| Research Article | ||
Open Vet. J.. 2024; 14(5): 1216-1223 Open Veterinary Journal, (2024), Vol. 14(5): 1216–1223 Research Article Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertensionOleynikov Dmitrij Arkadievich*Veterinary clinic “Belij Klyk”, Moscow, Russian Federation *Corresponding Author: Oleynikov Dmitrij Arkadievich. Veterinary clinic “Belij Klyk”, Moscow, Russian Federation, Russia. Email: wolfberg.guard [at] gmail.com Submitted: 28/02/2024 Accepted: 11/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
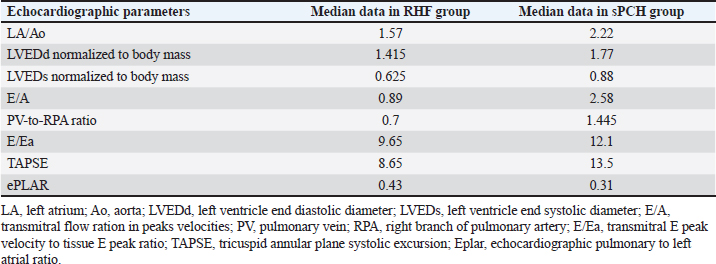
AbstractBackground: Pulmonary capillary hemangiomatosis (PCH) is an idiopathic disease with the anomalous proliferation of a small capillary-like vessel in the pulmonary tissue, which can lead to a severe form of PH. There are only several cases of PCH described in veterinary literature: 27 cases in dogs and 2 cases in cats. In veterinary medicine, PH is mostly recognized as a consequence of left heart failure as a progression of the postcapillary PH to the precapillary form. PCH is mostly described as a primary disease, but resistant postcapillary PH with the high possibility of pulmonary edema raises speculation that PCH could be a secondary malformation to the left heart disease. Aim: Discover the features associated with the shift between left- and right-sided heart disease in the context of PH development. Methods: Retrospective analysis of materials from cats and dogs with histological markers of PCH (sPCH) versus those with right heart failure (RHF). Results: Animals with histological and immunohistochemistry markers of PCH had a previous history of disease with left heart volume overload. There were no differences between the groups in radiography and gross pathology. Histologically, pulmonary fibrosis and arteriopathy could be found in RHF; in sPCH—a duplication of capillaries in alveolar septa and bizarre proliferation in surrounding structures. Conclusion: PCH could be a secondary pattern of vascular remodeling due to volume overload. Keywords: Pulmonary hypertension, canine, feline, pulmonary capillary hemangiomatosis. IntroductionPulmonary hypertension (PH) is a complex syndrome associated with primary or secondary vascular disorders accompanied by increased pulmonary arterial pressure higher than 25–30 mm Hg. Wide specter of causes predisposed to complex classification subtyped syndrome in 6 groups based on ethiopathological principle: 1) primary pulmonary arterial hypertension and pulmonary veno-occlusive disease (PVOD) or pulmonary capillary hemangiomatosis (PCH); 2) PH due to left heart disease (LHD); 3) PH due to respiratory disease; 4) pulmonary (thrombo) emboli; 5) parasitic disease; 6) multifactorial or unclear mechanisms. Hemodynamically it is subdivided into 3 types: precapillary (increased pulmonary arterial pressure and normal pulmonary arterial wedge pressure, with increased pulmonary vascular resistance); postcapillary (increased pulmonary arterial pressure and increased pulmonary arterial wedge pressure with normal pulmonary vascular resistance); combined (increased pulmonary arterial pressure and increased pulmonary arterial wedge pressure with