| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 512-524 Original Research Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migrationSheren A. Al-Zahaby*, Sahar S. Hassan and Eman H. ElsheikhZoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt *Corresponding Author: Sheren A. Al-Zahaby. Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt. Email: Shalahmady [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
AbstractBackground: Catadromous fishes have well-developed elongated olfactory organs with numerous lamellae and different types of receptor neurons related to their breeding migration. Aim: The current study showed how the olfactory system adapted to the catadromous life. Our work declared the need of the migratory fishes for the sense of smell that is exhibited by a higher number of the olfactory lamellae and the receptor neuron verification in the olfactory epithelium. Methods: Ten specimens of fully grown, but pre-matured, silver eels of Anguilla vulgaris were captured at the outlet of Edco Lake, overlooking the Mediterranean Sea, east of Alexandria. Olfactory rosettes were dissected and fixed for scanning electron microscope (SEM) and transmission electron microscope (TEM). Results: Our study gave a morphological description of the olfactory system of A. vulgaris. At the ultrastructural level using SEM and TEM, one olfactory rosette was provided with 90–100 flat radial olfactory lamellae. The nasal configuration allowed water to enter and exit, transferring odorant molecules to olfactory receptor cells which comprise long cylindrical ciliated and microvillous receptors as well as rod-tipped cells. These cells are bipolar neurons with upward dendritic knobs. The olfactory epithelia also include crypt receptor cells. Interestingly, the olfactory neurons are delimited by nonsensory supporting cells, including long motile kinocilia and sustentacular supporting cells beside mucus secretory goblet cells and ionocytes or labyrinth cells that contribute to the olfaction process. Conclusion: Olfaction is crucial in all vertebrates, including fishes as it involves reproduction, parental, feeding, defensive, schooling, and migration behaviors. Here, A. vulgaris is an excellent model for catadromous fishes. It has a well-developed olfactory organ to cope with the dramatic climate change, habitat loss, water pollution, and altered ocean currents effect during their catadromous life for reproduction. Keywords: Olfactory rosette, olfactory epithelium, olfactory receptors, Anguilla vulgaris, electron microscope. IntroductionFish enjoy well-developed chemical sensors and olfactory and gustatory pathways that allow them to withstand aquatic habitats. Olfaction is typically distinguished by gustation is a close-quarters sensation with great sensitivity and selectivity. The sense of olfaction is vitally important in fish survival since it is necessary for locating food, evading predators, caring for young, migrating, and reproducing (Nikonov et al., 2017). The histological and fine structure of the olfactory mucosa of Vertebrata, including Pisces, was first described by Schultze (1862). These preliminary investigations demonstrated that nearly all vertebrates have three cellular components that make up their olfactory mucosa: sensory receptors, nonsensory supporting cells, and basal stem cells. Olfaction in fish takes place completely in the aquatic environment, so stimulant odor molecules detected by olfaction must be soluble to be carried in water. Therefore, fish have a wide variety of developed, well-developed olfactory organs (macrosmatic), such as those of the present studied eel, or they are poorly developed (microsmatic), such as Carangoides bajad (Salem, 2013). The olfactory organs of fish vary in gross anatomy as well as fine morphology, despite certain broad traits that are common to most species. Almost in all teleosts, a pair of nasal or olfactory chambers are symmetrically located in the head just below its dorsal surface and in front of the eyes (Zeiske et al., 1992). Nonetheless, the paired olfactory cavities in Elasmobranchii are located on the head's ventral surface, with the cavity accessible through just one opening, however, a complex skin fold separates the cavity into two chambers (Døving et al., 1977). However, the European eel is an isosmat since it does not have accessory nasal sacs, so ventilation is performed only by ciliated nonsensory cells, as proposed by Døving et al. (1977). Generally, the olfactory chambers in teleosts perform only olfactory functions, unlike air-breathing vertebrates, in which nasal chambers perform both olfactory and breathing functions (Cox, 2008). The olfactory rosettes are built up of linguiform lamellae that embrace sensory and nonsensory epithelia. The lamellae’s number within each rosette varies greatly from one only in Atheriniformes such as Hemiramphus sajori (Doroshenko and Motavkin, 1986) to much more than 130–230 in Anguillidae (Yamamoto and Ueda, 1978). The multiple lamellae are adopted in quite different arrangements; in one, they are called as a longitudinal array as the axis connecting the anterior and posterior nostrils is parallel to the lamellae. But in others, the lamellae radiate from a median support (raphe) and run parallel to the anterior–posterior nasal axis. (Cox, 2008). As in all vertebrates, the fish olfactory epithelium comprises receptor, supporting, basal, and goblet mucus-secreting cells. In addition to other specific cell that is found in particular circumstances, such as labyrinth and rod-tipped cells (Ghosh, 2021) and even other infrequently present receptor cells such as crypt neurons (Cheung et al., 2021), as well as Kappe neurons (Cheung et al., 2021). Anguilla vulgaris is one of the true freshwater eels, belonging to the order: Anguilliformes, family Anguillidae, has only one genus, Anguilla. All of them are migratory and found in marine, brackish, and freshwater environments. They are bottom dwellers and hide in burrows, crevices, plant masses, and other types of obstacles (Nandlal, 2005). Of these 19 species, only the European (Anguilla anguilla), North American (Anguilla rostrata), and Asian or Japanese (Anguilla japonica) are temperate, having more flexibility in habitat. Their life histories show a striking resemblance in characteristics since they are catadromous and migrate from freshwater to spawn in deep oceanic waters (Cresci, 2020). Due to his twice-very-long trip of migration across the European eel in the Atlantic, A. anguilla, is critically threatened. The cumulative effects of global change, such as climate change, increased pollution, dramatic reductions in available spawning habitats, and the appearance of parasitic nematodes that impair spawning, all can lead to the collapse of Anguilla’s population and not only the overfishing progression (Dekker, 2019). Geba et al. (2016) assigned the pre-adult phase (yellow eel) of A. vulgaris inhabiting freshwater migrates to the Sargasso Sea to spawn far out in the ocean and does not reproduce in captivity. Nonetheless, dramatic Eel populations are adversely affected by water pollution, habitat loss, climate change, and altered ocean currents (Wang et al., 2022). On the other hand, despite the direction mechanisms of glass and yellow eel’s migration toward the fresh and marine ecosystems, the recently arriving glass eels from the sea into estuaries are able to distinguish between water types by odors. Important to Anguilla's life cycle is the upstream migration stage, or glass eel, which is obviously influenced by odor stimuli, which provoke choices about entry into certain water types (Jellyman et al., 1999). Nonetheless, recently Cresci (2020) revealed that the earth's magnetic field and lunar cues are also utilized by eels in addition to water odor to be oriented during estuarine migration to the coast and freshwater in rivers. Therefore, in the present investigation, the olfactory organ ultrastructure of A. vulgaris (particularly the glass eel phase) was studied to elucidate their cellular components with the use of scanning and transmission electron microscopy in addition to light to shed high light on the olfaction's important role in the peculiar eel’s spawning voyage, going to the Sargasso Sea, and coming back. Materials and MethodsFish sampling and experiment designTen specimens of fully grown, but pre-matured, silver eels of A. vulgaris (Family: Anguillidae) were captured at the outlet of Edco Lake, overlooking the Mediterranean Sea, east of Alexandria, in December 2021 (Fig. 1 A). The selected eel specimens, measuring about 55 ± 7 cm long, were brought in oxygenated tanks to the laboratory at the Zoology Department in the Faculty of Science, Zagazig University, Egypt. Scanning electron microscopyThe anesthetized fish specimens were sacrificed right away by beheading. From the dissected heads, olfactory rosettes were excised from the nasal chambers and immediately immersed in saline solution to remove the adhering mucus. After being rinsed with 0.1 M phosphate buffer, the rosettes were primarily fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 hours at 4℃. After fixation, some rosettes were rinsed secondarily in the same buffer (pH 7.4) for 10 minutes and then post-fixed in 1% OsO4 in 0.1 M phosphate buffer, pH 7.4, for 2 hours. The rosettes were then washed carefully in the same buffer and dehydrated through a graded series of acetone, followed by isoamyl acetate. The rosettes, after being dried to the critical point, were mounted on metal stubs, gold-coated, scanned, and examined on a Joel IT200 scanning electron microscope (SEM) of the Faculty of Science, Alexandria University, Egypt. Semi-thin sections preparationsFrom the above primarily fixed rosettes, some solitary lamellae were carefully detached and cut into small pieces which were immediately post-fixed in 2.5% glutaraldehyde solution and 1% osmium tetroxide in the same phosphate buffer (pH 7.4) for 2 hours at room temperature. The post-fixed lamella’s pieces were then dehydrated in a graded series of ethanol and embedded in an Epon-Araldite mixture, which, by using a Reichert ultra-microtome, produced semithin sections of 1.5 mm. These semithin sections were stained with toluidine blue and contrasted in a 50% alcohol-uranyl acetate solution and lead citrate, as recommended by (Camilieri-Asch et al., 2020). With a transmission Philips EM 400 electron microscope at Alexandria University, Egypt, the olfactory lamellae were examined and photographed.
