| Case Report | ||
Open Vet. J.. 2024; 14(5): 1302-1308 Open Veterinary Journal, (2024), Vol. 14(5): 1302–1308 Case Report Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case reportRadka Garnoeva*, Rumen Roydev and Radina VasilevaDepartment of Veterinary Surgery, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria *Corresponding Author: Radka Garnoeva. Department of Veterinary Surgery, Faculty of Veterinary Medicine, Stara Zagora, Bulgaria. Email: dr.garnoeva [at] abv.bg Submitted: 08/01/2024 Accepted: 16/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
AbstractBackground: Fractures with large bone defects and non-unions are a great challenge for veterinary orthopaedists. In small dog breeds, this complication is commonly encountered in fractures of the radius and ulna due to poorer vascularisation of the distal antebrachium region. Case Description: A case of radius/ulnar non-union in a 1.5-year-old Pinscher occurring after trauma and two successive unsuccessful osteosyntheses is described. During the operative revision, after the removal of existing bone implants, the bone defect was filled with cortical autologous bone graft. Autocancellous bone mixed with erythropoietin was applied proximally and distally to the cortical autograft for stimulation of bone healing. The post-operative period was without complications. As early as the 9th post-operative week, the animal was able to bear weight on the limb, without signs of lameness, pain, and swelling. Radiologically, a very good bridging of the graft was observed. Fifteen weeks after the operative revision, the fracture was completely healed with excellent clinical outcome. Conclusion: The application of autogenous cortical bone graft and cancellous autograft mixed with erythropoietin demonstrated an excellent therapeutic effect and resulted in complete regeneration of the large bone defect over a 15-week period. Keywords: Dog, Radius and ulna fracture, Erythropoietin, Cancellous and cortical bone grafts, Non-union. IntroductionNon-union is one of the most serious complications of fractures in dogs. The reported rate of nonunion fractures of the radius in small breed dogs is up to 60% (Munakata et al., 2018), possibly by reason of the poorer blood supply of the distal radius (Welch et al., 1997; Della Nina et al., 2007; Decamp et al., 2016). Surgery is mandatory as a treatment option in all non-union fractures. In case of uncontrolled infection or extensive bone resorption, limb amputation may be considered. Another option is the application of various types of bone grafts and/or promoters of bone healing. Bone grafts speed up the early formation of a bridging callus, consolidate delayed union or non-union, and fill the bone defect. Owing to their osteoinductive, osteoconductive, and osteogenic potential, autografts are considered the gold standard treatment (Kanczler and Oreffo, 2008; Decamp et al., 2016). Cortical bone grafts are applied to fill large bone defects, whereas cancellous grafts serve for additional stimulation of osteogenesis (dos Santos et al., 2016). Sometimes, in fractures with so-called critical-size defects, bone grafting may not lead to proper healing due to sequestration or resorption of the autograft. To address this issue, various substances to promote successful engraftment have been proposed: β-tri-calcium phosphate (Szponder et al., 2018), recombinant human bone morphogenetic protein-2 (Castilla et al., 2023), and platelet-rich plasma (Choi and Yoon, 2022; Cho et al., 2023). Erythropoietin is a growth factor with an important role in the differentiation and proliferation of hemopoietic progenitor and stem cells (Wang et al., 2018). An increasing number of orthopedic research studies confirm that erythropoietin (EPO) has also osteogenic and angiogenic properties (Jaquet et al., 2022). Its independent application improves bone healing, stimulates the differentiation of osteoblasts, the ingrowth of new blood vessels, and mineralization at the fracture site (Rolfing, 2014), whereas the combination of erythropoietin with a bone graft results in faster graft integration with the host bone (Wan et al., 2014; Vasileva, 2022). Thе presentation of this case report was aimed at the detailed description of the combined use of an autogenous cortical bone graft harvested from the contralateral ulna and cancellous autograft soaked with erythropoietin and evaluation of their effects on fracture healing of a distal radial non-union in a small-breed dog. Case DetailsCase historyA 1.5-year-old intact female Pinscher, weighing 1.8 kg, was referred for examination and treatment to the University Veterinary Hospital, Faculty of Veterinary Medicine, Stara Zagora, Bulgaria. The dog has been diagnosed in another clinic with a traumatic fracture of antebrachial bones (radius and ulna). It has undergone two bone surgeries; the first osteosynthesis of the radius with a Kirschner wire failed due to wire breakage. Two months later, a second osteosynthesis with a plate and screws was made, but again with post-operative complications (fracture non-union). One month after the second osteosynthesis, the dog was brought for an orthopedic examination with high-grade lameness in the right thoracic limb, swelling, and severe pain in the distal part of the antebrachium. Clinical examination, complete blood counts, and serum biochemical profile revealed no abnormal findings. After deep sedation with 0.075 mg/kg medetomidine hydrochloride (Dorbene vet®, 1 mg/ml, Syva, Spain) and 7.5 mg/kg ketamine hydrochloride (Anaket®, 100 mg/ml, Richter Pharma, Austria), applied intramuscularly in the quadriceps femoris muscle, a mediolateral radiograph was made (Philips, Bucky Diagnost CS4, Holland; exposure data 50 kV, 10 mAs). The radiograph demonstrated osteolytic areas around the fixation elements, part of a broken Kirschner wire in the proximal fragment, radiolucent areas, paucity of bone callus, and obvious fracture line (Fig. 1). Bone ends were atrophied, the proximal fragment of the radius was substantially thinned and cone-shaped, suggesting atrophic non-union. In this non-union type, the only chance for stimulation of osteogenesis is the removal of fixation elements and altered bone fragments, and bone graft application.
