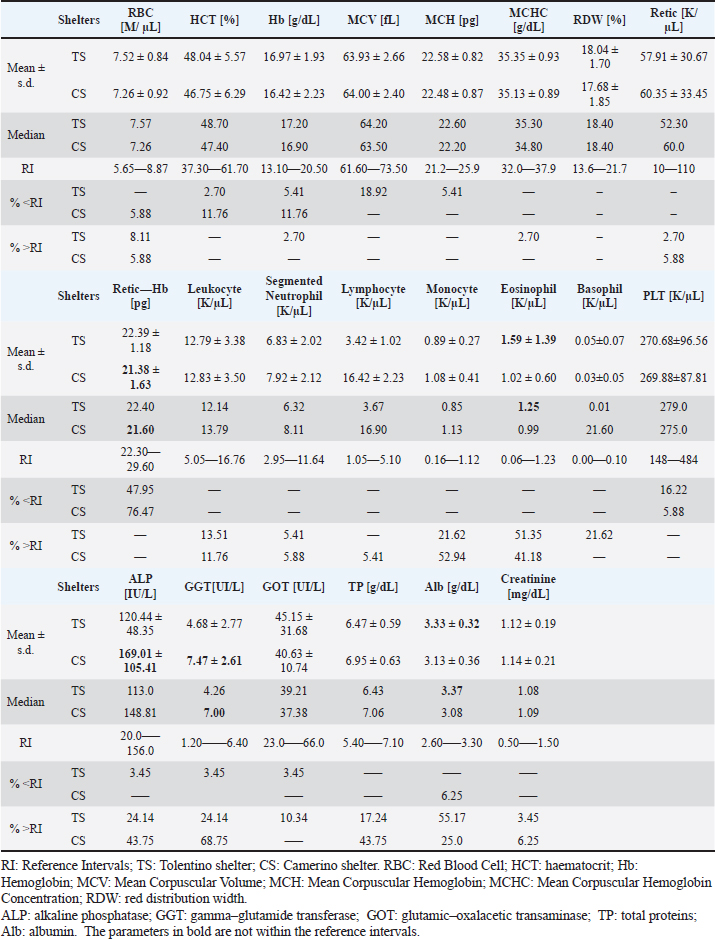
Table 3 provides a comprehensive overview of the selected populations and their respective characteristics, encompassing both TS and CS. Blood type typing and laboratory testsThe percentage of the dogs that resulted to be DEA1 positive was higher [TS=75%; CS=70.59%] than DEA1 negative [TS=25%; CS=29.41%]. Table 4 reports the CBC parameters evaluated in TS and CS and only the biochemical values whose mean did not fall within the reference intervals for the parameter. A urine test was not considered because, in a situation like the shelter, it is quite impossible to collect the urine individually with a non-invasive method. The SNAP tests conducted in both the TS and CS groups yielded negative results for Ehrlichia canis, Borrelia burgordferi, Dirofilaria immitis, Anaplasma phagocytophilum, and Leishmania infantum. A singular dog, exhibiting clinical conditions of Leishmaniasis, underwent serum electrophoresis, but the results were normal and did not show hypergammaglobulinemia. All dogs subjected to indirect immunofluorescence serological tests (IFATs) as part of Leishmania infantum (Surveillance plan) resulted in negative. Simultaneously, random tests for Babesiosis and Brucellosis resulted in a negative for this parasitosis. All the fecal flotation tests showed negative results. DiscussionThis study aims to delineate the health status and characteristics of shelter dogs as eligible blood donors. Upon evaluation, the majority of excluded dogs had behavioural pathologies, rending them not eligible for donation, as opposed to presenting clinical and organic diseases (Dufour et al., 2005; Descamps et al, 2023).
Fig. 1. Flow diagrams of the dogs populations Table 3. Description of the 45 selected dogs for the study from the shelter populations and their main characteristics.
Table 4. Mean and median of the hematological and biochemical parameters evaluated of the selected dogs for the study from the shelter populations.