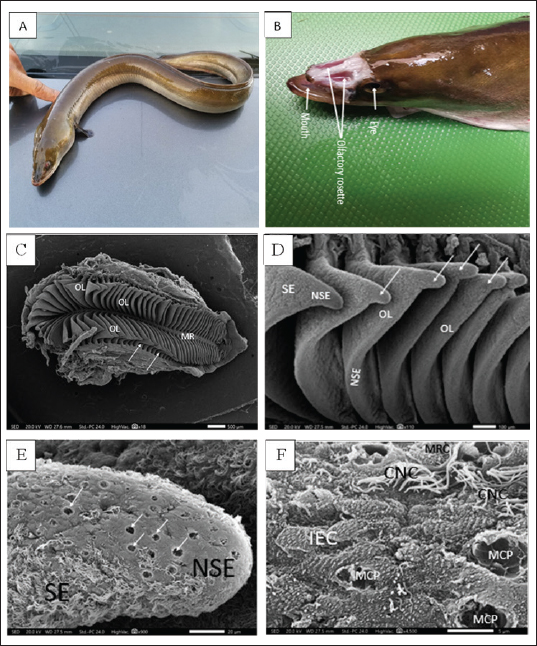
Figure 1. A. Photograph of the whole mount (lateral view) of the studied migratory fish stage of Anguilla vulgaris. B. Photograph of the head of Anguilla vulgaris (top view) with removed skin showing the two olfactory rosette and eyes. C. Scanning Electron Micrograph (SEM) of the olfactory rosette of Anguilla vulgaris showing a whole olfactory rosette with their approximately (90–100) olfactory lamellae (OL) arranged on both sides of the median raphe (MR). Notice the connection between the wall’s capsule and lamellae (arrows). Scale bar = 500μm. D. Scanning Electron Micrograph (SEM) of the olfactory lamellae (OL) of Anguilla vulgaris showing the glandular zoon (GZ) and ciliary zoon (CZ) of nonsensory epithelium (NSE) and the sensory one (SE), linguiform process of the olfactory lamellae (arrows). Scale bar = 100μm. E. Scanning electron micrograph (SEM) of the olfactory rosette of Anguilla vulgaris showing the median central glandular zoon (GZ) and the peripheral ciliary zoon (CZ) of nonsensory epithelial area (NSE) and the sensory area (SE) in-between on the linguiform process (LP) of olfactory lamellae (OL). Scale bar = 20μm. F. SEM micrograph showing; Glandular zoon (GZ) is formed of indifferent epithelial cells (IEC) with regularly arranged microridges and wide mucous cell pores (MCP) at middle center of the linguiform process (arrow) of an olfactory lamellae (OL), however the sensory area (SE) is appeared as isolated island in-between with its ciliated receptor (CRC) and microvillous receptor (MRC). Scale bar = 5μm. Transmission electron microscopyAnother post-fixed (in 1% osmium tetroxide for 2 hours at 4℃) olfactory lamella’s pieces were washed anew in the same buffer, dehydrated through ascending grades of ethanol, cleared in toluene, and embedded in epoxy resin. Ultrathin sections were obtained by using glass knives on a Reichert ultra-microtome. These ultrathin sections were stained with uranyl acetate and lead citrate and consequently photographed under a JEOL 100 CX transmission electron microscope (TEM) operated at an emissive mood of 60 KV in the Faculty of Science, Alexandria University, Egypt. Ethical approvalAll steps of this study were approved by the Ethical Committee of Animals of Zagazig University (ZU-IACUC/1/F/29/2023). ResultsThere are two large, elongated olfactory chambers of A. vulgaris are located just behind the eyes. Each chamber exhibits an olfactory rosette, so the two elongated rosettes in each chamber are remarkably far apart from each other (Fig. 1A and B). SEM observationsIn the present investigation, the olfactory organ of A. vulgaris is symbolized by a pair of elongated, laterally constricted rosette-like structures. They occupy most of the space of the two olfactory chambers, which open externally via an anterior inlet and a posterior outlet for entering and leaving water, respectively (Fig. 1A and B). Each olfactory rosette is made up of almost 90–100 flat radial olfactory lamellae close together and positioned perpendicularly in a plane on either side of a long, narrow support median raphe. It is recognized that the inner side of the lamellae adheres to the raphe while the outer comes into contact with the olfactory chamber walls. The middle lamellae of the rosette are somewhat larger and broader, with an outer concave edge linguiorm process, but they are diminished in size toward the posterior rosette’s end (Fig. 1C). This indicates new lamellae may be added by time at the posterior ends of the rosettes, elucidating why the number of lamellae increases with the fish's age and length. Each lamella has been walled by olfactory epithelia, which comprises a discontinuous nonsensory and sensory epithelial area. The nonsensory one is mostly restricted to the lamellae's margin, but the sensory area is apparent on the median side surfaces of each lamella (Fig. 1D). The non-sensory epithelial areaThe discontinuous nonsensory epithelial area is composed of indifferent, stratified epithelial cells with microridges and a compact assemblage of supporting cells either of long cilia, ciliated nonsensory, or nonciliated sustentacular supporting cells interposed with mucus secretory goblet cells. Therefore, this nonsensory area is regarded as the ciliary zone and glandular zone, respectively. The glandular zone of the nonsensory epithelial area extends around the periphery of the lamella. It is mainly built up of indifferent epithelial cells with overfull microridges mostly arranged in concentric ridges as a fingerprint pattern, in addition to many wide mucous cell pores (arrows) (Fig. 1E and F). However, the ciliary zone of the nonsensory epithelial area is built up of ciliated nonsensory cells with a dense coat of tufted long motile cilia that cover the majority of the lamellar periphery side surfaces. These long cilia are commonly inclined in the same direction, indicating their role in water circulation in the inter-lamellar spaces. This ciliary zone is mostly distributed throughout the indifferent epithelium as a minute ciliary island or at the edge of the glandular zone bordering it from the sensory area (Figs. 1E and F; and 2A and B). According to SEM, the free surface of the olfactory epithelium is in between receptor and supporting cells, with prominent longitudinal folds leaving long furrows in between. This represents the apical surface of the scarcely found labyrinth cells. (Fig. 2F). The sensory epithelial areaThe sensory epithelial area comprises mainly olfactory receptor cells, supporting cells, and a few mucus secretory goblet