| Case Report | ||
Open Vet. J.. 2024; 14(5): 1309-1312 Open Veterinary Journal, (2024), Vol. 14(5): 1309–1312 Case Report A novel approach to ear pain in the horse: A case reportElena Lardone* , Alessandra Landi and Paolo FranciDepartment of Veterinary Science, University of Turin, Grugliasco, Italy *Corresponding Author: Elena Lardone. Department of Veterinary Science, University of Turin, Grugliasco, Italy. Email: elena.lardone [at] unito.it Submitted: 23/01/2024 Accepted: 18/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
AbstractBackground: During electrochemotherapy (ECT), a chemotherapeutic drug is injected into the tumor and then an electroporation is provided. In horses, ear manipulation may be very painful, and combining a loco-regional technique with sedation might be a good option to avoid anesthesia-related risks. A two-injection-point block of the internal and external pinna and acoustic meatus was described in horse cadavers, and it permitted complete stain of all three branches of the great auricular nerve (GAN), internal auricular nerve branch (IAB), lateral auricular branch (LAB), and caudal auricular nerve (CAN), suggesting a lower risk of intra-parotid injection during the IAB and LAB block. Case Description: An 8-year-old Italian jumping gelding presented for ECT to treat a fibroblastic sarcoid in the left medial pinna. After intravenous sedation with acepromazine, romifidine, and butorphanol, a two-injection-point block was provided as previously described. The block of the GAN was blind, whereas an electrical nerve locator was used for the IAB, LAB, and CAN. A total of 12 ml of 0.5% ropivacaine was injected. The ECT was safely performed without any difficulties. The horse well tolerated the procedure and completely recovered 75 minutes after sedation. No complications were detected. Conclusion: The described approach seems feasible and suitable for the blockade of the sensory innervation of the equine ear in the case of ECT. Keywords: Auricular blocks, Electrochemotherapy, Ear, Horse, Ropivacaine. IntroductionElectrochemotherapy (ECT) is a technique in which a chemotherapeutic drug is injected into the sarcoid and then electric pulses are applied at high voltage (electroporation). This leads to an increase in drug concentration in the sarcoid cells and an increase in its effect. Horses usually undergo the procedure under a brief general anesthesia because of the electric shock (Tamzali et al., 2012), if sedation combined with locoregional techniques is not a viable option. In equids, ear manipulation may be very painful. Many procedures, such as otoscopy and surgery, often require general anesthesia as sedation may not be effective. Combining a locoregional technique with sedation might be a good option to avoid anesthesia-related risks (Johnston et al., 2001), as standing procedures seem to carry less risks than general anesthesia in equines (Gonzalo-Marcilla et al., 2021). In horses, the anatomy of the sensory innervation of the ear is characterized by differences in nomenclature and descriptions (Sommeraurer et al., 2012). The great auricular nerve (GAN), facial nerve, and vagal nerves play a role in the innervation of the auricular region, whereas the presence of the auriculotemporal nerve is still controversial in horses (Ellenberger and Baum, 1943; Barone and Simoens, 2010; Sommeraurer et al., 2013; Cerasoli et al., 2017). The external and internal surface of the pinna is innervated by the GAN (a branch of the second cervical nerve), whereas the internal auricular nerve branch (IAB, a branch of the facial nerve) innervates the internal faces of both the auricle and acoustic meatus. The other two branches of the facial nerve the lateral auricular branch (LAB), and the caudal auricular nerve (CAN), contribute to the sensory and motor innervation of the external pinna with the GAN. The sensory component is provided by the fibers of the Vagus nerve that join the LAB and IAB. Few locoregional techniques are reported to desensitize the ear in horses and some of them are not completely effective or may have accidental puncture of the parotid gland and guttural pouch as possible collateral effects (McCoy et al., 2007; Sommeraurer, 2012). In 2017, Cerasoli and colleagues described a new approach for the block of the internal and external pinna and acoustic meatus in adult warm-blood horse cadavers (Cerasoli et al., 2017). This two-injection-point approach permitted complete stain of all three branches of GAN and suggested a lower risk of intra-parotid injection during the IAB and LAB block.