In the selected populations, approximately 70% of dogs tested positive for DEA1, indicating they could therefore donate blood exclusively to DEA1+ animals. This result aligns with previous studies on the prevalence of blood groups in both mongrels and purebreds’ dogs in Italy (Carli et al., 2017; Medina Valentin et al., 2017), Europe (Paleckaitis et al., 2018), and worldwide (Mangiaterra et al., 2021), where the percentage of dogs DEA1+ where higher than DEA1-. On the contrary, studies on greyhounds, frequently employed as blood donors in clinical practice due to their favourable physical and behavioural characteristics, showed a significantly lower percentage of DEA1-positive dogs (Iazbik et al., 2010; Mesa-Sanchez et al., 2014). In terms of hematological findings, the majority of dogs had no significant alterations in RBC, Hct and Hb levels, which is a positive factor in relation to their potential suitability as donors. However, the average MCV values observed in both TS and CS, were approximately 64 fL, just above the minimum limit of the reference interval. Notably, MCV indicates the size of RBCs which is already determined at the medullary level. Consequently, any condition that slows erythrocyte maturation or Hb formation can generate smaller cells, called microcytes. Microcytosis is often associated with iron deficiency and can have different etiologies; when iron levels decrease, the body tends to produce smaller erythrocytes, ensuring that the limited Hb is concentrated within them, maintaining a concentration of Hb at saturation. This trend may serve as an indicator of subclinical pathologies not yet manifested or non-optimal nutritional status (Harvey, 2012). Despite this, the parameters found closely align to the reference intervals. Considering the challenging management conditions in shelters, characterized by a high number of animals and consistent environmental factors, these results are commendable. Indeed, iron deficiency anaemia is prevalent in dogs and cats, often stemming from factors such as malabsorption with impaired gastroenteric pH, chronic bleeding (bleeding ulcers, parasites especially in puppies) and gastroenteric tract neoplasms leading to continuous dripping. This deficiency cannot be compensated by the introduction of iron through the digestion and intestinal absorption processes. Consequently, these anaemias are chronic haemorrhagic conditions, and over time can become iron deficiency (Naigamwalla et al., 2012). Retic-Hb coupled with reduced MCV emerges as a good indicator for detecting subclinical states of iron deficiency, allowing for early diagnosis, potentially as soon as 3–4 days into the pathological process (Steinberg et al., 2005). The association between clinical syndromes and Retic-Hb lacks specificity, focusing solely on the insufficient Hb production level rather than the underlying mechanism (Fuchs et al., 2017). In both shelters, the mean of Retic-Hb is almost overlaps with the minimum value of the reference range; moreover, in CS, the average of Retic-Hb dogs is 21.38 pg, slightly below the lower limit of the RI set at 22.30 pg. Unfortunately, both MCV and Retic-Hb have limitations as relatively late indicators of iron deficiency, incapable of distinguishing an iron deficiency anaemia, stemming from continuous blood loss, from an inflammatory anaemia due to iron sequestration. It is worth acknowledging that reticulocyte haemoglobin concentration can be influenced by inflammatory conditions, impacting both anaemic and non-anaemic dogs (Meléndez-Lazo et al., 2015; Schaefer et al., 2015). In this study, the dogs considered did not present an anaemic condition. Even if the faecal flotation tests resulted negative and the dogs were treated for gastrointestinal parasites, the high animal density in shelters, leading to increased environmental faecalization a potential stress-induced immunosuppression, often resulted in a higher prevalence of direct-life-cycle parasites in shelter dogs compared to family dogs, even when properly treated with endo and ectoparasite medications (Scaramozzino et al., 2018). Eosinophils were identified as the only leukocyte parameter that was