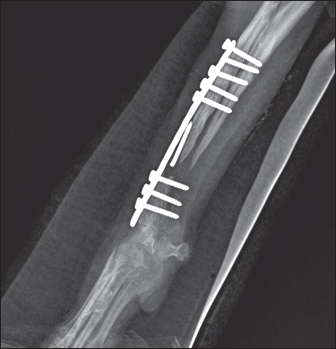
Fig. 1. Mediolateral radiograph of antebrachial bones, demonstrating osteolytic areas around the fixation elements, part of a broken Kirschner wire in the proximal fragment, radiolucent areas, bone callus paucity, and fracture nonunion. Operative revisionA third operative intervention was undertaken for the salvation of the limb. Due to the size of the bone defect (4.7 сm), it was decided to implant an autogenous cortical bone graft harvested from the contralateral ulna combined with cancellous autograft soaked with erythropoietin for stimulation of osteogenesis. The anesthesia protocol included i.m. premedication with acepromazine maleate (Neurotranq®, 10 mg/ml, Alfasan International, Netherlands) at 0.2 mg/kg and buprenorphine (Bupaq®, 0.3 mg/ml, Richter pharma, Austria; Vetergesic®, 0.3 mg/ml, Ceva, UK) at 0.01 mg/kg. Thirty minutes apart, 5 mg/kg propofol (Propofol Fresenius®, Fresenius Kabi GmbH, Germany) was administered intravenously for induction of anesthesia. After endotracheal intubation, inhalation anesthesia was maintained with 1.5–2 vol% isoflurane (Forane®, Abbott Laboratories Limited, United Kingdom (Isoflurin®, 1,000 mg/g, Vetpharma Animal Health, Barcelona, Spain) with 100% oxygen. Fluid therapy comprised Ringer lactate (Ringer Braun, B. Braun Melsungen AG, Germany) at a rate of 10 ml/kg/hours. After routine limb preparation and a medial approach to the distal radius, the plate, screws, and the broken Kirschner wire were removed from the proximal fragment. The proximal and distal ends of the radius were resected with an oscillating saw. To find the proper length of the graft needed, the distance (4.8 сm) between the two bone ends was intraoperatively measured by means of a caliper. То harvest the autogenous cortical graft, a caudal approach to the contralateral ulnar diaphysis was used (Fig. 2). The subcutaneous tissue was bluntly dissected, and thereafter retraction was maintained using Gelpi retractors. A Hohmann retractor was placed between the ulna and radius to prevent iatrogenic damage to the radius. A 4.8 cm long bone segment of the ulnar diaphysis was removed by means of an oscillating saw. The ostectomy and periosteum were removed en bloc and transferred into the surgical gap of the right radius. At both ends of the cortical autograft, a cancellous graft soaked with 1,000 IU recombinant human erythropoietin (Binocrit®, 2000 IU, Sandoz GmbH, Kundl, Austria) was applied for optimization of osteoinduction and angiogenesis. To collect the cancellous graft, a small incision was made over the craniolateral aspect of the greater trochanter. A 3.5-mm hole in the proximal humeral metaphysis was created with a drill bit and a bone curette was used to harvest the cancellous graft. Then, it was inserted into the recipient site as soon as possible to minimize cell death. After proper alignment of bone fragments, they were fixed with a dynamic compression plate (1.5 mm thickness) and 8 cortical screws: 2 in the distal radial fragment, 3 in the cortical graft, and 3 in the proximal radial fragment (Fig. 3). The intradermal layer was closed with an absorbable monofilament suture material (PDX, 3-0, VetSuture, France), followed by non-absorbable skin sutures (Nylon, 3-0, Kruuse, Denmark).
