| Research Article | ||
Open Vet. J.. 2024; 14(8): 1912-1920 Open Veterinary Journal, (2024), Vol. 14(8): 1912–1920 Research Article Detection of Crimean-Congo hemorrhagic fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina FasoLaibané Dieudonné Dahourou1,2*, Madi Savadogo3,4, Mikhailou Kiswend-Sida Dera5, Lamoussa Roland Abga6, Bruno Lalidia Ouoba7, Rayangnéwêndé Stéphane Arnaud Tapsoba2, Bernadette Yougbare2, Salimata Akio6, Lamouni Habibata Zerbo7, Amadou Traore2 and Rianatou Bada Alambedji61Environmental Sciences and Rural Development Institute (ISEDR), Daniel Muezzin Coulibaly University, Dedougou, Burkina Faso 2Animal Biology and Health Laboratory (LABIOSA), Institute of Environment and Agricultural Research (INERA), Ouagadougou, Burkina Faso 3Influenza National Reference Laboratory (LNR-G), Epidemic Potential diseases, Emerging Diseases and Zoonoses Unit, Medical Biology and Public Health Department, Research Institute of Health Sciences (IRSS/CNRST), Ouagadougou, Burkina Faso 4Fundamental and Applied Research for Animals and Health (FARAH), Faculty of Veterinary Medicine, University of Liege, Quartier Vallée 2 avenue de Cureghem, Liege, Belgium 5Insectarium of Bobo Dioulasso, Bobo-Dioulasso, Burkina Faso 6Microbiology, Immunology and Infectious Diseases Service, Public Health and Environment Department, Interstate School of Sciences and Veterinary Medicine, (EISMV), Dakar, Senegal 7National Livestock Laboratory (LNE), Ouagadougou, Burkina Faso *Corresponding Author: Laibané Dieudonné Dahourou. Environmental Sciences and Rural Development Institute (ISEDR), Daniel Muezzin Coulibaly University, Dedougou, Burkina Faso. Email: d_dahourou [at] yahoo.fr Submitted: 01/05/2024 Accepted: 26/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Crimean-Congo hemorrhagic fever (CCHF) is a zoonotic disease caused by an Orthonairovirus of the Nairoviridae family transmitted by tick bites and also contact with infected blood, tissues, or body fluids. Until now, fewer studies have been conducted on animals in Burkina Faso. Aim: This study was conducted to investigate the seroprevalence and risk factors of CCHF in two provinces of Burkina Faso. Methods: Serum samples were collected from 371 bovine animals. In addition, questionnaire surveys were performed with cattle breeders. The double-antigen sandwich enzyme-linked immunosorbent assay test was used to determine the presence of antibodies against the CCHF virus in serum samples. Results: The results showed an overall prevalence of 72.2% [95% confidence interval (CI): 67.6%–76.7%)]. Within the 74 herds included in the study, a herd prevalence of 96% (95% CI: 91.4%–100%) was obtained. The prevalence was significantly higher in Mouhoun province (80%, 95% CI: 74%–86%) compared to Kénédougou province (65.6%, 95% CI: 59.1%–72.2%). Multivariable regression analysis showed that females were more likely to be infected (OR=1.99, 95% CI: 1.1–3.6, p=0.023) than males. In addition, cross-breed animals (OR=6.42, 95% CI: 1.71–24.14, p=0.006) were more likely to be infected compared to local-bred animals. This study revealed the presence of antibodies of the CCHF virus in cattle in the study area, indicating the need to implement control measures in the veterinary sector. Conclusion: Despite the importance of CCHF in public health, no study has been implemented regarding this condition in animals in Burkina Faso. This study described evidence of cattle exposure to the virus in Burkina Faso. Keywords: Burkina Faso, Cattle, Haemorrhagic fever virus, Crimean-Congo, Seroepidemiologic studies. IntroductionBurkina Faso is a Sahelian country located in West Africa with several potentialities in the livestock sector. Animal breeding is the socio-economic activity of more than 80% of families from which they take part or all their revenues. The livestock sector contributed, in 2019, for 10% of the GDP. In Burkina Faso, around 4 million of people live mainly from livestock and 38.8% of the monetary income of rural households is generated from the