increased pulmonary vascular resistance) (Reinero et al., 2019). The last combined type is difficult to differentiate from the precapillary type because of the limited ability to provide right heart catheterization, unless there is an opportunity to follow patients from the start of (LHD, such as myxomatose mitral valve disease) to the development of right heart disease (RHD) and phenotype shift. PCH is an idiopathic disease characterized by anomalous proliferation of small capillary-like vessels in the pulmonary tissue, leading to a severe form of PH. The prominent morphological marker is the infiltrative growth of capillaries in pulmonary structures, including arteriolas, bronchi, alveolar septa, and venulae. PCH has morphological features similar to those of PVOD, another rare primary vessel disease. However, in PVOD, occlusive obliteration of the venula lumen is the dominant manifestation; in human medicine, signs of PVOD and PCH are present in 75% of cases at the same time (Frazier et al., 2007; Langleben, 2014). Only a few several cases of PCH or PVOD have been described in veterinary literature: 27 cases in dogs and 2 cases in cats (Williams et al., 2016; Jaffey et al., 2017; Jenkins and Jennings, 2017; Toom et al., 2018; Reinero et al., 2019). PCH and PVOD do not have specific symptoms, but their common features are respiratory distress, dyspnea, fatigue, syncope, endocardial murmurs, crepitation as well as low saturation. Radiography shows cardiomegaly and lung parenchymal opacity in the form of an unstructured interstitial pattern with a diffuse or asymmetric distribution. In human medicine, high-resolution computer tomography is used to differentiate PH from PCH/PVOD (Lawler and Askin, 2005). This method is not widely represented in veterinary clinics, but routine computer tomography scanning could show multiple patchy ground glass opacities diffusely distributed in the lung parenchyma, main pulmonary artery, and its branches are dilated (Reinero et al., 2019). Today, echocardiography is the most extensively used method for PH diagnosis. The probability of PH is based on right heart enlargement, underfilling of the left chambers, pulmonary artery enlargement, decreased index of right pulmonary artery distensibility, and tricuspid regurgitation, which are used as surrogates of pulmonary arterial pressure (Reinero et al., 2020). In veterinary medicine, PH is mostly recognized as a consequence of LHD with a phenotype shift to RHD which is described as a progression of postcapillary PH to the precapillary form. This fact should be kept in mind because, in human literature, PCH is mentioned in the pathogenesis of chronic LHD, resulting in a prolonged state of increased pulmonary venulae pressure and vascular proliferation (Jing X et al., 1998). The prognosis of the mentioned diseases usually ranges from poor to grave. In humans, the only alternative is lung transplantation, but several medications are used to win some time before the operation: imatinib, doxycycline, and interferon (White, 1990; Ginns et al., 2003; Adachi et al., 2014; Nayyar et al., 2014). In this study, we decided to analyze several cases of mixed PH and