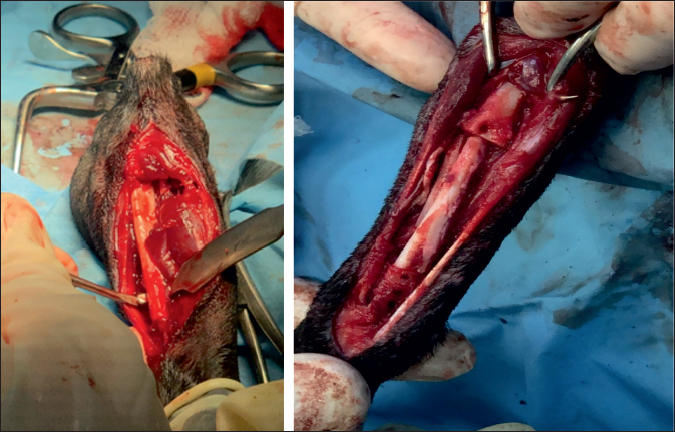
Fig. 2. Collection of bone autograft after caudal approach to the contralateral ulnar dyaphysis (left) and placement of the bone graft in the radial defect (right).
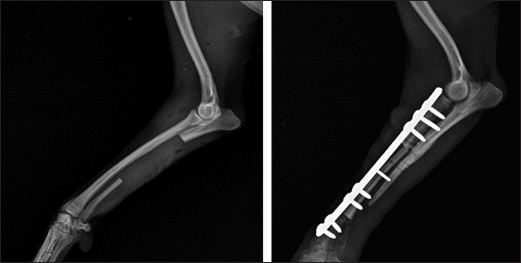
Fig. 3. Mediolateral radiograph of antebrachial bones after the revision surgery. Left: the ulna of the healthy thoracic limb after harvesting of the cortical autograft. Right: fixation of the cortical autograft with dynamic compression plate. Post-operative period and control examinationsIn the post-operative period, movements were confined in a cage for 24 days. Post-operative pain and infection were respectively controlled with s.c. administration of meloxicam (Meloxidolor, Produlab Pharma B.V., Netherlands) at 0.3 mg/kg for 3 days and i.v. ceftriaxone (Tercef, Actavis, Bulgaria) at 30 mg/kg for 7 days. By the 30th day, the patient started bearing weight with the operated limb and was discharged without signs of infection.
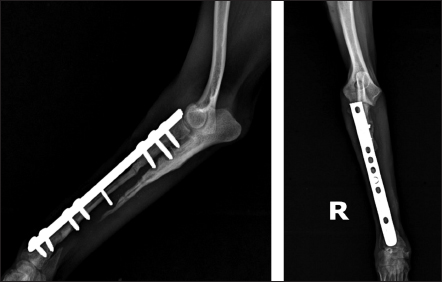
Fig. 4. Mediolateral and craniocaudal radiographs of antebrachial bones, 9 weeks after the revision surgery showing very good cortical graft bridging.