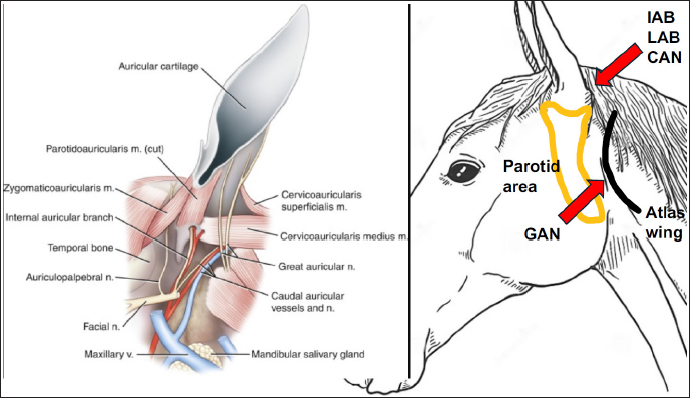
Fig. 1. After palpating the cranial aspect of the atlas wing, the GAN and its branches were localized, and the first injection of 0.5% ropivacaine (6 ml) was performed. A second injection at the caudal base of the ear was performed to block the IAB, LAB, and CAN. The needle was inserted through the skin once the parotid gland was palpated, then it was advanced in a sagittal direction, parallelly to the ear canal until its tip was just above the parotid gland (about 2 cm deep). When medio-lateral twitch of the pinna was well elicited at a current of 0.4 mA, 6 ml of 0.5% ropivacaine was slowly administered. Case DetailsAn 8-year-old Italian jumping gelding presented for ECT to treat a fibroblastic sarcoid in the left medial pinna. The medical history was unremarkable, except for the tumor; the preanesthetic physical examination revealed a physiological systolic aortic murmur and complete blood analyses displayed no abnormalities. After obtaining owner’s consent, the horse’s weight was estimated at 600 kg using a weight tape wrapped around its girth, directly behind the elbow. Being the horse’s temperament agitated, 25 mcg/kg of acepromazine (Prequillan, 10 mg/ml, Fatro SPA, Italy) was intravenously (IV) injected, and a 14-gauge IV catheter (Introcan safety, BBraun, USA) was placed in the left jugular vein. After 40 minutes, the horse was conducted to the box and premedicated with butorphanol (0.05 mg/kg IV, Dolorex 10 mg/ml, MSD Animal Health, USA), and romifidine (60 mcg/kg IV, Sedivet 10 mg/ml, Boehringer, Germany). The sedation was effective, and the horse was standing with a minimal degree of ataxia that completely disappeared after a few minutes. Following the disinfection of the auricular area, a block of the GAN (Fig. 1) was blindly executed as described by Cerasoli and colleagues (2017): after palpating the cranial aspect of the atlas wing, the GAN and its branches were localized, and the first injection of 0.5% ropivacaine (Ropivacaina 7.5 mg/ml, Bioindustria, Italy) was performed. Six ml of the local anesthetic was slowly administered subcutaneously using a 21-gauge needle (Sterican 0.8 × 50 mm, BBraun, USA). Afterward, a second injection at the caudal base of the ear was performed to block the IAB, LAB, and CAN (Fig. 1) using an electrical nerve locator (Stimuplex HSN 12, BBraun, USA). The needle was inserted through the skin once the parotid gland was palpated, then it was advanced in a sagittal direction, parallelly to the ear canal until its tip was just above the parotid gland (about 2 cm deep). When medio-lateral twitch of the pinna was well elicited at a current of 0.4 mA, 6 ml of 0.5% ropivacaine was slowly administered through an insulated needle with an extension set (Stimuplex D 22G × 80 mm, BBraun, USA). The total dose of ropivacaine administered was 0.1 mg/kg. After each injection, the area was gently massaged. The duration of the entire locoregional technique was 4 minutes and the technique was easily executed without any reaction of the horse. After 5 minutes, the electroporation started, and cisplatin was administered to the sarcoid. The horse tolerated the application of the high-voltage electric pulses, but he slightly shook his head when the chemotherapeutic drug was injected. The procedure lasted 9 minutes and it was safely performed without any difficulties. The horse completely recovered 75 minutes after sedation and was discharged from the hospital after 2 hours of observation. No complications were detected. DiscussionThis case report describes the sedation combined with the block of the internal and external pinna in a horse undergoing ECT to treat a sarcoid in the left medial pinna. The approach described by Cerasoli and colleagues (Cerasoli et al., 2017) was effective in successfully desensitizing the motor and sensitive components of the pinna and allowed them to easily and safely execute the ECT. In literature, there are few locoregional techniques desensitizing the equine ear: a ring block around the auricular base, which is often not tolerated by the horses and may result in an incomplete block (McCoy et al., 2007); a blind GAN and IAB block which