precapillary PH to discover the features associated with the shift between left- and right-sided heart disease in the context of PH development. Material and Methods18 animals were included in this study (dogs n=16, cats n=2). We compared animals with a shifting phenotype from left to right heart failure (RHF) and suspected PCH (n=9; 2 cats and 7 dogs) with dogs that had only the RHF at the presentation (n=9). Instrumental diagnosticsAll patients included in the study underwent a full clinical study as well as standard blood analyses. Radiography was provided in 5 dogs and 2 cats in the PCH suspected group before euthanasia and in 9 dogs with only dominating RHF. In addition, an echocardiographic study was conducted (or performed) in the standard protocol by an experienced operator. After euthanasia, gross pathology was performed and the lung-heart organ complex was analyzed. Following the macroscopic study, tissue samples were collected. The lung sections were fixed in 10% buffered formalin. The tissue samples were then sent to the pathomorphological laboratory for histological and immunohistochemical analyses. Samples were routinely processed, sectioned, and stained with hematoxylin and eosin (HE), and Masson’s Trichrome. Immunohistochemical labeling was performed on sections of the lung from all dogs and cats. In the laboratory, adequate portions of lung tissue were incubated with an anti-CD31 antibody (Clone 1A10, Leica Biosystems Newcastle Ltd, United Kingdom) and an anti-factor von Willebrand antibody (Clone E980.1., Leica Biosystems Newcastle Ltd, United Kingdom). The process was performed using a Leica bond system and buffered saline for negative control. Ethical approvalNot needed for this Retrospective study. ResultsDemography Group suspected with PCH (sPCH) included 6 dogs with myxomatous mitral valve disease suspected of postcapillary PH, 1 dog with myxomatous mitral valve disease and acquired interatrial septal defect, 1 cat with patent ductus arteriosus and PH, and 1 cat with hypertrophic cardiomyopathy. Dogs from the RHF included: 3 dogs with myxomatoses mitral valve disease and pulmonary fibrosis, 1 dog with tetrad of Fallot, 1 dog with pneumonitis, and 4 dogs with chronic interstitial pneumonia. The duration of patient monitoring ranged from 5 days to 6 months with variations of the symptom intensity. Clinical signs included progressing dyspnea, acute dyspnea, chronic cough, weakness, lameness, cyanosis, and syncope. Chest radiographs obtained on the day of presentation with symptoms showed a diffuse interstitial unstructured pattern with loci of alveolar pattern, cardiomegaly, and in some cases, with left atrial enlargement (Fig. 1). All animals had echocardiography which showed signs of PH: high-velocity tricuspid regurgitation, pulmonary artery regurgitation, decreased pulmonary vein to right branch pulmonary artery ratio, decreased right pulmonary artery distensibility index, dilated right chambers (in some cases biventricular and biatrial dilation). Reverse analyses of echocardiographic markers were performed, based on ratio-associated parameters, due to variability in animal size (Table 1). Gross pathology Dogs with