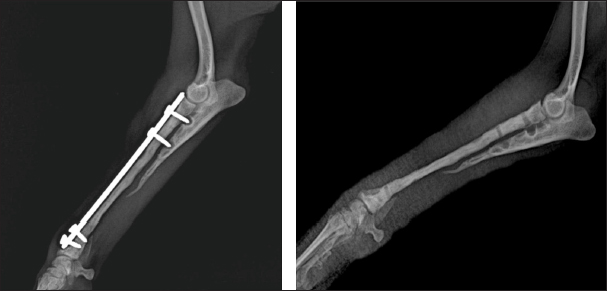
Fig. 5. Left—mediolateral radiograph of antebrachial bones 12 weeks after the revision surgery after the removal of screws from the cortical autograft; Right—mediolateral radiograph by the 15th week demonstrating complete union of the radial fracture and bone callus remodeling. During the control examination by the 9th post-operative week, the weight bearing with the limb was steady, whereas lameness, pain, and swelling were absent. Radiologically, an excellent bridging of the cortical graft without loosening of fixation implants was demonstrated (Fig. 4). After the second control examination by the 12th post-operative week, because of the good radiological and clinical outcome, the three screws in the cortical graft were removed, leaving only screws in the proximal and distal bone fragments. By the 15th week, due to excellent clinical outcomes, all implants were removed (Fig. 5). Through interaction by phone 13 months after the third control examination, the owner informed us that the dog was ambulating with no apparent lameness and that he was very satisfied with the outcome. Ethical approvalAll procedures described in the case report were conducted after obtaining the written content of the patient’s owner. DiscussionThe main factors with adverse impact on fracture healing are impaired blood supply in the fracture line region, presence of foreign bodies, necrotic tissues, bone sequestra, infection, malalignment of bone fragments, and inappropriate internal fixation technique (Marshall et al., 2022). In this case, the bone union had not occurred two months after the first surgery due to the unsuitable method of osteosynthesis (intramedullary osteosynthesis does not provide the necessary stability of bone fragments in fractures of distal radius/ulna), inadequately performed fixation (impaired compact bone integrity of the proximal fragment) and implant breakage. In fractures with large bone defects resulting from trauma, infection, or non-union, the regeneration potential of bone tissue is compromised (Mao and Mooney, 2015). In them, bone healing may not occur without the application of grafts for restoration of bone anatomic integrity and acceleration of healing. In dogs, autologous bone grafts are most commonly harvested from the proximal ilium wing, the tibia, the ribs, and coccygeal vertebrae (Decamp et al., 2016; Choi and Yoon, 2022; Goto and Ikeda, 2022). In small breed dogs, it is sometimes challenging to harvest a graft of sufficient size for the treatment of large bone defects. For this patient, the ulna of the healthy thoracic limb was used as donor bone. The ulna was chosen because the shape, size, and diameter of the obtained ulnar cortical autograft matches the anatomical configuration of the radius, the operative approach to this bone is easy and graft harvesting does not prolong considerably the time under anaesthesia. Being a non-weight bearing bone, ulna allows collecting a graft of larger size. Anyway, graft collection is one of the critical stages in such type of surgery, as it requires a well-trained team and sometimes, may prolong considerably the surgery duration. The application of large bone grafts however does not always result in bone union due to the lack of adequate vascularisation, which may compromise the supply of the callus with vital oxygen, nutrients, and growth factors (Vidal et al., 2020; Menger et al., 2022). Lately, EPO has been found effective as a promoter of bone regeneration of critical-size defects (Wan et al., 2014). Experimental studies on the application of EPO in fractures report promising data about stimulation of new bone tissue formation, and increased mechanical strength of bones (Eggold and Rankin, 2019; Vasileva, 2022). Histological evidence that erythropoietin increases the formation of fibrous tissue and blood vessels in treated vs untreated bone defects is provided (Rölfing et al., 2014; Khairy et al., 2019; Mahmoud, 2019; Vasileva et al., 2023). That is why, proximally and distally to the cortical autograft, autocancellous bone soaked with erythropoietin was applied. Most probably, the addition of erythropoietin was the reason for the faster engraftment, as demonstrated by radiographs made 9 weeks after the revision surgery. In this patient, the combination of cortical and cancellous autografts with erythropoietin had a marked beneficial effect on new bone formation. The fixation of bone grafts in the distal radius region may be performed by means of an external fixator or dynamic compression plates (Milovancev and Ralphs, 2004; Goto and Ikeda, 2022). External coaptation, however, is not a good