cells. Concerning the receptor cells, they are differentiated on the basis of their free terminal dendrites into three specific bipolar neurons: ciliated, microvillus, and scarcely rod-tipped cells. Their dendritic lumps protrude slightly above the surface of the neighboring cells, forming a terminal swelling, or dendritic knob. From each ciliated receptor cell, a varying number (4–6) of somewhat short and relatively thick primary nonmotile cilia radially emitted from their olfactory knob (Fig. 2D and E). However, the microvillous receptor cells release plentiful compressed microvilli-like short projections from their relatively elevated hump (Fig. 2D–F). Nonetheless, rod-tipped cells are occasionally seen, enlarged as compound tips at the epithelium's free surface forming a middle dendritic knob that emerges out of a single thick, prominent rod-like structure of varied lengths. It is much thicker than a normal cilium of neighboring ciliated cells and tapers progressively on the epithelial surface into the olfactory space (Fig. 2F). Histological semi thin section observationsHistologically, the semi thin sections of the olfactory epithelia of Anguilla’s lamellae display that they are made up of two pseudostratified columnar neuroepithelia. Each is composed of many cell types resting on a compact fibrous connective tissue, the basal lamina, which borders a central core of dense fibrous connective tissue enclosing blood vessels and nerve fibers. These olfactory epithelia comprise three olfactory receptor cell types: supporting cells, basal cells, and scattering mucous goblet cells (Fig. 3A and B).
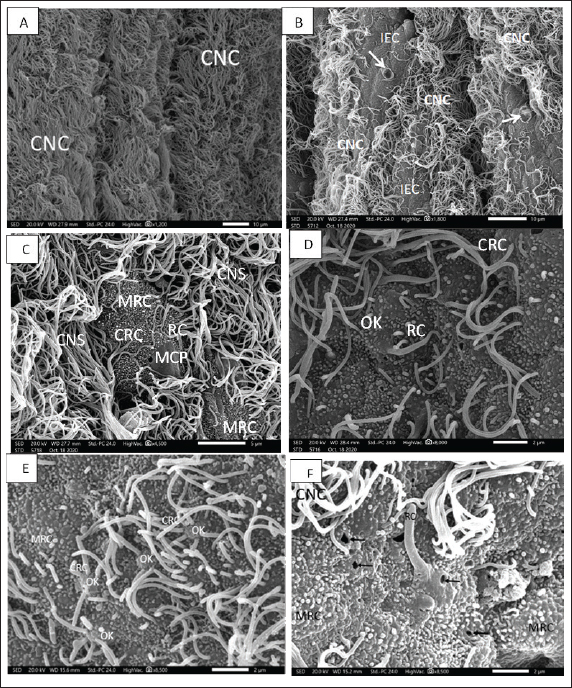
Figure 2. A. SEM micrograph showing; Strands of ciliated nonsensory cell (CNS) with their copies of tufted long cilia directed in one direction on the marginal edges of an olfactory lamella. Scale bar = 10μm. B. SEM micrograph showing; Sensory island of ciliated receptors (CRC), microvillous receptors (MRC), and rod receptors cell (RC) as well as other nonsensory island of indifferent (IEC) and mucous cell pores (MCPs) in-between copies of tufted long cilia of ciliated nonsensory cell (CNS) on an olfactory lamella. Scale bar = 10μm. C. SEM micrograph showing; two sensory islands of ciliated receptors (CRC), microvillous receptors (MRC), and rod receptors cell (RC) encountered with mucous cell pores (MCPs) in-between copies of tufted long cilia of ciliated nonsensory cell (CNS) on an olfactory lamella. Scale bar = 5μm. D. SEM micrograph of high magnification showing; sensory area of ciliated receptors (CRC), microvillous receptors (MRC), and rod-tipped receptors cell (RC) all of these dendrites emitted from a characteristic olfactory dendritic knob (OK) and encountered with mucous cell pores (MCPs) on an olfactory lamella. Scale bar = 2μm. E. SEM micrograph of high magnification showing; show; ciliated receptors cells (CRCs) with 3–5 thick somewhat short sensitive cilia and microvillous receptor cells (MRCs) with short sensitive microvilli (MV), both cilia and microvilli are emitted from a dendritic knob (OK). Scale bar = 2μm. F. SEM micrograph of high magnification showing; rod-tipped receptors cell (RC) with a very thick complex rod (R) also emitted from a dendritic knob (OK) and microvillous receptor cells (MRCs) and labyrinth cells (LCs). Scale bar = 2μm. Olfactory receptor cellsThe olfactory receptor cells are bipolar neurons occupying the entire thickness of the olfactory epithelium and distinguishing into ciliated, microvillus receptor cells, and rod-tipped cells. They are distinguished by their mounded apex and their strongly stained elongated or oval nuclei (Fig. 3A). Olfactory supporting cellsThe olfactory supporting columnar cells are distinguished by their small and weakly stained nuclei. They are either with long, abundant thin cilia erupted from their flat surface and denoted ciliated nonsensory cells or with outward microridges, indifferent epithelial cells. Both supporting cells are found mostly between the receptor cells (Fig. 3B). Goblet cellsThese cells are abundant and dispersed throughout the superficial olfactory epithelial layer. Their ovoid or rounded nuclei are moved toward the cell's basal ends (Fig. 3A and B). Basal cellsThe basal cells are smaller than all other cells and are found in the deepest area of the epithelial layers. They are oval or rounded in shape and have distinct, almost rounded nuclei. These cells constitute a reservoir for the invention of the other olfactory epithelial cells as they move upward in the epithelium (Fig. 3A and B). TEM observationsBy TEM, the olfactory epithelia with their entire cellular structure, described above by SEM and semithin sections, are also pronounced and illustrated. In addition to the olfactory receptor cells, olfactory supporting cells, rod cells, mucus secretory goblet cells, basal cells, hardly observed receptor crypt cells, and ionocyte labyrinth cells are also illustrated in the eel’s olfactory epithelia. The olfactory receptor cells are bipolar sensory receptors (neurons) with an unmyelinated axon and are regarded as ciliated, microvillous, as well as infrequently detected rod-tipped and crypt cells. Their cylindrical cell body comprises electron-lucent cytoplasm, abundant vesicular mitochondria, and bundles of cytoplasmic microfilaments at their third top portion. In addition to these cellular organelles, prominent cisternae of the endoplasmic reticulum and Golgi complex are obvious around the upper border of their basal, elongated, dense nucleoplasm. All three first receptor cells have a basal terminal long