livestock sub-sector. Many cattle breeders use the income generated from the sale of animals to meet the costs associated with children’s education, wedding ceremonies, baptisms as well as traditional and religious celebrations. Among the livestock species, poultry, goats, sheep, cattle, and pigs occupy the first, second, third, fourth, and fifth ranks, respectively, in terms of population size (INSD, 2020). Indeed, the national livestock population was estimated at 10,237,000 for cattle, 11,078,000 for sheep, and 16,587,000 for goats (INSD, 2021). With an estimated production of 35,000,000 tonnes of meat, 264,000,000 liters of milk, and 6,000 tonnes of eggs per year (FAO, 2019), animal breeding is the largest provider of protein. Despite this socio-economic importance, livestock farming in Burkina Faso, like in other Sahelian African countries, faces several constraints that limit its development. These constraints include high-impact animal diseases as well as emerging and re-emerging zoonoses. Among these diseases, Crimean-Congo hemorrhagic fever (CCHF), a tick-borne zoonotic disease, distributed in several countries could be an important threat. It is caused by the CCHF virus, an Orthonairovirus of the Nairoviridae family. Humans are infected by tick bites or by contact with biological fluids from infected animals or subjects. The disease was previously described in cattle in some countries neighboring Burkina Faso, including Mali (prevalence of 66%) (Maiga et al., 2017), Niger (prevalence of 9.1%) (Maïna et al., 2020); and Ghana (prevalence of 5.7%) (Akuffo et al., 2016). In addition, a recent CCHF outbreak resulted in human deaths in Mali (Temur et al., 2021). In Burkina Faso, livestock farming techniques are characterized by the movement of cattle through transhumance to neighboring countries (Cote d’Ivoire, Mali, Benin, Togo, Niger, and Ghana), which could lead to the circulation of the disease in cattle across borders. However, seroepidemiological evidence-based data on CCHF in animals in Burkina Faso remains poor (Temur et al., 2021). Therefore, this study was conducted to contribute to a better understanding of the epidemiology of CCHF in cattle in Burkina Faso. Material and MethodsStudy areaThe study was conducted in two provinces of Burkina Faso; the Kénédougou province (located in the region of Hauts-Bassins where cattle population size was estimated at 1,699,916) and the Mouhoun province (located in the region of Boucle du Mouhoun where cattle population size was estimated at 903,916). The investigation covered 12 villages belonging to six communes in the two studied provinces (Fig. 1). The province of Kénédougou is in the South Soudanian climate area between 4°30′ and 5°30′ West longitude and 10°5′ and 12°5′ North latitude. Animal breeding is the second main socio-economic activity of the inhabitants after agriculture. The extensive breeding system is dominant, with a significant movement of transhumant herds during the dry season, coming from the north of the country and from Mali. In this province, the study was conducted in three villages of the commune of Ndorola (N’Dorola, Torokoto, and Sefina) and three villages of the commune of Sindo (Lêrasso, Sindou and Blini). The Mouhoun province is located at 12°27′ North latitude and 3°28′ West longitude. It is characterized by a Sudano-Sahelian climate and is composed of seven communes. In this province, the study was implemented in three villages of the commune of Dédougou (Noakuy, Passakongo, and Kari) and three villages in the communce of Safané (Nounou, Safané, and Sodien). Study design and animals samplingThis cross-sectional study was conducted from October to December 2021. A multistage sampling method was performed. First, the two provinces were selected regarding the importance of animal movements between these provinces and Mali [where a recent CCHF outbreak was reported (Temur et al., 2021)]. For this purpose, the province of Kenedougou characterized by important contacts with animals from