option as up to 83% of radial fractures in small and toy breed dogs treated with external fixation develop serious complications including malunions and non-unions (Waters et al., 1993). This is attributed to decreased intraosseous blood supply to the distal third of the radius, decreased vascular density, and arborisation of vessels in the distal metaphysis of these breeds (Welsh et al., 1997). That is why plate osteosynthesis was preferred to ensure better stability of bone fragments and faster regeneration of the bone defect, which was confirmed by follow-up radiographs by the 12th post-operative week. Good results from these fixation techniques have been also reported in earlier studies (Wingerter et al., 2007). Finally, the limitations of the presented case report deserve to be mentioned. They are associated with the retrospective design, lack of control cases, lack of possibility to establish a cause-effect relationship and therefore risk from overinterpretation, and lack of regular follow-up after patient’s discharge. ConclusionIn conclusion, the application of cortical bone autograft together with cancellous autograft mixed with erythropoietin demonstrated an excellent therapeutic effect and managed to regenerate completely the extensive bone defect over a period of 15 weeks. The local application of erythropoietin was beneficial for engraftment and bone union, with radiological evidence by the 12th week and excellent clinical outcome 15 weeks after the revision surgery. AcknowledgmentsThe authors express their profound gratitude for the exceptional support of the Faculty of Veterinary Medicine, Stara Zagora, Bulgaria in their teaching, research, and clinical activities. Our most sincere thanks to the National Program, Young Scientists and Postdoctoral Students-2, of the Bulgarian Ministry of Education and Science. Authors’ contributionPatient treatment and surgical management: RG, RR, RV; post-operative diagnostic imaging evaluations: RG, RR; manuscript drafting: RG, RV, RR. All authors contributed to the final version of the manuscript. FundingNone. Conflict of interestThe authors declare no conflict of interest. Data availabilityAll data pertaining to the study findings are available within the manuscript. ReferencesCastilla, A., Filliquist, B., Spriet, M., Garcia, T.C., Arzi, B., Chou, P.Y. and Kapatkin, A.S. 2023. Long-term assessment of bone regeneration in nonunion fractures treated with compression-resistant matrix and recombinant human bone morphogenetic protein-2 in dogs. Vet. Comp. Orthop. Traumatol. 36, 29–38. Cho, K., Lee, K., Kang, K. and Kim, M. 2023. Treatment of a large defect induced by atrophic nonunion of femoral fracture in a dog with autogenous coccygeal bone grafting. Vet Sci. 10, 388. Choi, J.Y. and Yoon, H.Y. 2022. Use of coccygeal vertebra autograft and platelet-rich plasma for treating a distal radial nonunion fracture in a small-breed dog. Can. Vet. J. 63, 689–694. Decamp, C.E., Johnston, S.A., Déjardin, L.M. and Schaefer S.L. 2016. Bone grafting. In Piermattei and Flo’s handbook of small animal orthopedics and fracture repair. Eds., Decamp, C.E., Johnston, S.A., Déjardin, L.M. and Schaefer, S.L., St. Louis, MO: Elsevier, pp: 153–162. Della Nina, M.I., Schmaedecke, A., Romano, L. and Ferrigno, C.R.A. 2007. Comparação de osteossíntese com placa e osteossíntese com placa associada a enxerto de proteína morfogenética óssea em fratura bilateral distal de rádio e ulna em cão - relato de caso. Braz. J. Vet. Res. Anim. Sci., 44, 297–303. dos Santos, J.F., Ferrigno, A., Ricardo, C., dos Santos. D.Í., Caquías, I. and Fabiana, D. 2016. Nonunion fractures in small animals—a literature review. Semin. Cienc. Agrar. 37, 3223–3230. Eggold, J.T. and Rankin, E.B. 2019. Erythropoiesis, EPO, macrophages, and bone. Bone 119, 36–41. Goto, M. and Ikeda, H. 2022. Use of an autologous bone graft derived from three caudal vertebrae for reconstruction in a dog with radioulnar atrophic nonunion and osteomyelitis. VCOT Open 05, e93–e97. Jaquet, K., Krause, K., Tawakol-Khodai, M., Geidel S. and Kuck, K.H. 2002. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc. Res. 64, 326–333. Kanczler, J.M. and Oreffo, R.O. 2008. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cell. Mater. 