involves two injection points (one at the base of the ear for the GAN and the other on the lateral side of the pinna, just above the parotid gland’s cranial border, for IAB) (McCoy et al., 2007). However, the success rate of this GAN block may be limited due to anatomical differences. The GAN travels dorso-ventrally starting from the second cervical vertebral nerve and ending at the base of the ear. In horses, the GAN path is characterized by a high inter- and intra-variance: two or three branches traveling either cranially or caudally to the cranial aspect of the atlas wings are reported, as well as further unpredictable bifurcations of these branches on the auricular pinna (Cerasoli et al., 2017). Using a more caudal approach to GAN, as in this clinical case, it can be assumed that the local anesthetic solution has more chances to completely bath all the branches of GAN, suggesting a better desensitization than that reported in the previously described blocks at the base of the ear (McCoy et al., 2007; Sommeraurer et al., 2013). The blind GAN and IAB block described by McCoy et al. (2007) was effectively used under ultrasound (US) guide to perform bilateral otoscopies in 23 horses (Sommeraurer et al., 2013). As the landmark for the IAB was the styloid process that is close to the parotid gland and guttural pouch, they reported that caution must be taken not to accidentally puncture these structures and advised the US evaluation before local anesthesia. In the current case, US guidance was not deemed necessary because our injection point for IAB was at the caudal auricular base, just above the parotid gland, lowering the risk of intra-parotid injection and the possibility of gland inflammation than the above-mentioned block. In the hectic pace of today’s practice or if a US machine is not available, a technique that does not require US guidance may be a suitable option. Facial nerve paralysis is a complication that must be considered when performing auricular blocks. Sommeraurer and colleagues (2013) reported mildly drooping ears and ptosis in 3 out of 23 horses receiving GAN and IAB block. In this perspective, ropivacaine may be a good choice because of its differential sensory/motor blocking capacities. Ropivacaine is a long-acting, amide-type local anesthetic that has a high pKa and low lipid solubility. Ropivacaine blocks the Ad- and C-nerve fibers involved in pain transmission. At low concentrations, ropivacaine causes a significant differential blockade between sensory and motor fibers (Casati et al. 2001). Ropivacaine has also fewer cardiotoxic side effects than bupivacaine (Graf et al., 2002). In equine anesthesia, different uses of 0.5% ropivacaine have been described: Ganidagli et al. (2004) used 0.1 mg/kg ropivacaine for caudal epidural administration in adult Thoroughbred horses; Gonzalez et al. (2023) in the subconjunctival space; Souto et al. (2020) for continuous block of median and ulnar nerves. The technique was easy and fast to perform. The electrolocation of the IAB was an advisable aid to perform the block, but if an electrical nerve locator is not present the block could be blindly executed. Further clinical studies are advised to determine the efficacy and safety of this technique. ConclusionThe described approach seems feasible and suitable for the blockade of the sensory innervation of the equine ear in the case of ECT. AcknowledgementsNone. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsEL: data collection, data management, and manuscript preparation; AL: data collection; PF: interpretation and correction of the manuscript. FundingThis research received no specific grant. Data availabilityAll data supporting the findings of this study are not available within the manuscript. ReferencesBarone, R. and Simoens, P. 2010. Neurologie II in Anatomie Compareģe des MammifereĢs domestiques. Tome 7, Vigot (eds) Paris, France, pp: 84–87. Casati, A., Santorsola, R., Cerchierini, E. and Moizo, E. 2001. Ropivacaine. Minerva Anesthesiologica 67, 15–19. Cerasoli, I., Cornillie, P., Gasthuys, F., Gielen, I. and Schauvliege, S. 2017. A novel approach for regional anaesthesia of the auricular region in horses: an anatomic and imaging study. Vet. Anaesth. Analg. 