axonal process that runs toward the basal lamina to communicate with the main olfactory nerve. But they are varied in their free terminal dendrites, which are protruded radially from the mounded apex, dendritic knob specific for all olfactory neurons, and form the receptor surface. The ciliated receptor cells bear topical numerated (4−6) somewhat short, thick nonmotile cilia of a typical axonemal pattern of microtubules (9 + 2), but the microvillous receptor cells outlook plentiful microvilli also project from an olfactory knob. However, the third receptor rod cell has a long, thick terminal rod-like protrusion formed from the fusion or combination of many cilia, as indicated in Figures 2F and 3C, which also protrude from a dendritic knob-like apex. This rod-like protrusion has longitudinally oriented microtubules aligned in the (9 + 2) order in the transverse section as those of other ciliated cells along its axis (Fig. 3C–E). Nonetheless, the fourth receptor crypt cell bears some occulted short cilia surrounding apical microvilli on a dendritic knob (Fig. 3F), hidden under the other long cilia of olfactory epithelia, so they are seldom or hardly observed. Generally, the four receptor cells have a higher electron density in the nucleoplasm. These above-mentioned receptor cells are supported by supportive cells in between (Fig. 4A and B). These olfactory supporting cells are nonsensory, cylindrical or columnar, extending from the basal lamina to the apical surface of olfactory epithelia, closely adjacent to the sensory cells. They are either ciliated or nonciliated; the first, ciliated nonsensory cells have plentiful, tufty, very long motile cilia directed in the same direction. These cilia have a typical axonemal pattern of (9 + 2) microtubules, made by nine fused pairings of microtubules on the outside forming a cylinder and two unfused microtubules in the center of these nine pairs, so they are entitled kinocilia. These cells have a flat top surface, deep globular dense nucleoplasm associated with the obvious rough endoplasmic reticulum, terminal copious of vascular or elongated mitochondria, and Golgi complex (Fig. 4A and B). Nonetheless, the second supporting cell has central globular nuclei, and its free surface does not have cilia (nonciliated) but has concentric microridges in a pattern that looks like fingerprints and appears to be indifferent epithelial cells. Supporting cells differ from receptors in the general morphology, form, position, and staining of their nuclei. In TEM, they are less electron opaque than the receptor cells; their cytoplasm contains numerous tubular or vesicular endoplasmic reticulum, Golgi complex, and mitochondrial elements. In addition to these latter cells, the olfactory epithelia comprise many other nonsensory cells, such as mucus secretory goblet cells, which are usually dispersed between the receptor and supporting cells throughout the free surface, they are of spheroid or ovoid shape, containing basally placed globular nuclei and a lot of granulated mucous discharge that is released on the epithelial surface. These mucus cells are more abundant in the nonsensory areas, but there are a few in the sensory ones. The young goblet cells occur in the middle part of both epithelia and are enlarged considerably as mucus is formed and charged (Fig. 4C and D). Basal cells are small in ovoid shape with prominent quite large globular nuclei, which occupy most of the cell volume, leaving very little space for cytoplasm. These cells lie in a deeper part of the whole olfactory epithelia, just above the basal lamina (Fig. 4A and B).
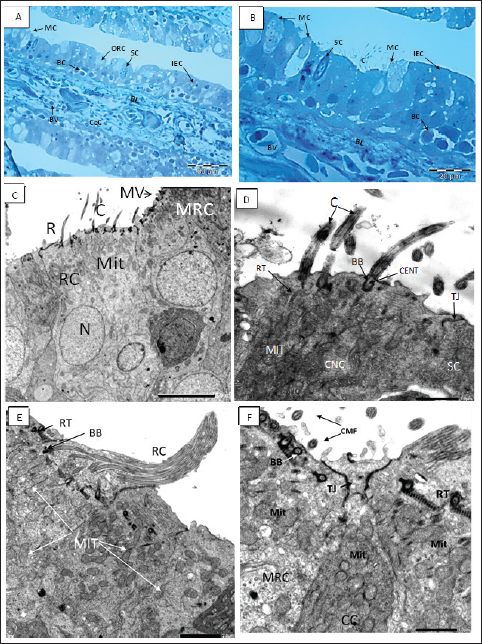
Figure 3. A, B Semithin section of the olfactory epithelium stained with Toluidine blue. (B) Ciliated nonsensory cells (CNCs) with cilia (C), distribution of many mucous cells (MCs), blood vessels (BVs). (C–D) The olfactory lamella consists of two layers of epithelium separated by central core (CeC) containing blood vessels (BVs) and basal lamina (BL); distribution of indifferent epithelium (IEC), olfactory receptor cell (ORC), supporting cell (SC), mucous cell (MC), basal cell (BC). C. TEM micrograph showing; Ciliated receptor (CRC) cell with short thick cilia (C), Microvillous receptor (MRC) cell with microvilli (MV), and Rod-tipped cell (RC) with compound cilium (R). All have a top mound surface, dendritic knob (OK) where their cilia, microvilli, and rod, respectively, are emitted radially. Both cells have prominent deeply positioned nucleus (N) and plentiful vesicular top-laying mitochondria (Mit). X–1,500, Scale bar: 5.0 μm. D. Higher magnification of the previous TEM micrograph showing; CRC with somewhat short thick cilia (C) emitted radially from a dendritic knob (OK), plentiful vesicular top-laying mitochondria in between two Microvillous receptor (MRC) cells. X–6,000, Scale bar: 1.0 μm. E. TEM micrograph showing; Rod-tipped receptor cell (RC) of compound cilium (R) protrudes also form a dendritic knob (OK) and has longitudinally oriented microtubules aligned in the 9 + 2 order in transverse section along its axis. This Rc is like other receptor cells has a prominent nucleus and plentiful vesicular top-laying mitochondria (Mit). X–3,000, Scale bar: 2.0 μm. F. TEM micrograph show; Crypt cell (CC) of ovoid shape bearing obscure or sunken short cilia (C) surround apical microvilli (MV). It has plentiful vesicular top-laying mitochondria (Mit) and manifested as an olfactory receptor cell. These cells are hardly detected in between the olfactory epithelia, so only are noticed in TEM figures. X–6,000, Scale bar: 1.0 μm.