Mali; and the province of Mouhoun characterized by lower contacts with animals from Mali were included in the study. Second, in each selected province, two communes were randomly selected. Third, in each selected commune, three villages were randomly selected. Finally, in each selected village, simple random sampling was applied to recruit herds and animals. In each herd, the number of sampled animals was according to the size of the herd, and 20% of animals in each herd were sampled. Thus, a total of 74 herds were visited from which 371 cattle were randomly sampled. Questionnaire survey and samples collectionA pre-tested structured questionnaire was administered to cattle owners to collect data on the control methods against ticks, practice of transhumance, mixing of animal with animals from Mali, the type of breed and the sex of animals, and the type of farming. The questionnaire had been administrated during a face-to-face interview. Observations have been made on the skin of each selected animal to collect data about the presence of ticks on animals. Blood samples were collected from the jugular of animals, using a 10 ml vacuum dry tube. The tubes were placed vertically and left overnight to allow for decantation. After decanting, the sera were collected with pipettes into well-identified eppendorf tubes. These samples were then sorted into coolers containing ice preservatives and sent under refrigeration to the National Livestock Laboratory (Laboratoire National d’Elevage, LNE) located in Ouagadougou. Then they were stored at −20°C until the day of the serological analyses. Laboratory analysisCCHF-positive samples were identified using the Enzyme-linked immunosorbent assay (ELISA) method, based on the detection of anti-CCHFV antibodies in the serum. For this purpose, an ELISA kit (ID Screen® CCHF Double Antigen Multi-species from IDvet laboratory) was used following the manufacturer’s instructions (Sas et al., 2018) quick and reliable multispecies assay for the detection of CCHFV-specific antibodies is needed. This work presents the development and validation of a novel CCHF double-antigen ELISA for the detection of anti-CCHFV nucleoprotein antibodies. The test requires 30 μl of serum, and results are obtained within 90 min. As the ELISA is based on recombinant N-protein of the IbAr10200 virus, it can be run under standard biosafety conditions. For assay validation, sera from 95 cattle and 176 small ruminants from CCHF-endemic regions (origin: Albania, Cameroon, Kosovo, Former Yugoslav Republic of Macedonia, Mauritania, Pakistan, Turkey. According to the manufacturer, the kit is characterized by a specificity of 100% and a sensitivity of 99%. This ELISA kit is based on the recombinant N protein of CCHFV strain IbAr10200 and can be safely used under standard biosafety conditions. In summary, 30 μl of the positive and negative controls and each sample were diluted with 50 µl of “dilution buffer 14” in wells. The plate was incubated for 45 minutes at 21°C. After a washing procedure, 50 µl of “1x conjugate” was added to each well of the plate and the plate was incubated for 30 minutes at 21°C. A second wash procedure was then performed and 100 µl of “Development Solution” was added, followed by incubation for 15 minutes at 25°C in the dark before stopping the reaction with the Stop Solution. An optical density value for each sample and the controls were determined using a “Multiskan Ex” ELISA plate reader at 450 nm. The reading was taken starting with the blank and then the test plates. According to the kit, the test was valid and definitive when the mean OD value of the positive control was greater than 0.350 (DOCP > 0.350) and the ratio of the mean OD value of the positive and negative controls was greater than 3 (DOCP/DOCN>3). For each sample, the S/P% was calculated using the following formula: S/P%=(OD of sample/OD of positive control) × 100. Serum samples with S/P% less than or equal to 30% were classified as negative and those with S/P% greater than 30% as positive.