myxomatous mitral valve disease had deformed atrioventricular valves on both sides and dilated left atrium and ventricle. The right chambers were also enlarged, but not as much as than the left ones. Animals with pneumonitis and Fallot tetrad had dilated right chambers and a small left chamber. The cat with patent ductus arteriosus had all four chambers dilated. At the same time, the cat with hypertrophic cardiomyopathy showed a severe dilatation of the left atrium and left ventricle wall hypertrophy. All animals had significant right ventricle wall hypertrophy. Dogs from RHF had patchy lesions across lung lobes, presented with loci of white pale tissue on the surface diffusely distributed in lobe tissue, and zoned venous edema diffusing around vessels. The sPCH group had a wide spectrum of findings, but the most common were wide zones of edematic tissue (“meat-like”) across all lung lobes, with a border from the normal tissue (Fig. 2).
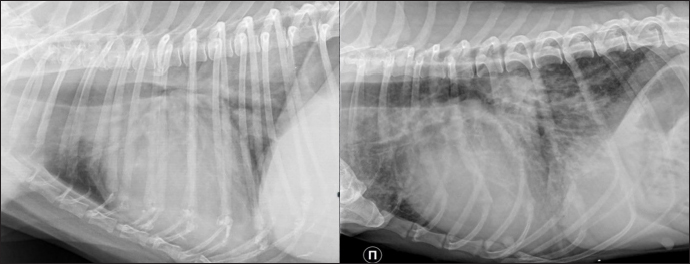
Fig. 1. Lateral thoracic radiographs of 2 dogs. Left one—RHF group, right -sPCH. Both presented with unstructured interstitial and local alveolar patterns. Table 1. Some echocardiographic parameters differed between groups in the median dataset.
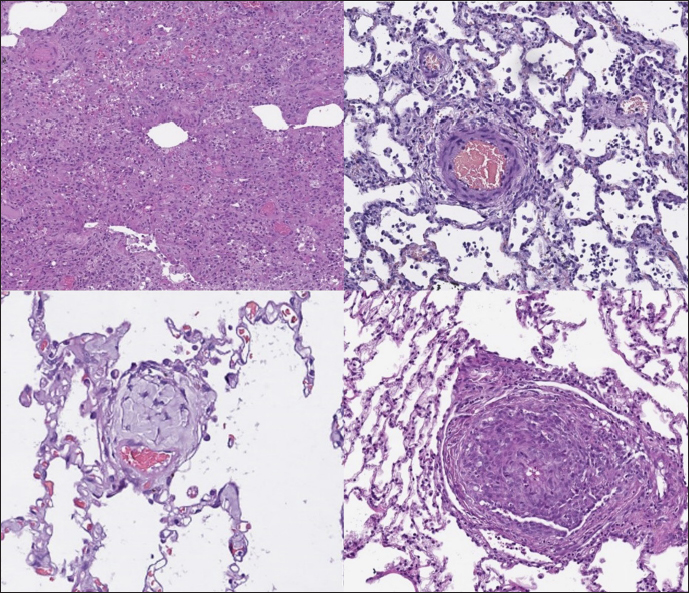
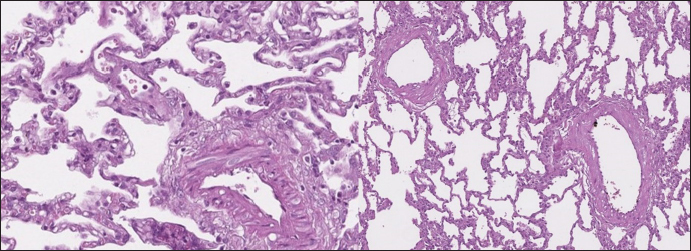
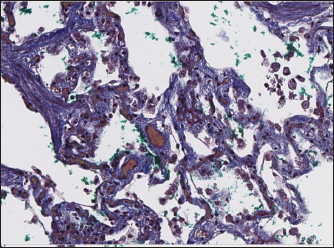
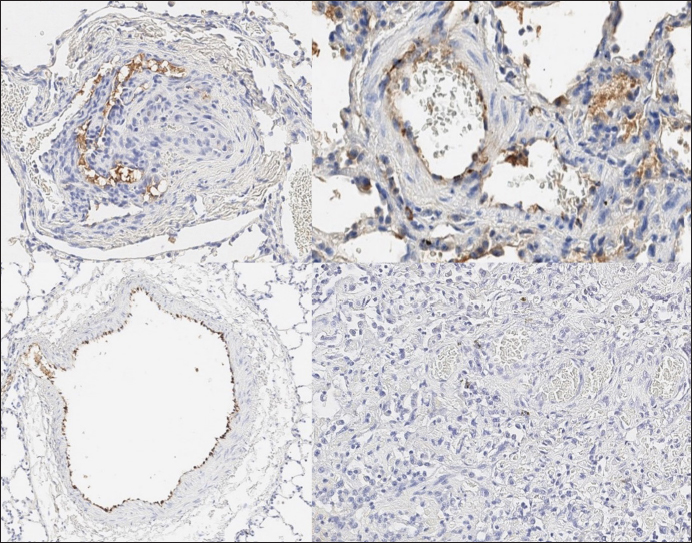
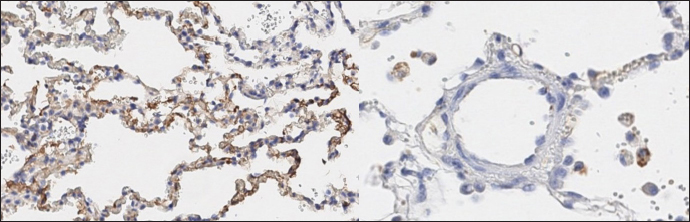
Histopathology In dogs with RHF, with both Masson’s trichrome and hematoxylin and eosin staining, we found wide fields of pulmonary fibrosis, arterial thickening due to tunica media proliferation, and plexiform vascular lesions with signs of revascularization. The dog with pneumonitis showed chronic interstitial histiocytic pneumonia (Fig. 3). In the sPCH group, we found zones of multifocal mild-to-moderate alveolar septa thickening with numerous capillary proliferation with signs of congestion. Endothelial cells were flattened, but had no signs of anisocytosis or anisokariosis. The growing capillaries expanded in pulmonary tissue, forming nodules and web-like anastomosis with bizarre vessel proliferation which affects zonal bronchi and arteriolas (Figs. 4 and 5). Immunohistochemical labeling with anti-CD31 antibody and anti-factor von Willebrand antibody showed wide dispersion between the groups. In the RHF group, we found a weak-to-absent reaction for the CD31 marker in most of the fibrotic fields, restricted to endothelial cells. Additionally, we discovered an intensive reaction to von Willebrand factor in most arterioles and in vessels with plexiform morphology. In sPCH animals, we observed significant expression of CD31 marker covering most of the fields and actively present in alveolar septae with numerous capillaries. However, the representation of von Willebrand factor was almost absent (Figs. 6 and 7).
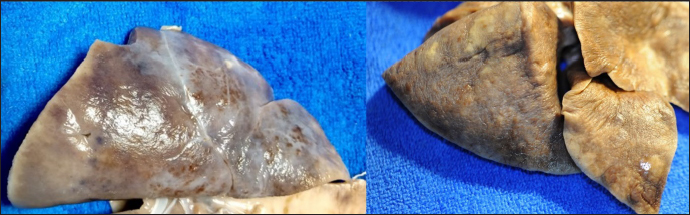
Fig. 2. Gross lungs pathology. Left one—sPCH, presented with numerous and patchy distributed blood-stained foci and zones with fibrous thickening of pleura. Right—RHF group, nodules and loci of white pale tissue on the surface diffusely distributed into the lobe tissue.