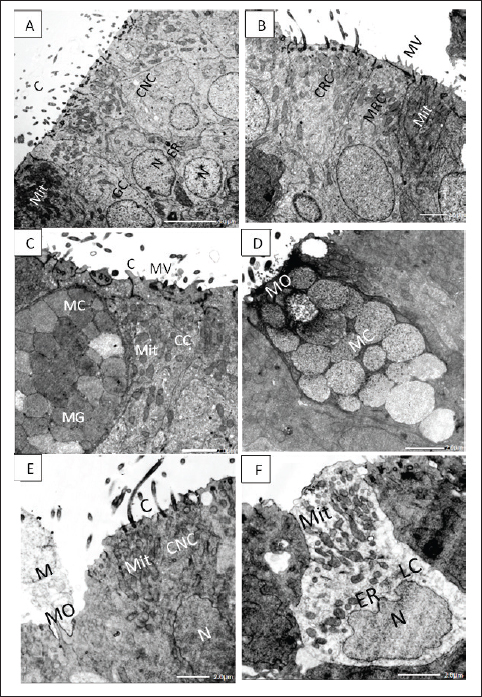
Figure 4. A. TEM micrograph showing; ciliated nonsensory cells (CNS) of flat top surface and long cilia (C), deep globular nuclei with dense nucleoplasm (N), and terminal plentiful vesicular or elongated mitochondria (Mit) associated with obvious rough endoplasmic reticulum (ER) and Golgi complex (GC). X–1,500, Scale bar: 5.0 μm. B. TEM micrograph showing; ciliated receptor cell (CRC) with somewhat short thick cilia, microvillous receptor cell (MRC) with short sensory microvilli (MV) both have central prominent nucleus (N) and multiple top-laying vesicular mitochondria (Mit) and tubular endoplasmic reticulum (ER) in the labyrinth cell. X–2,000, Scale bar: 2.0 μm. C.TEM micrograph showing; mucous cell (MC) with large mucous granules (MG) in the vicinity of crypt cell (CC) bears obscure short cilia (C) surrounding apical microvilli (MV) and has plentiful vesicular top-laying mitochondria (Mit). X–2,500, Scale bar: 2.0 μm. D. TEM micrograph showing; mucous cell (MC) with large mucous granules directly opens in the olfactory surface with mucous orifice (MO). X–2,500, Scale bar: 2.0 μm. E. TEM micrograph showing; ciliated nonsensory cell (CNC) with of flat top surface and long cilia (C), central prominent nucleus (N), and plentiful vesicular mitochondria (Mit). Mucous cell orifice (MO) with discharged mucus (M). X–2,500, Scale bar: 2.0 μm. F. TEM micrograph showing; ovoid or columnar labyrinth (LC) of basely located lopsided nucleus and plentiful top-laying mitochondrial vesicles (Mit) interconnected with tubular endoplasmic reticulum (ER). X–3,000, Scale bar: 2.0 μm. SEM investigation also showed prominent longitudinal folds, leaving some small furrows in between the olfactory epithelial surface, representing the apical surface of labyrinth cells. Labyrinth cells are elongated and scarcely observed in between the olfactory epithelial cells. They have an interconnected tubular structure of endoplasmic reticulum that is intimately linked to multiple mitochondrial vesicles (Fig. 4F). DiscussionAmong the most crucial senses for long-distance communication is smell, especially in dimly lit areas. The fish's olfactory organs are skilled at detecting water-soluble compounds for food finding, predator avoidance, social interaction, reproductive synchrony, and the pattern and direction of different migrations (Aicardi et al., 2022). The present study aims to illustrate anatomical and ultrastructural features of the peripheral olfactory organs of a very popular and interesting fish species, A. vulgaris. It is composed of two wide olfactory chambers, each with two specialized far-away external olfactory nares, but is not connected to the buccal cavity and so performs only olfactory functions, unlike the air-breathing vertebrates, which perform both olfactory and respiratory functions (Cox, 2008). The two external nares, inlet, and outlet, even when the fish is not actively swimming, its olfactory rosettes are in direct contact with an endless, massive volume of water that carries odorant molecules to the olfactory receptors (Aicardi et al., 2022). The size, shape, and arrangement of the olfactory organs (rosettes) and their constituting olfactory lamellae vary considerably in different fish species. Anguilla vulgaris has a keen sense of smell; it is a macrosmatic fish ‘‘nose-fishes” since its olfactory surface area is about 59.9% of the eye's retinal surface area (Atta, 2013). This finding is in accordance with what was also detected Hypophthalmichthys molitrix (El-Attar and Al-Zahaby, 2010). The two olfactory rosettes of the studied fish, A. vulgaris, are elongated and have been established as about 90–100 foliate olfactory lamellae. These are transversely arrayed bilaterally on both sides of a narrow, long support, the median raphe, affording a very large olfactory surface area. This configuration of olfactory rosettes and their array of lamellae awards uniform space between the closely parallel lamellae. Therefore, an odorant has to travel a lot less distance to reach the olfactory epithelium (Cox, 2008). Anguilla vulgaris has one of the most advanced olfactory organs among fishes. In the present studies, the epithelia covering its olfactory lamellae are pseudostratified based on a central core made up of dense fibrous connective tissue congested with blood vessels and nerve fibers to nourish its cellular components. The latter is overcrowded with many receptors and supporting cells in addition to scattering mucus secretory goblet cells as well as deeply positioned basal cells, all of which are not regularly aligned in the olfactory mucosa. The olfactory receptor cells are categorised into four types according to their apical dendritic protrusions into ciliated receptor cells and microvillous receptor cells, in addition to the infrequently occurring rod-tipped cells and the rarest crypt receptor cells. Otherwise, the nonsensory components comprise olfactory supporting cells which are either ciliated nonsensory cells and/or sustentacular supporting cells as well as less visible labyrinths and indifferent epithelial cells that appear to be specific to vertebrates and likely aid in preserving the ideal ion balance that is vital for fish migration (Bertmar, 1972). In the olfactory epithelia of all Anguiliformes species, even at their post-larval, glass eel stage, the olfactory mucosa of all vertebrates is composed of four main cellular components: receptor, supporting, basal, and mucus secretory goblet cells. The first ones are bipolar neurons, with an apical end with variable appearance, and number of dendritic protrusions, and a basal axon end. Therefore, they are categorized into four cell types: ciliated, microvillous, rod-tipped, and crypt cells (Triana-Garcia et al., 2021), in addition to the Kappe neuron (Ahuja et al., 2014) and the pear-shaped neurons (Wakisaka et al., 2017), but both were not represented in eels. These receptor cells have nearly similar morphologies and ultrastructures but are variously present in different fish species, from Cyclostomata (Lampreys), Elasmobranchs, and Osteichthyes to teleosts (Zeiske et al., 1992). The olfactory receptor neuronsThe olfactory epithelium of A. vulgaris contains ciliated and microvillous receptor cells in addition to the infrequently present rod cells and ciliated receptor cells which are all insulated from each other by supporting cells and intermingled with supporting cells and mucus secretory cells. All animals have olfactory receptor neurons, which are exemplary sensory neurons with strikingly similar cellular and molecular characteristics (Axel, 2005). The olfactory receptor cells are bipolar neurons distinguished by their cell dendritic protrusions. From their apical free surface, a single process expands into a mound-like projection, an olfactory or dendritic knob, from which several olfactory cilia or microvilli radiate. The olfactory receptor neurons of the cilia or of the microvilli possess the necessary properties to transform an odorant-receptor stimulus into an electrical signal. Receptor elements sensing the odorants are concentrated on the dendritic protrusion, either cilia or microvilli, of the olfactory sensory neurons (Othman et al., 2022). The microvilli are short, extremely thin protrusions of uniform length released from