significantly higher (Scaramozzino et al., 2018). Specifically, in CS, the average eosinophil count fell within the RI, while in TS it exceeded the upper limit. Elevated eosinophilic granulocytes may be indicative of allergic diseases and parasitosis, with recorded eosinophilia suggesting ongoing gastro-intestinal subclinical parasitosis (Raza et al., 2018). Regarding the biochemical profile, nearly all parameters, with rare exceptions, fell within the RIs. Notably, attention was drawn to the trend of GGT, an enzyme associated with different tissues’ membranes (bile ducts, acini and pancreatic ducts, renal tubular cells and those of the breast epithelium). GGT serves as a marker for liver cholestasis, demonstrating lower sensitivity but higher specificity than ALP, whose mean in CS also exceeded the RI. GGT plasma concentration typically increases in conditions such as hepatobiliary diseases, hyperadrenocorticism, glucocorticoid, or anticonvulsant therapies (although the ALP increase is much more significant), cholangiohepatitis, diabetes mellitus, pancreatitis, liver neoplasms. While an increase in GGT plasma concentration is less sensitive than ALP in signalling hepatobiliary diseases, it is more specific, although cases of possible enzymatic induction by endogenous or exogenous and barbiturate corticosteroids should be excluded. It is mainly associated with increased bile production resulting from bile stasis and/or bile cell hypertrophy (Paltrinieri et al., 2017). The average GGT in CS was above the RI, whereas in TS it remained within the RI. The causes of this increase in GGT may be attributed to nutritional imbalances and inflammatory processes of the gastroenteric apparatus, with consequent cholestasis phenomena. Finally, all SNAP tests performed for hemoparasitosis and protozoan infection yielded negative results. This is highly encouraging as the Marche Region is an endemic territory for Leishmaniasis (Mendoza-Roldan et al., 2020). The prophylaxis and controls carried out by the shelters, such as IFAT for Leishmania, have proven effective in maintaining the health of the host dogs and limiting this infectious disease. In fact, in 2021, within the dog shelters in the province of Macerata, where TS and CS are located, the IFAT test identified four positive dogs (3.2%) with antibody titres equal to or greater than 1:160. The test also detected 12 dogs (9.7%) with a “dubious” infection, featuring antibody titres between 1:40 and 1:80. According to regulations, these dogs are monitored over time with clinical and serological control every 8-16 weeks (Website VeSa Marche). However, selecting healthy blood donor canines in a region where vector-borne illnesses are prevalent poses significant challenges. It is crucial to opt for a serological and biomolecular inquiry panel that best fits the donor’s surroundings (Antognoni et al., 2022). The prevalence and distribution of many canine vector-borne disease are present and evolving all over Europe and are constantly monitored (Miró et al., 2012, 2022). The SNAP tests, accepted by the Ministerial Guideline on Veterinary Transfusion Medicine, resulted in negative for the hemoparasitoses. Despite being less sensitive than molecular and immunofluorescence tests for non-diseased animals, previous studies on rapid tests such as the SNAP® 4Dx® Plus Test Kit have demonstrated very high sensitivity and specificity (Chandrashekar et al., 2010; Stillman et al., 2014; Liu et al., 2018). This suggests that in daily clinical practice, rapid tests for hemoparasitoses can be used due to their ease of use and the provision of reliable quick results (Proverbio et al., 2016). Moreover, the Surveillance Plan of Marche region ensures the confirmation of dogs’ negativity for Leishmaniasis through the IFAT technique. A limitation of the study was the inability to perform the Babesiosis test (examined only on the blood smear) and urine analyses for all dogs. In addition, in a One Health approach, in future, it will be necessary to use more specific tests for hemoparasitosis, such as PCR in favour of the epidemiological supervision of the shelter dogs and reducing the transmission risk of