Fig. 3. RHF group. Left top—aggressive lung remodeling, resembling organizing diffuse alveolar damage with collapse and fusion of the alveoli and loss of typical structure. Right top—tunica media and adventitia proliferation around pulmonary vessel. Left bottom—partial occlusion of pulmonary vessel. Right bottom—plexiform vasculopathy with lumen obstruction and revascularization, presented with onion-like structure. Stained with H&E.
Fig. 4. sPCH group. Left (dog)—portions of alveolar septae with duplicated capillaries and capillaries proliferation into arterial walls. Right (cat)—less prominent septal alteration, significant arterial walls capillary proliferation. Stained with H&E.
Fig. 5. sPCH group. Net-like capillaries organization in alveolar septa (cat). DiscussionPrimary PCH is an idiopathic vessel disease with a complex pathogenesis that leads to the development of PH Clinical signs are usually associated with dyspnea, syncope, and fatigue. However, these symptoms are non-specific, which makes differentiation very challenging. Another drawback is the contraindication of typical therapy with phosphodiesterase inhibitors, as it results in pulmonary edema (Humbert et al., 1998). The main feature of PCH is capillary proliferation presented in bizarre infiltration of the pulmonary parenchyma, including all sizes of bronchi, alveolar septa, and arteries. In human medicine, this disease can be evaluated using high-resolution computed tomography (in veterinary practice, its use is limited due to lack of availability) and biopsies (including incisive variant). In veterinary medicine, there are constraints for performing such studies due to economic odds and suspected high invasiveness of tissue sampling associated with anesthetic risks. In human literature, there are some notes that similar to PCH morphology could be found in cases of severe pulmonary circulation overload due to congenital heart disease or heart failure (Richard and Kradin, 2016). In this context, one case reports similar findings in a 15-year-old cat with hypertrophic cardiomyopathy, which potentially could exclude congenital vascular disease and shift opinion to an acquired state (Jaffey et al., 2017). This thesis is supported by data from a report of diagnosed PCH in a human patient with hypertrophic cardiomyopathy (Jing et al., 1998). Another case described in feline was found in a 1-year-old cat with unexpected death and post-mortem analysis showed pulmonary tissue with PVOD-like and PCH-like alterations (Jenkins and Jennings, 2017). As mentioned above, PCH and PVOD could be found in one patient as a co-existing disease and there is an opinion that PCH could be a form of PVOD (Lantuéjoul et al., 2006). In veterinary medicine, rare canine cases have been described as a mixed form of PVOD and PCH (Williams et al., 2016). In this study, we did not observe mixed phenotypes that can be explained by the non-uniform distribution of alterations and, possibly, the period of the disease. We found low expression of von Willebrand factor in the lung endothelium and consequently a low grade of possible thrombosis in arterial and venous blood flow, which decreased the formation of the venous occlusion. The causes of both PVOD and PCH in dogs and cats remain unknown. Some data shows the absence of left heart failure in cases of PVOD (Williams et al., 2016). Meanwhile, signs of these diseases have been described in other species with conditions of elevated pulmonary venous pressure (Williams et al., 2008). In human medicine, it is believed that PVOD and PCH are multifactorial processes. These include genetic predisposition, exposure to exogenic substrates (chemical agents), infections, and immune-mediated reactions (Stenmark et al., 2016).
Fig. 6. RHF group. Immunohistochemistry. Left top right top, left bottom—factor von Willebrand active expression on endothelial surface in pulmonary vessels. Right bottom—absence of reaction on CD31 marker of endothelial cells.