the cell surface, increasing its surface area and providing a critical role as a protective barrier since they are supported by parallel actin bundles (Barr-Gillespie, 2015). Their core is formed of microfilaments lacking (9 + 2) ultrastructure microtubules and does not arise from the basal granules, so they are nonmotile but sense the external environment as mechanosensory (Houdusse and Titus, 2021). In addition to these two olfactory receptors in A. vulgaris and even in all Anguilliformes, two other infrequently occurring forms of receptor neuron rod-tipped cell and crypt cell are detected (Schulte, 1972). The rod cell is a steady rod-like or compound cilium, with ciliary or microvillar components partially fused and emitted from the entire free margin of a dendritic knob, so the cell is often called a rod-tipped cell. These cells occur singly or in groups in some places where their rod-like cilia are sparse on the olfactory lamellae with a relatively long and thick rod. Despite the fact that the ciliated and microvillous receptor cells are inclusively found in practically all fishes' olfactory epithelia, the RC is also detected more rarely in some fish species from the Cypriniformes: Danio rerio (Cheung et al., 2021). Otherwise, Yamamoto and Ueda established that the number of rod cells increased during the smoltification period of Salmonid Oncorhynchus masou before entering salt water from fresh water (anadromous fish), which may be like the present studied eel during its regular migration (Yamamoto and Ueda, 1978). The fourth olfactory receptor, crypt cells, has been hardly detected in the olfactory epithelium of A. vulgaris. They have a superficially positioned, ovoid-shaped soma with an apical crypt bearing microvilli and cilia. Crypt cells also showed up in many other teleost species with the same uniform morphology (Triana-Garcia et al., 2021) They were also detected even in elasmobranch Rajiformes (Ferrando et al., 2007). Crypt cells are much less abundant than ciliated receptor cells or microvillous receptor cells, if they represent any fish species (Cheung et al., 2021; Al-Zahaby et al., 2023). The olfactory system of fish is remarkably effective in detecting and differentiating between a wide range of water-soluble substances, including steroids, prostaglandins, amino acids, bile acids, and nucleotides (Yoshihara, 2014). Every receptor neuron carries unique chemical stimuli that, when combined with olfactory cues related to fish vital life processes, produce unique behaviors. The olfactory neurons are specific receptors adjusted to detect definite odorant ligands (Bazáes et al., 2013). This may be due to the submergence of both microvillous receptor neurons and ciliated receptor neurons in the thickness of cilia copies of both sensory and nonsensory cells (Lazzari et al., 2022). To allow fish to detect food, bile salt, and amino acid odorants (food that has an odor) are the main stimulants of ciliated receptor neurons (Chakrabarti and Ghosh, 2011b). They are concentrated after fish starvation, which may be performed during breeding migration (Furne and Sanz, 2018). While ciliated and microvillous olfactory receptor neurons are activated by amino acid odorants, ciliated receptor neurons are primarily responsible for detecting bile salt odorants. Bile salts are potent olfactory stimulants, some of which act as chemical signals for fish communication. Nucleotides released from food into ambient water, however, are thought to serve as feeding cues and are recognized by Wakisaka et al. (2017). Generally, the ability and direction of migratory movements are influenced by the smell of silver-phase eels, and the loss of smell does not prohibit movement to the sea, but it substantially reduces orientation (Barbin et al., 1998). The olfactory nonsensory cellsThe olfactory nonsensory epithelia basically comprise olfactory supporting cells, labyrinth cells, mucus secretory goblet cells, and basal cells. The olfactory supporting cellsThe olfactory supporting cells are nonsensory cells, so they are not labeled as sensory neurons (Triana-Garcia et al., 2021). They occupy most of the olfactory epithelium, comparable to neural glial cells, so they surround the different receptor cell bodies and dendrites. They are cylindrical cell bodies stretched throughout the entire thickness of the olfactory epithelia and taper basally to the basal lamina. Their top surface is relatively flat, bearing either long motile cilia or minute microvilli (Yamamoto, 1978), so they are of two types: ciliated nonsensory cells and sustentacular supporting cells. Other than the receptor cilia, the driving force behind ciliary beating is provided by the dynein arms on kinocilia (Zeiske et al., 1992). The ciliated nonsensory cell architecture is similar to the respiratory epithelial cells of a tetrapod’s nasal cavities, which are often described as respiratory cells. Their cilia beat in unison, demonstrating their active function in promoting fluid flow across the olfactory epithelium (Reiten et al., 2017). In the A. vulgaris of the present studies, ciliated nonsensory cells occur singly or in aggregates throughout the olfactory lamella’s surface. It seems likely that the motile kinocilia of ciliated nonsensory cells are longer and more involved in the water flow through and around olfactory lamellae. The beating action of olfactory cilia was likely to favor efficient odorant transport to the olfactory epithelium (Døving et al., 1977). Furthermore, Cox (2008) also specified that the vigorous water flow of synchronized beating of cilia alone is sufficient both to draw water into the olfactory chambers and to circulate water within and around olfactory lamella. This vigorous water flow also removes foreign minute particles and microorganisms from the lamellae along with the mucus (Reiten et al., 2017). Mucous or Goblet cellsThe name "goblet" refers to the cup-shaped form of the mucus secretory goblet cells, which are unicellular glands that are specialized for the synthesis and secretion of mucus and are packed full of mucin granules as described by Birchenough et al. (2015). They are abundantly scattered within the olfactory nonsensory areas in between the indifferent epithelial cells and ciliated nonsensory cells of the studied fish, A. vulgaris. They are found in the top layer of the olfactory epithelia and can vary widely in size. Mucigen, which is produced by their numerous rough endoplasmic reticulum and Golgi complexes, as well as mitochondria encircled in the basal dense cytoplasm around their global nuclei, are all found in their apical cytoplasm. This mucigen is converted in turn into vesicles mucins (mucus) granules at the apical cell pole to be released onto the olfactory epithelial surface. The mucus covering the olfactory lamellae surfaces constitutes an important medium in which the odorants are diffused. Furthermore, the secreted mucus protects the olfactory epithelia from mechanical abrasion and possibly helps in hunting microscopic rubbish remains, which retains the receptor neurons get for new stimuli. Rygg et al. (2013) later surmised that fish may have a higher chance of detecting an odor if the mucus layer is evenly spread across the olfactory neurons. This cell surface mucin surrounds and hangs on the cilia, forming a periciliary layer