zoonosis. Moreover, utilizing shelter dogs for blood donation presents various challenges due to the rapid and heterogeneous changes in their populations, coupled with the difficulty of obtaining a comprehensive clinical history. ConclusionThis study demonstrates that both the chosen populations of TS and CS could be suitable candidates for blood donations. Nevertheless, a significant limitation was found to be the large percentage of behavioral pathologies among the dogs, hindering the execution of clinical checks and blood sampling. Additionally, the dogs’ advanced age and the greater number of DEA1-positive dogs than DEA1-negative ones create obstacles. This could potentially enhance the adoption rate for older and larger dogs, often overlooked in adoption processes. In conclusion, the feasibility of using shelters for transfusion medicine in relation to the progress of research in this field, especially in the monitoring of vector-borne diseases, remains to be assessed. At the same time, the shelters, already integral to the health landscape of the territory, can play a crucial role in advancing veterinary practices and contributing to the overall well-being of both animals and the community. AcknowledgmentWe would like to thank the personnel of “Monti Azzurri” and “Colle Altino” shelters, for their kindness, trust, collaboration and care of the dogs. The authors are grateful to Niccolò Rossi for the English revisions. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsConceptualization, A.G., M.Q., G.R.; methodology, A.G., M.Q., AR., M.C., L.G., S.M., G.R.; software, M.Q.; data curation A.G., M.Q., S.M.; writing—original draft preparation, M.Q., A.G.; revision, A.G., M.Q., A.R., M.C., L.G., S.M., G.R; supervision, A.G. FundingThis research received no external funding. Data availabilityData supporting the results of this study are available from authors and are available on request. ReferencesAntognoni, M.T., Vascellari, M., Da Rold, G., Toniolo, F., Sgubin, S., Zanardello, C., Carminato, A. and Miglio, A. 2022. Looking for dog blood donors in an endemic area for vector-borne infections of Central Italy. Animals. 12, 817. Ashall, V. and Hobson-West, P. 2017. ‘Doing good by proxy’: human-animal kinship and the ‘donation’ of canine blood. Sociol Health Illn. 39, 908–922. Carli, E., Carminato, A., Ravagnan, S., Capello, K., Antognoni, M.T., Miglio, A., Furlanello, T., Proverbio, D., Spada, E., Stefani, A., Mutinelli, F. and Vascellari, M. 2017. Frequency of DEA 1 antigen in 1,037 mongrel and pure breed dogs in Italy. BMC Vet. Res. 13, 364. Chandrashekar, R., Mainville, C.A. and Beall, M.J. 2010. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am. J. Vet. Res. 71, 1443–1450. DeLuca, L.A., Glass, SG., Johnson, R.E. and Burger, M. 2006. Description and evaluation of a canine volunteer blood donor program. J. Appl. Anim. Welf. Sci. 9(2), 129–141. Descamps, M. and Humm, K. 2023. Why some canine and feline blood donors do not make the cut: A cohort study. Vet. Rec. 193. e2993. Dufour, A.B., Viggiano, E., Palme, R., De Palma, C., Natoli, E., Fantini, C. and Barillari, E. 2005. Evaluating the temperament in shelter dogs. Behaviour. 142, 1307–1328. Fuchs, J., Moritz, A., Grußendorf, E., Lechner, J., Neuerer, F., Nickel, R., Rieker, T., Schwedes, C., DeNicola, D.B., Russell, J. and Bauer N. 2017. Canine reticulocyte hemoglobin content (RET-He) in different types of iron-deficient erythropoiesis. Vet. Clin. Pathol. 46(3), 422–429. Harvey, J.W. 2012. Introduction to veterinary hematology. Vet. Hematol. 2012, 1–10. Iazbik, M.C., O’Donnell, M., Marin, L., Zaldivar, S., Hudson, D. and Couto, C.G. 2010. Prevalence of dog erythrocyte antigens in retired racing Greyhounds. Vet. Clin. Pathol. 39(4), 433–435. Liu, J., Drexel, J., Andrews, B., Eberts, M., Breitschwerdt, E. and Chandrashekar, R. 2018. Comparative evaluation of 2 in-clinic assays for vector-borne disease testing in dogs. Top. Companion Anim. Med. 33, 114–118. Mangiaterra, S., Rossi, G., Antognoni, M.T., Cerquetella, M., Marchegiani, A., Miglio, A. and Gavazza, A. 2021. Canine blood group prevalence and geographical distribution around the world: an updated systematic review. Animals (Basel). 