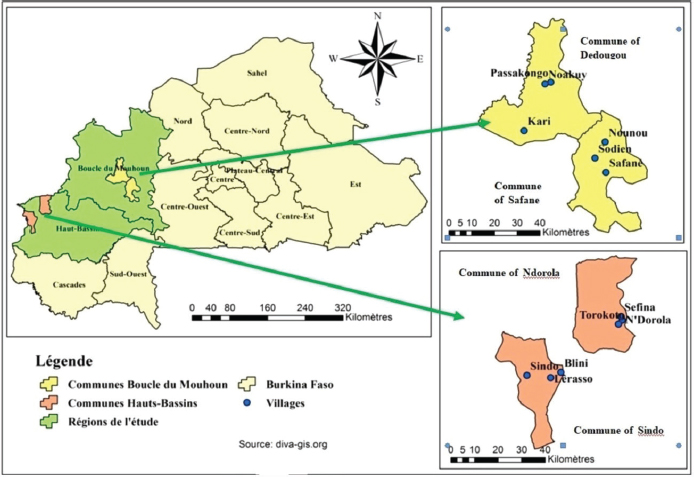
Fig. 1. Location of the study sites in the targeted provinces and regions. Statistical analysisData from the questionnaire survey and serological analysis were entered in a Microsoft Office 2016 Excel spreadsheet. A herd was considered positive if there was at least one positive cattle detected in that herd. For each seroprevalence value, a 95% confidence interval (CI) was calculated. Statistical analyses were performed using R 3.5.2 software for the analysis of associations (Chi-square test and Fisher exact test). The serological status of the animal was considered as the dependent variable and the questionnaire variables (related to herd and animal characteristics) were considered as independent variables. To investigate the risk factors associated to seroprevalence, univariable logistic regression was performed using STATA 13 software to determine unadjusted odds ratios. Following the univariable logistic regression, variables with a p-value less than or equal to 0.20 were included in a multivariable logistic regression model to identify adjusted odds ratios. The manual forward stepwise selection was applied to select the final model using the Akaike’s information criterion (AIC) and the model with the smallest AIC was kept as the best. The identification of the risk factors associated with CCHF virus infection was performed by analyzing the odds ratios, their CIs, and the p-values associated with each variable. For all these calculations, the significance level was set at 0.05. Ethical approvalEthical approval was obtained from the Research Ethics Committee of the University Joseph KI ZERBO (agreement reference CE-UJKZ/2020-03). In addition, prior to interviews and animal sampling, the cattle breeders were informed of the context and purpose of the study, emphasizing that participation was voluntary, and data collected would remain confidential. Therefore, only cattle breeders who provided oral consent to participate were included in the study. All data were collected and reported in accordance with ARRIVE, UK Animals (Scientific Procedures) Act, and EU Directive 2010/63/EU for animal experiments guidelines. ResultsCharacteristics of sampled herds and cattleTable 1 presents the characteristics of sampled cattle and visited herds. Most of sampled cattle were females (71.7%). Most of the cattle (95.1%) were bred under an extensive breeding system and transhumance was practiced by 47.2% of interviewed breeders. Most sampled cattle were acquired through legacy (94.8%). The age of interviewed cattle breeders ranged from 20 to 71 years. Data regarding control methods against tick infestation were not accurate and have not been included in the present study. Table 1. Variation of CCHF seroprevalence in the province of Mouhoun and Kénédougou provinces.
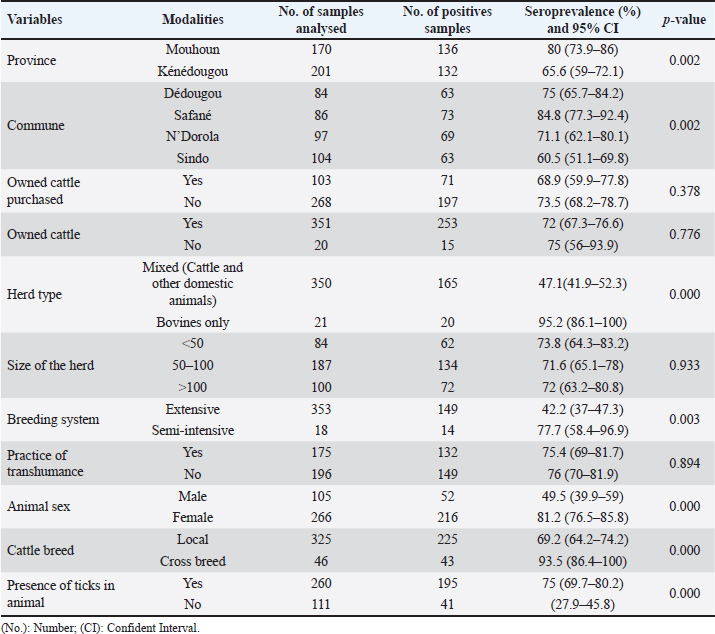
Seroprevalence of CCHF in cattleThe results showed that 268 sera were positive, corresponding to an overall seroprevalence of 72.2% (95% CI: 67.6%–76.7%) as described in Table 1. Within the 74 herds, 96% were detected positive (95% CI: 91.4%–100%). In Mouhoun province, the seroprevalence was significantly higher compared to Kénédougou province (p=0.002). The variation of the seroprevalence was significantly higher in the herds composed of cattle only (95.2%, 95% CI: 86.1–100) compared to herds in which other domestic species (sheep, goats) (p=0.000). The seroprevalence was significantly lower in the extensive breeding system (47.1%, 95% CI: 37–47.3; p=0.003) and a significant variation was noted for animal sex with a higher seroprevalence in females (81.2%, 95% CI: 76.5–85.8; p=0.000). Finally, the seroprevalence was significantly higher in cattle infested by ticks (75%, 95% CI: 69.7–80.2; p=0.000). Table 2. Univariable logistic regression of CCHF virus infection in relation to the different variables.