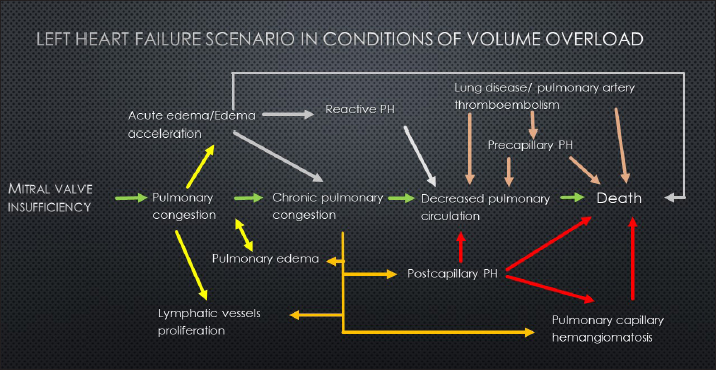
Fig. 7. sPCH group. Immunohistochemistry. Left—active expression of CD31 marker in numerous endothelial cells in alveolar septae. Right—almost absent of factor von Willebrand expression in cells. In light of the described facts, we proposed a united scenario of left-side heart failure development, including the possibility of secondary PCH development (Fig. 8).
Fig. 8. Schematic representation of volume overload heart failure development. In our study, we have found some data that supports the possibility of a secondary development of PCH-like changes. In this group of cases, specific histological markers were found in patients with a shifting left-heart failure phenotype to right-side failure. This intermediate condition shows that chronic volume overload of pulmonary circulation stimulates small vessel proliferation to provide enough space for increased blood volume until the celling effect is reached. At this point, microvascular vessel remodeling starts affecting both venulae and arteriolas, worsening pulmonary circulation, increasing pulmonary artery pressure, and decreasing flow to the left chambers, with dilatation of the right chambers. The absence of venula thrombus suggests that PCH-like changes are less likely to be associated with PVOD. Despite the all-accepted classification representing primary PCH as a primary vascular disease, we can suspect the presence of secondary PCH-like lesions due to chronic pulmonary congestion, which could be the consequence of postcapillary PH and shifting to the precapillary form. LimitationThis study is retrospective. Unfortunately, we cannot provide a histopathological study of all patients with suspected PH due to owners’ concerns regarding postmortem pathology or the inability to provide a biopsy study in vivo. Additionally, we cannot perform right heart catheterization to obtain straight pulmonary pressure and use only standard echocardiography techniques. Echocardiographic parameters have serious limitations in the estimation of mixed PH. Our “reverse diagnosis” is only an observation in several patients and cannot be a significant marker until more serious studies are provided. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Data availabilityAll data are provided in the manuscript. ReferencesAdachi, S., Hirashiki, A., Kondo, T., Nakaguro, M., Ogawa, A., Miyaji, K., Matsubara, H., Yokoi, T. and Murohara, T. 2014. Imatinib is partially effective for the treatment of pulmonary capillary hemangiomatosis. Intern. Med. 53(6), 603–607. Frazier, A.A., Franks, T.J., Mohammed, T.L.H., Ozbudak, I.H. and Galvin, J.R. 2007. From the archives of the AFIP: pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. RadioGraphics. 27, 867–82. Ginns, L.C., Roberts, D.H., Mark, E.J., Brusch, J.L. and Marler, J.J. 2003. Pulmonary capillary hemangiomatosis with atypical endotheliomatosis: successful antiangiogenic therapy with doxycycline. Chest. 124(5), 2017–2022. Humbert, M., Maitre, S., Capron, F., Rain, B., Musset, D. and Simonneau, G. 1998. Pulmonary edema complicating continuous intravenous prostacyclin in pulmonary capillary hemangiomatosis. Am. J. Respir. Crit. Care Med. 157, 1681–1685. Jaffey, J.A., Williams, K.J., Masseau, I., Krueger, M. and Reinero, C. 2017. Vasoproliferative process resembling pulmonary capillary hemangiomatosis in a cat. BMC Vet. Res. 13(1), 72. Jenkins, T.L. and Jennings, R.N. 2017. Pulmonary capillary hemangiomatosis and hypertrophic cardiomyopathy in a Persian cat. J. Vet. Diagn. Invest. 29(6), 900–903. Jing, X., Yokoi, T., Nakamura, Y., Nakamura, M., Shan, L., Tomimoto, S., Hano, T. and Kakudo, K. 1998. Pulmonary capillary hemangiomatosis: a unique feature of congestive vasculopathy associated with hypertrophic cardiomyopathy. Arch. Pathol. Lab. Med. 122(1), 94–96. Langleben, D. 2014. Pulmonary capillary hemangiomatosis: the puzzle takes shape. Chest. 145(2), 197–199 Lantuéjoul, S., Sheppard, M.N., Corrin, B., Burke, M.M. and Nicholson, A.G. 2006. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: a clinicopathologic study of 35 cases. Am. J. Surg. Pathol. 30(7), 850–857. Lawler, L.P. and Askin, F.B. 2005. Pulmonary capillary hemangiomatosis: multidetector row CT findings and clinic-pathologic correlation. J. Thorac. Imaging. 20, 613. Nayyar, D., Muthiah, K., Kumarasinghe, G., Hettiarachchi, R., Celermajer, D., Kotlyar, E. and Keogh, A. 2014. Imatinib for the treatment of pulmonary arterial hypertension and pulmonary capillary hemangiomatosis. Pulm. Circ. 4(2), 342–345. Reinero, C., Visser, L.C., Kellihan, H.B., Masseau, I., Rozanski, E., Clercx, C., Williams, K., Abbott, J., Borgarelli, M. and Scansen, B.A. 2020. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 34(2), 549–573. Reinero, C.R., Jutkowitz, L.A., Nelson, N., Masseau, I., Jennings, S. and Williams, K. 2019. Clinical features of canine pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. J. Vet. Intern. Med. 33(1), 114–123. Richard, L. and Kradin, M. 2016. Understanding pulmonary pathology: applying pathological findings in therapeutic decision making 1st Edition. Cambridge, MA: Academic Press. Stenmark, K.R., Krafsur, G.M. and Tuder, R.M. 2016. Pulmonary veno-occlusive disease and pulmonary hypertension in dogs: striking similarities to the human condition. Vet. Pathol. 53(4), 707–10 Toom den, M.L., Grinwis, G., van Suylen, R.J., Boroffka, S.A., de Jong, P., van Steenbeek, F.G. and Szatmári, V. 2018. Pulmonary veno-occlusive disease as a cause of severe pulmonary hypertension in a dog. Acta Vet. Scand. 60(1), 78. White, C.W. 1990. Treatment of hemangiomatosis with recombinant interferon alfa. Semin Hematol. 27(3 suppl. 4), 15–22. Williams, K., Andrie, K., Cartoceti, A., French, S., Goldsmith, D., Jennings, S., Priestnall, S.L., Wilson, D. and Jutkowitz, A. 2016. Pulmonary veno-occlusive disease: a newly recognized cause of severe pulmonary hypertension in dogs. Vet. Pathol. 53(4), 813–822. Williams, K.J., Derksen, F.J., de Feijter-Rupp, H., Pannirselvam, R.R., Steel, C.M. and Robinson, N.E. 2008. Regional pulmonary veno-occlusion: a newly identified lesion of equine exercise-induced pulmonary hemorrhage. Vet Pathol. 45(3), 316–326. | ||
| How to Cite this Article |
| Pubmed Style Oleynikov Dmitrij Arkadievich. Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Vet. J.. 2024; 14(5): 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 Web Style Oleynikov Dmitrij Arkadievich. Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. https://www.openveterinaryjournal.com/?mno=191815 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.17 AMA (American Medical Association) Style Oleynikov Dmitrij Arkadievich. Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Vet. J.. 2024; 14(5): 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 Vancouver/ICMJE Style Oleynikov Dmitrij Arkadievich. Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 Harvard Style Oleynikov Dmitrij Arkadievich (2024) Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Vet. J., 14 (5), 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 Turabian Style Oleynikov Dmitrij Arkadievich. 2024. Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Veterinary Journal, 14 (5), 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 Chicago Style Oleynikov Dmitrij Arkadievich. "Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension." Open Veterinary Journal 14 (2024), 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 MLA (The Modern Language Association) Style Oleynikov Dmitrij Arkadievich. "Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension." Open Veterinary Journal 14.5 (2024), 1216-1223. Print. doi:10.5455/OVJ.2024.v14.i5.17 APA (American Psychological Association) Style Oleynikov Dmitrij Arkadievich (2024) Retrospective analysis of dogs and cats with a mixed form of pulmonary hypertension and suspected pulmonary capillary hemangiomatosis in comparison to animals with predomination of precapillary pulmonary hypertension. Open Veterinary Journal, 14 (5), 1216-1223. doi:10.5455/OVJ.2024.v14.i5.17 |