essential for the help and lubrication of the ciliary beat. It regulates and ensures efficient transport of water among olfactory lamellae, permits the infusion of immune factors, and excludes toxins and pathogens. Basal CellsThe basal cells are small polygonal cells having globular, centrally located nuclei that interpose intermediate tonofilaments, they are spread in a monolayer adjacent to the basal lamina of olfactory epithelia and comprise two categories: basal cells proper and globose basal cells (Suzuki and Takeda, 1993). Nonetheless, Holbrook et al. (1995) suggested that by aiding in the olfactory epithelium's healing from wounds, the basal cells themselves, which act as a link between the basal processes of some sustentacular supporting cells and the basal lamina, contribute to the preservation of the olfactory epithelium's structural integrity. Generally speaking, throughout a fish's life, the olfactory epithelium is still capable of neurogenesis. Because these basal neural stem cells are present, the olfactory epithelia undergo lifelong renewal as ciliated nonsensory cells and mucous cells eventually degenerate and die, to be replaced by new cells derived from the mitotically active basal cells (Goss et al., 2016). Labyrinth cellsThe superficial layer of the olfactory epithelium contains labyrinth cells, which are bulbous in shape and located between sensory and nonsensory cells. They are basal nuclei with copious interconnected tubular endoplasmic reticulum intimately connected with numerous filamentous mitochondria and homogeneous fine granules. Morphologically, labyrinth cell is not modified sensory, nonsensory-supporting, or goblet cells but have quite another fine structure (Bertmar, 1972). By SEM, the olfactory epithelial surface of the eel under study displayed noticeable longitudinal folds with lengthy furrows between, which symbolized the apical surface of labyrinth cells, as those declared by Chakrabarti and Ghosh (2011a) in freshwater catfish and tigerperch, respectively. They are easily recognizable as rather large columnar cells crossing the entire olfactory epithelium thickness with clear cytoplasm and apical cup morphology (Camilieri-Asch et al., 2020). It is plausible that fish can develop the ion balance required for optimal olfactory functions by having their labyrinth cells excrete electrolytes during the sea phase and absorb them during the freshwater phase. Their concern lies in the active ion absorption in freshwater environments by Anguilla japonica (Shirai and Utida, 1970). Chakrabarti and Ghosh (2011a) speculated that the labyrinth cells play the chief role in osmoregulation, incentivizing the olfactory epithelium to work optimally in water of different salinities, particularly in migratory fish like Anguilliformes species. They also play a part in preserving the ideal ionic and acid-base balance in the gills, mucosa, and skin of different fish. ConclusionAnguilla vulgaris of the present study acquires well-developed olfactory sense organs for migration overseas and oceans and is able to determine the chemical changes in the surroundings. It possesses one of the most sensitive olfactory senses among fish, which plays a central role in their catadromous lives. AcknowledgmentThe authors are very grateful to Professor Abdelbadie E El-Attar for his efforts in the practical section, also authors thank Professor Al-Ahmady Al-Zahaby for his efforts in the manuscript writing items, also authors thanks Dr. Suzan Attia Mawed for the final manuscript editing and revision. Conflicts of interestThere is no conflict of interest, according to the authors. Author contributionsConceptualization, S.A.A. and E.H.E., methodology, S.A.A and S.S.H. Pictures capturing and adjustment, S.S.H. investigation, E.H.E. resources, E.H.E and S.A.A., original draft preparation, S.A.A., and E.H.E.; writing-review and editing S.A.A and S.S.H. Data availabilityUpon request, the corresponding author can provide the data presented in this study. ReferencesAhuja, G., Nia, S.B., Zapilko, V., Sh iriagin, V., Kowatschew, D., Oka, Y. and Korsching, S.I. 2014 Kappe neurons, a novel population of olfactory sensory neurons. Sci. Rep. 4, 4037. Aicardi, S., Bozzo, M., Amaroli, A., Gallus, L., Risso, B., Carlig, E., Di Blasi, D., Vacchi, M., Ghigliotti, L. and Ferrando, S., 2022. The arrangement of the peripheral olfactory system of Pleuragramma antarcticum: a well-exploited small sensor, an aided water flow, and a prominent effort in primary signal elaboration. Animals 12, 663. Al-Zahaby, S.A., Farag, M.R., Alagawa ny, M., Taha, H.S., Varoni, M.V. and Crescenzo, G., Mawed, S.A., 2023. Zinc oxide nanoparticles (ZnO-NPs) induce cytotoxicity in the zebrafish olfactory organs via activating oxidative stress and apoptosis at the ultrastructure and genetic levels. Animals 13, 2867. Atta, K., 2013. Morphological, anatom ical and histological studies on the olfactory organs and eyes of teleost fish: Anguilla anguilla in relation to its feeding habits. J. Basic. Appl. Zool. 66, 101–108. Axel, R., 2005. Scents and sensibility : a molecular logic of olfactory perception (Nobel lecture). Angew. Chem. Int. Ed. Engl. 44, 6110–6127. Barbin, G.P., Parker, S.J. and McCleave , J.D. 1998. Olfactory clues play a critical role in the estuarine migration of silver-phase American eels. Environ. Biol. Fish. 53, 283–291. Barr-Gillespie, P.-G., 2015. Assembly of hair bundles, an amazing problem for cell biology. Mol. Biol. Cell. 26, 2727–2732. Bazáes, A., Olivares, J. and Schmachtenbe rg, O., 2013. Properties, projections, and tuning of teleost olfactory receptor neurons. J. Chem. Ecol. 39, 451–464. Bertmar, G., 1972. Labyrinth cells, a new c ell type in vertebrate olfactory organs. Z. Zellforsch. Mikrosk. Anat. 132, 245–256. Birchenough, G.M., Johansson, M.E., Gustafss on, J.K., Bergström, J.H. and Hansson, G., 2015. New developments in goblet cell mucus secretion and function. Mucosal. Immunol. 8, 712–719. Camilieri-Asch, V., Caddy, H.T., Hubbard, A., Rigby, P., Doyle, B., Shaw, J.A., Mehnert, A., Partridge, J.C., Yopak, K.E. and Collin, S.P., 2020. Multimodal imaging and analysis of the neuroanatomical organization of the primary olfactory inputs in the brownbanded Bamboo shark, Chiloscyllium punctatum. Front. Neuroanat. 14, 560534. Chakrabarti, P. and Ghosh, S.K., 2011a. The st ructural organization and functional aspects of the olfactory epithelium of tigerperch, Terapon jarbua (Forsskål, 1775) (Perciformes: Terapontidae). Turk. J. Zool. 35, 793–799. Chakrabarti, P. and Ghosh, S., 2011b. Histologic al and ultrastructural studies of the olfactory epithelium of spotted butter fish Scatophagus argus (Linnaeus). Folia. Morphol. 70, 74–79. Cheung, K.Y., Jesuthasan, S.J., Baxendale, S., Va n Hateren, N.J., Marzo, M., Hill, C.J. and Whitfield, T.T., 2021. Olfactory rod cells: a rare cell type in the larval zebrafish olfactory epithelium with a large actin-rich apical projection. Front. Physiol. 12, 626080. Cox, J.P., 2008. Hydrodynamic aspects of fish olf action. J. Royal. Soc. Interface. 