11(2), 342. Medina Valentin, A.A., Gavazza, A. and Lubas, G. 2017. Prevalence of dog erythrocyte antigen 1 in 7,414 dogs in Italy. Vet. Med. Int. 2017, 5914629. Meléndez-Lazo, A., Tvarijonaviciute, A., Cerón, J.J., Planellas, M. and Pastor, J. 2015. Evaluation of the relationship between selected reticulocyte parameters and inflammation determined by plasma C-reactive protein in dogs. J. Comp. Pathol. 152(4), 304–312. Mendoza-Roldan, J., Benelli, G., Panarese, R., Iatta, R., Furlanello, T., Beugnet ,F., Zatelli, A. and Otranto, D. 2020. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: changing distribution patterns. Parasit. Vectors. 13(1), 193. Mesa-Sanchez, I., Ruiz de Gopegui-Fernández, R., Granados-Machuca, M.M. and Galan-Rodriguez, A. 2014. Prevalence of dog erythrocyte antigen 1.1 in galgos (Spanish greyhounds). Vet. Rec. 174(14), 351. Ministerial Guideline on Veterinary Transfusion Medicine. 2016. “Guidelines on the exercise of health activities concerning transfusion medicine in the veterinary field”. Official Journal of the Italian Republic n.25, 1 February 2016. Available online: https://www.fnovi.it/sites/default/files/LineeGuidaMedicinaTrasfus_0.pdf (Accessed on 29 June 2023). Miró, G., Checa, R., Montoya, A., Hernández, L., Dado, D. and Gálvez, R. 2012. Current situation of Leishmania infantum infection in shelter dogs in northern Spain. Parasit. Vectors. 5, 60. Miró, G., Wright, I., Michael, H., Burton, W., Hegarty, E., Rodón, J., Buch, J., Pantchev, N. and Von Samson-Himmelstjerna, G. 2022. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit. Vectors. 15, 189. Morganti, G., Miglio, A., Moretta, I., Misia, A.L., Rigamonti, G., Cremonini, V., Antognoni, M.T. and Veronesi, F. 2022. Retrospective longitudinal survey on canine vector-borne pathogens: trends and challenges of 10 years of activities of a veterinary blood bank. Vet. Sci. 9(6), 274. Mutinelli, F., Carminato, A. and Vascellari, M. 2013. Blood donation by dogs in shelters. Vet. Rec. 172(10), 274. Naigamwalla, D.Z., Webb, J.A., and Giger, U. 2012. Iron deficiency anemia. Can. Vet. J. 53(3), 250–256. Paleckaitis, M., Rakickaitė, G., Tolpežnikaitė, E., Buckiūnienė, V., Racevičiūtė-Stupelienė, A., Alijošius, S. and Trepėnaitienė, R., 2018. Breed and gender dependency of blood type in dogs. Vet. IR Zootech. 76, 71–74. Paltrinieri, S., Bertazzolo, W. and Giordano A. 2017. Pathophysiology and interpretation of laboratory tests. In Clinical pathology of dog and cat-practical approach to laboratory diagnostics. Milano, Italy: Edra S.p.A, pp: 90–91. Proverbio, D., Spada, E., Perego, R., Baggiani, L., Bagnagatti De Giorgi, G., Migliazzo, A. and Vitale, F. 2016. Comparison of a rapid immunochromatographic assay with an immunofluorescent antibody test for detection of Leishmania infantum antibodies in dogs. Vet. Clin. Pathol. 45, 623–626. Raza, A., Rand, J., Qamar, A.G., Jabbar, A. and Kopp, S. 2018. Gastrointestinal parasites in shelter dogs: occurrence, pathology, treatment and risk to shelter workers. Animals (Basel). 8(7), 108. Reine, N.J. 2004. Infection and blood transfusion: a guide to donor screening. Clin Tech Small Anim Pract. 19(2), 68–74. Regional Law 20 April 2015, n.18 concerning: Amendments to the regional law 20 January 1997, n.10 “Norms in matter of animals from affection and prevention of the straying”. Available via https://www.izs.it/IZS/Engine/RAServeFile.php/f/pdf_normativa/Iuvene_normativa_regionale_animali_affezione/Marche/Mar_20aprile2015_n18.pdf (Accessed 3 July 2023). Scaramozzino, P., Carvelli, A., Iacoponi, F. and De Liberato, C., 2018. Endoparasites in household and shelter dogs from Central Italy. Int. J. Vet. Sci. Med. 6, 45–47. Schaefer, D.M. and Stokol, T. 2015. The utility of reticulocyte indices in distinguishing iron deficiency anemia from anemia of inflammatory disease, portosystemic shunting, and breed-associated microcytosis in dogs. Vet. Clin. Pathol. 44(1), 109–119. Steinberg, J.D. and Olver, C.S. 2005. Hematologic and biochemical abnormalities indicating iron deficiency are associated with decreased reticulocyte hemoglobin content (CHr) and reticulocyte volume (rMCV) in dogs. Vet. Clin. Pathol. 