44(3), 656–664. Ellenberger, W. and Baum, H. 1943. Handbuch der Vergleichende anatomie der haustiere, Auchtzehnte auflage. Berdlin Springer-Verlag Ed, 16 ed., pp: 884. Ganidagli, S., Cetin, H., Biricik, H.S. and Cimtay, I. 2004. Comparison of ropivacaine with a combination of ropivacaine and fentanyl for the caudal epidural anaesthesia of mares. Vet. Rec. 154, 329–332. Gonzalez, G.A., Betbeze, C., Wills, R., Eddy, A., Mochal-King, C. and Fontenot, R.L. 2023. Effects of subconjunctival ropivacaine, liposomal bupivacaine, and mepivacaine on corneal sensitivity in healthy horses. Vet. Surg. 52, 1041–1049. Gozalo-Marcilla, M., Bettschart-Wolfensberger, R., Johnston, M., Taylor, P.M. and Redondo, J.I. 2021. Data collection for the fourth multicentre confidential enquiry into perioperative equine fatalities (CEPEF4) study: new technology and preliminary results. Animals 11, 2549. Graf, B.M., Abraham, I., Ebernach, N., Kunst, G., Stowe, D.F. and Martin, E. 2002. Differences in cardiotoxicity of bupivacaine and ropivacaine are the result of physicochemical and stereoselective properties. Anesthesiology 96, 1427–1434. Johnson, G.M., Eastment, J.K. and Wood, J. 2002. The confidential enquiry into perioperative 298 equine fatalities (CEPEF): mortality results of Phase 1 and 2. Vet. Anaesth. Analg. 29, 159–170. McCoy, A., Schaefer, E. and Malone, E. 2007. How to perform effective blocks of the equine ear. AAEP Proceedings 53, 397–398. Sommerauer, S., Muelling, C.K., Seeger, J. and Schusser, G.F. 2012. Anatomy and anaesthesia of the equine external ear canal. Anat. Histol. Embryol. 41, 395–401. Sommerauer, S., Snyder, A., Breuer, J. and Schusser G. 2013. A technique for examining the external ear canal in standing sedated horses. J. Equine Vet. Sc. 33, 1124–1130. Souto, M.T.M., Fantoni, D.T., Hamaji, A., Hamaji, M., Vendruscolo, C.P., Otsuki, D.A., Pinto, A.C.B. and Ambrósio, A.M. 2020. Ultrasound-guided continuous block of median and ulnar nerves in horses: development of the technique. Vet. Anaesth. Analg. 47(3), 405–413. Tamzali, Y., Borde, L., Rols, M.P., Golzio, M., Lyazrhi, F. and Teissie, J. 2012. Successful treatment of equine sarcoids with cisplatin electrochemotherapy: a retrospective study of 48 cases. Equine Vet. J. 44, 214–220. | ||
| How to Cite this Article |
| Pubmed Style Lardone E, Landi A, Franci P. A novel approach to ear pain in the horse: A case report. Open Vet. J.. 2024; 14(5): 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 Web Style Lardone E, Landi A, Franci P. A novel approach to ear pain in the horse: A case report. https://www.openveterinaryjournal.com/?mno=187617 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.26 AMA (American Medical Association) Style Lardone E, Landi A, Franci P. A novel approach to ear pain in the horse: A case report. Open Vet. J.. 2024; 14(5): 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 Vancouver/ICMJE Style Lardone E, Landi A, Franci P. A novel approach to ear pain in the horse: A case report. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 Harvard Style Lardone, E., Landi, . A. & Franci, . P. (2024) A novel approach to ear pain in the horse: A case report. Open Vet. J., 14 (5), 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 Turabian Style Lardone, Elena, Alessandra Landi, and Paolo Franci. 2024. A novel approach to ear pain in the horse: A case report. Open Veterinary Journal, 14 (5), 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 Chicago Style Lardone, Elena, Alessandra Landi, and Paolo Franci. "A novel approach to ear pain in the horse: A case report." Open Veterinary Journal 14 (2024), 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 MLA (The Modern Language Association) Style Lardone, Elena, Alessandra Landi, and Paolo Franci. "A novel approach to ear pain in the horse: A case report." Open Veterinary Journal 14.5 (2024), 1309-1312. Print. doi:10.5455/OVJ.2024.v14.i5.26 APA (American Psychological Association) Style Lardone, E., Landi, . A. & Franci, . P. (2024) A novel approach to ear pain in the horse: A case report. Open Veterinary Journal, 14 (5), 1309-1312. doi:10.5455/OVJ.2024.v14.i5.26 |