5, 575–593. Cresci, A., 2020. A comprehensive hypothesis on th e migration of European glass eels (Anguilla anguilla). Biol. Rev. 95, 1273–1286. Dekker, W., 2019. The history of commercial fisheri es for European eel commenced only a century ago. Fish. Manag. Ecol. 26, 6–19. Doroshenko, M. and Motavkin, P., 1986. Olfactory epi thelium of marine fishes in scanning electron microscopy. Acta. Morphol. Hung. 34, 143–155. Døving, K.B., Dubois-Dauphin, M., Holley, A. and Jour dan, F., 1977. Functional anatomy of the olfactory organ of fish and the ciliary mechanism of water transport. Acta. Zoologica. 58, 245–255. El-Attar, A. and Al-Zahaby, S.A. 2010. Structural organi za tion of the chemoreceptor organs in silver carp. In Proceedings of International Congress of Biological Science and Zoology, Egypt, pp: 103–118. Ferrando, S., Bottaro, M., Pedemonte, F., De Lorenzo, S., Gallus, L. and Tagliafierro, G., 2007. Appearance of crypt neurons in the olfactory epithelium of the skate Raja clavata during development. Anatom. Rec. 290, 1268–1272. Furne, M. and Sanz, A. 2018. Starvation in fish–sturgeon a nd ra inbow trout as examples. In Handbook of famine, starvation, and nutrient deprivation. Eds., Preedy, V. and Patel, V. pp: 1–16. Geba, K.M., Hassab, El-Nabi, S.E.-S. and El-Desoky, M.S. 201 6. Development of cytochrome-c-oxidase 1 specific primers for genetic discrimination of the European eel Anguilla anguilla (Linnaeus, 1758). J. Biosci. Appl. Res. 2, 258–262. Ghosh, S.K. 2021. The olfactory organ of schilbid catfish Eut ropiichthys vacha (Hamilton, 1822): morphological and ultrastructural studies. J. Basic. Appl. Zool. 82, 1–11. Goss, G.M., Chaudhari, N., Hare, J.M., Nwojo, R., Seidler, B., Saur, D. and Goldstein, B.J. 2016. Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing. Develop. Neurobiol. 76, 241–251. Holbrook, E.H., Szumowski, K.E.M. and Schwob, J.E., 1995. An imm unochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J. Compar. Neurol. 363, 129–146. Houdusse, A. and Titus, M.A. 2021. The many roles of myosins in f ilopodia, microvilli and stereocilia. Curr. Biol. 31, R586–R602. Jellyman, D., Chisnall, B., Bonnett, M. and Sykes, J., 1999. Seaso nal arrival patterns of juvenile freshwater eels (Anguilla spp.) in New Zealand. New Zealand J. Mar. Freshwater. Res. 33, 249–261. Lazzari, M., Bettini, S., Milani, L., Maurizii, M.G. and Franceschi ni, V. 2022. Response of olfactory sensory neurons to mercury ions in zebrafish: an immunohistochemical study. Microsc. Microanal. 28, 227–242. Nandlal, S., 2005. Catching eels in Pacific Island countries and ter ritories. Fish. Newsletter-South. Pac. Comm. 115, 44. Nikonov, A.A., Butler, J.M., Field, K.E., Caprio, J. and Maruska, K. P. 2017. Reproductive and metabolic state differences in olfactory responses to amino acids in a mouth brooding African cichlid fish. J. Exper. Biol. 220, 2980–2992. Othman B.A., Maulud S.Q., Jalal P.J., Abdulkareem S.M., Ahmed J.Q., D hawan M. and Choudhary O.P., 2022. Olfactory dysfunction as a post-infectious symptom of SARS-CoV-2 infection. Ann. Med. Surg. 75, 103352. Reiten, I., Uslu, F.E., Fore, S., Pelgrims, R., Ringers, C., Verdugo, C.D., Hoffman, M., Lal, P., Kawakami, K. and Pekkan, K., 2017. Motile-cilia-mediated flow improves sensitivity and temporal resolution of olfactory computations. Curr. Biol. 27, 166–174. Rygg, A.D., van Duin, A.C. and Craven, B.A. 2013. Molecular dynamics s imulations of water/mucus partition coefficients for feeding stimulants in fish and the implications for olfaction. PLoS One 8, e72271. Salem, M., 2013. Comparative study on the structure of the eye and olf actory organ in two bony fishes in relation to their feeding habits. Egypt. J. Zool. 60, 189–222. Schulte, E., 1972. Untersuchungen an der Regio olfactoria des Aals, Ang uilla anguilla L. Z. Zellforsch. Mikrosk. Anat. 125, 210–228. Schultze, M.J.S., 1862. Untersuchungen über den Bau der Nasenschleimhaut : namentlich die Structur und Endigungsweise der Geruchsnerven bei dem Menschen und den Wirbelthieren HW Schmidt. Shirai, N. and Utida, S., 1970. Development and degeneration of the chlor ide cell during seawater and freshwater adaptation of the Japanese eel, Anguilla japonica. Z. Zellforsch. Mikrosk. Anat. 103, 247–264. Suzuki, Y. and Takeda, M. 1993. Basal cells in the mouse olfactory epithel ium during development: immunohistochemical and electron-microscopic studies. Develop. Brain. Res. 73, 107–113. Triana-Garcia, P.A., Nevitt, G.A., Pesavento, J.B. and Teh, S.J., 2021. Gro ss morphology, histology, and ultrastructure of the olfactory rosette of a critically endangered indicator species, the Delta Smelt, Hypomesus transpacificus. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 207, 597–616. Wakisaka, N., Miyasaka, N., Koide, T., Masuda, M., Hiraki-Kajiyama, T. and Y oshihara, Y. 2017. An adenosine receptor for olfaction in fish. Curr. Biol. 27, 1437–1447. Wang, H., Wan, H.T., Wu, B., Jian, J., Ng, A.H., Chung, C.Y.-L., Chow, E.Y.-C ., Zhang, J., Wong, A.O. and Lai, K.P., 2022. A chromosome-level assembly of the Japanese eel genome, insights into gene duplication and chromosomal reorganization. Gigascience 11, 120. Yamamoto, M. 1978. Comparative morphology of fish olfactory epithelium-II clu peiformes. Nippon Suisan Gakkaishi 44, 855–859. Yamamoto, M. and Ueda, K., 1978. Comparative morphology of fish olfactory epit helium. Iv: Anguilliformes And Myctophiformes. Bull. Jpn. Soc. Sci. Fisheries. 44(11), 1207–1212. Yoshihara, Y. 2014 Zebrafish olfactory system. The olfactory system: from odor molecules to motivational behaviors, (ed. Mori, K.), Tokyo: Springer, pp: 71–96. Zeiske, E., Theisen, B. and Breucker, H., 1992. Structure, development, and evol utionary aspects of the peripheral olfactory system. Fish chemoreception. Chapmann & Hall, London, pp: 13–39. | ||
| How to Cite this Article |
| Pubmed Style Al-zahaby SA, Hassan SS, Elsheikh EH. Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 512-524. doi:10.5455/OVJ.2024.v14.i1.46 Web Style Al-zahaby SA, Hassan SS, Elsheikh EH. Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. https://www.openveterinaryjournal.com/?mno=182416 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.46 AMA (American Medical Association) Style Al-zahaby SA, Hassan SS, Elsheikh EH. Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 512-524. doi:10.5455/OVJ.2024.v14.i1.46 Vancouver/ICMJE Style Al-zahaby SA, Hassan SS, Elsheikh EH. Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 512-524. doi:10.5455/OVJ.2024.v14.i1.46 Harvard Style Al-zahaby, S. A., Hassan, . S. S. & Elsheikh, . E. H. (2024) Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 512-524. doi:10.5455/OVJ.2024.v14.i1.46 Turabian Style Al-zahaby, Sheren A., Sahar S. Hassan, and Eman H. Elsheikh. 2024. Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 512-524. doi:10.5455/OVJ.2024.v14.i1.46 Chicago Style Al-zahaby, Sheren A., Sahar S. Hassan, and Eman H. Elsheikh. "Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration." Open Veterinary Journal 14 (2024), 512-524. doi:10.5455/OVJ.2024.v14.i1.46 MLA (The Modern Language Association) Style Al-zahaby, Sheren A., Sahar S. Hassan, and Eman H. Elsheikh. "Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 512-524. Print. doi:10.5455/OVJ.2024.v14.i1.46 APA (American Psychological Association) Style Al-zahaby, S. A., Hassan, . S. S. & Elsheikh, . E. H. (2024) Ultramicroscopic organization of the exterior olfactory organ in Anguilla vulgaris in relation to its spawning migration. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 512-524. doi:10.5455/OVJ.2024.v14.i1.46 |