34(1), 23–27. Stillman, B.A., Monn, M., Liu, J., Thatcher, B., Foster, P., Andrews, B., Little, S., Eberts, M., Breitschwerdt, E.B., Beall, M.J. and Chandrashekar, R. 2014. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J. Am. Vet. Med. Assoc. 245(1), 80–86. Surveillance Plan. 2012. Resolution of the Council of Marche Region n. 1652 (DGR 1652/2012), 26 November 2012 “Canine leishmaniasis surveillance and control plan”. Available via https://www.norme.marche.it/Delibere/2012/DGR1652_12.pdf (Accessed 3 July 2023). Wardrop, K.J., Birkenheuer, A., Blais, M.C., Callan, M.B., Kohn, B., Lappin, M.R. and Sykes, J., 2016. Update on canine and feline blood donor screening for blood-borne pathogens. J. Vet. Intern. Med. 30, 15–35. Wardrop, K.J. and Davidow E.B. 2023. Laboratory testing in transfusion medicine. Vet. Clin. North Am. Small Anim. Pract. 53(1), 265–278. Wardrop, K.J., Reine, N., Birkenheuer, A., Hale, A., Hohenhaus, A., Crawford, C. and Lappin, M.R. 2005. Canine and feline blood donor screening for infectious disease. J. Vet. Intern. Med. 19, 135–142. Wilder, A. and Humm, K. 2019. Pet owners’ awareness of animal blood banks and their motivations towards animal blood donation. Vet. Rec. 185(16), 509. Website Britannica.com. Available via https://www.britannica.com/place/Marche-region-Italy (Accessed 6 April 2024). Website VeSa Marche. Available via https://veterinariaalimenti.sanita.marche.it/Articoli/category/sanita-animale/leishmaniosi-canina-nelle-marche-sorveglianza-e-tasso-di-incidenza-annuale (Accessed 6 July 2023). Yagi, K. and Bean, L.B. 2016. Canine donor selection. In Manual of veterinary transfusion medicine and blood banking, USA: Wiley Blackwell, pp: 189, 193–196. | ||
| How to Cite this Article |
| Pubmed Style Quagliardi M, Rossi G, Cerquetella M, Roncarati A, Galosi L, Mangiaterra S, Gavazza A. Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Vet. J.. 2024; 14(8): 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 Web Style Quagliardi M, Rossi G, Cerquetella M, Roncarati A, Galosi L, Mangiaterra S, Gavazza A. Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. https://www.openveterinaryjournal.com/?mno=181417 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.5 AMA (American Medical Association) Style Quagliardi M, Rossi G, Cerquetella M, Roncarati A, Galosi L, Mangiaterra S, Gavazza A. Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Vet. J.. 2024; 14(8): 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 Vancouver/ICMJE Style Quagliardi M, Rossi G, Cerquetella M, Roncarati A, Galosi L, Mangiaterra S, Gavazza A. Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 Harvard Style Quagliardi, M., Rossi, . G., Cerquetella, . M., Roncarati, . A., Galosi, . L., Mangiaterra, . S. & Gavazza, . A. (2024) Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Vet. J., 14 (8), 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 Turabian Style Quagliardi, Martina, Giacomo Rossi, Matteo Cerquetella, Alessandra Roncarati, Livio Galosi, Sara Mangiaterra, and Alessandra Gavazza. 2024. Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Veterinary Journal, 14 (8), 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 Chicago Style Quagliardi, Martina, Giacomo Rossi, Matteo Cerquetella, Alessandra Roncarati, Livio Galosi, Sara Mangiaterra, and Alessandra Gavazza. "Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors." Open Veterinary Journal 14 (2024), 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 MLA (The Modern Language Association) Style Quagliardi, Martina, Giacomo Rossi, Matteo Cerquetella, Alessandra Roncarati, Livio Galosi, Sara Mangiaterra, and Alessandra Gavazza. "Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors." Open Veterinary Journal 14.8 (2024), 1779-1788. Print. doi:10.5455/OVJ.2024.v14.i8.5 APA (American Psychological Association) Style Quagliardi, M., Rossi, . G., Cerquetella, . M., Roncarati, . A., Galosi, . L., Mangiaterra, . S. & Gavazza, . A. (2024) Evaluation of the dog population in two Italian shelters in Central Italy (Marche region) as potential blood donors. Open Veterinary Journal, 14 (8), 1779-1788. doi:10.5455/OVJ.2024.v14.i8.5 |