15, 100–114. Khairy, M.A., Ahmed, A., Elmagd, I.A. and Fahmi, A.N. 2019. Effects of erythropoietin on the healing of calvarial bone defect. Egypt. Dent. J. 65, 3283–3294. Mahmoud, N. 2019. The effect of Erythropoietin on bone regeneration “an experimental study.” Egypt. Dent. J. 65, 2199–2208. Mao, A.S. and Mooney D.J. 2015. Regenerative medicine: current therapies and future directions. PNAS, 112, 14452–14459. Marshall, W.G., Filliquist, B., Tzimtzimis, E., Fracka, A., Miquel, J., Garcia, J. and Dalla Fontana, M. 2022. Delayed union, non-union and mal-union in 442 dogs. Vet. Surg. 51, 1087–1095. Menger, M.M., Laschke, M.W., Nussler, A.K., Menger, M.D. and Histing, T. 2022. The vascularization paradox of non-union formation. Angiogenesis 25, 279–290. Milovancev, M. and Ralphs, S.C. 2004. Radius/ulna fracture repair. Clin. Tech. Small Anim. Pract. 19, 128–133. Munakata, S., Nagahiro, Y., Katori, D., Muroi, N., Akagi, H., Kanno, N., Harada, Y., Yamaguchi, S., Hayashi, K. and Hara, Y. 2018. Clinical efficacy of bone reconstruction surgery with frozen cortical bone allografts for nonunion of radial and ulnar fractures in toy breed dogs. Vet. Comp. Orthop. Traumatol. 31, 159–169. Rölfing, J., Jensen, J., Jensen, J.N., Greve, A., Lysdahl, H., Chen, M., Rejnmark L. and Bünger, C. 2014. A single topical dose of erythropoietin applied on a collagen carrier enhances calvarial bone healing in pigs. Acta Orthop. 85, 201–209. Rölfing, J.H.D. 2014. The effect of erythropoietin on bone. Acta Orthop. 85(suppl. 353), 1–29. Szponder, T., Wessely-Szponder, J., Sobczyńska-Rak, A., Żylińska, B., Radzki, R.Р. and Polkowska, I. 2018. Application of platelet-rich plasma and tricalcium phosphate in the treatment of comminuted fractures in animals. In Vivo 32, 1449–1455. Vasileva, R. 2022. Local application of erythropoietin and xenograft for enhancing bone regeneration. Trad. Modern. Vet. Med. 7, 38–43. Vasileva, R., Chaprazov, T., and Sivrev, D. 2023. Histological evaluation of erythropoietin application on bone healing in rat calvaria. Egypt. J. Histol. 46, 635–640. Vidal, L., Kampleitner, C., Brennan, M.Á., Hoornaert, A. and Layrolle, P. 2020. Reconstruction of large skeletal defects: current clinical therapeutic strategies and future directions using 3D printing. Front. Bioeng. Biotechnol. 8, 61. Wan, L., Zhang, F., He, Q., Tsang, W.P., Lu, L., Li, Q., Wu, Z., Qui, G., Zhou, G. and Wan, C. 2014. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS One 9, e102010. Wang, L., Wu, F., Song, Y., Duan, Y. and Jin, Z. 2018. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 MAPK pathway. Int. J. Mol. Med. 41, 829–835. Waters, D.J., Breur, G.J. and Toombs, J.P. 1993. Treatment of common forelimb fractures in miniature and toy breed dogs. J. Am. Anim. Hosp. Assoc. 29, 442–448. Welch, J.A., Boudrieau, R.J., DeJardin, L.M. and Spodnick, G.J. 1997. The intraosseous blood supply of the canine radius: implications for healing of distal fractures in small dogs. Vet. Surg. 26, 57–61. Wingerter, S., Calvert, G., Tucci, M., Tsao, A., Russell, G. and Benghuzzi, H. 2007. Comparison of two different fixation techniques for a segmental defect in a rat femur model. J. Invest. Surg. 20, 149–155. | ||
| How to Cite this Article |
| Pubmed Style Garnoeva R, Roydev R, Vasileva R. Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Vet. J.. 2024; 14(5): 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 Web Style Garnoeva R, Roydev R, Vasileva R. Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. https://www.openveterinaryjournal.com/?mno=184362 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.25 AMA (American Medical Association) Style Garnoeva R, Roydev R, Vasileva R. Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Vet. J.. 2024; 14(5): 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 Vancouver/ICMJE Style Garnoeva R, Roydev R, Vasileva R. Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 Harvard Style Garnoeva, R., Roydev, . R. & Vasileva, . R. (2024) Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Vet. J., 14 (5), 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 Turabian Style Garnoeva, Radka, Rumen Roydev, and Radina Vasileva. 2024. Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Veterinary Journal, 14 (5), 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 Chicago Style Garnoeva, Radka, Rumen Roydev, and Radina Vasileva. "Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report." Open Veterinary Journal 14 (2024), 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 MLA (The Modern Language Association) Style Garnoeva, Radka, Rumen Roydev, and Radina Vasileva. "Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report." Open Veterinary Journal 14.5 (2024), 1302-1308. Print. doi:10.5455/OVJ.2024.v14.i5.25 APA (American Psychological Association) Style Garnoeva, R., Roydev, . R. & Vasileva, . R. (2024) Erythropoietin as promoter of engraftment for treatment of radius/ulnar non-union fracture in a dog: Case report. Open Veterinary Journal, 14 (5), 1302-1308. doi:10.5455/OVJ.2024.v14.i5.25 |