Table 3. Multivariable logistic regression of CCHF virus infection in relation to the different variables.
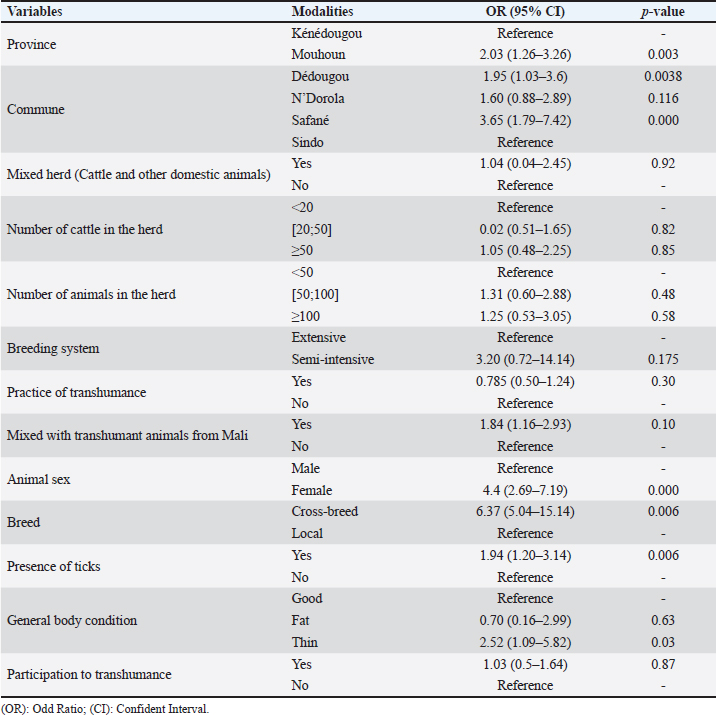
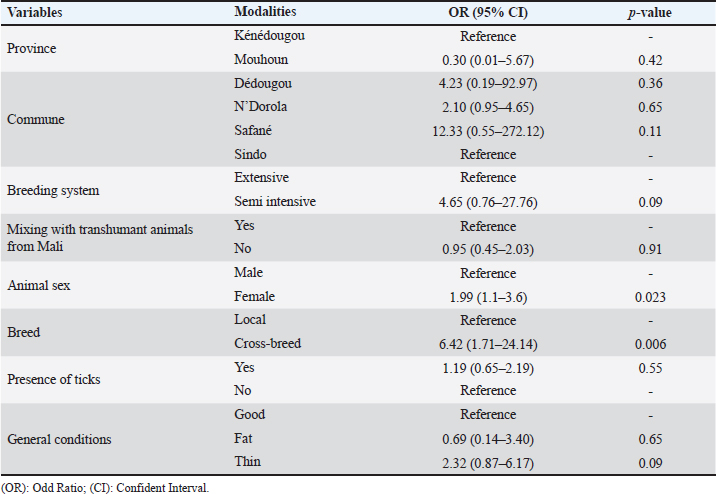
Risk factors associated with CCHF seroprevalence in cattleAfter the univariable analysis, different factors were associated with the CCHF seroprevalence. Cattle from Mouhoun province (OR=2.03, 95% CI: 1.26–3.26, p=0.003) were more likely to be infected compared to cattle from Kénédougou province. Also, cattle bred in Safané (OR=3.65, 95% CI: 1.79–7.42, p= 0.000) and Dédougou (OR=1.95, 95% CI: 1.03–3.6, p=0.003) were more likely to be seropositive compared to cattle in the commune of Sindo (Table 2). In addition, female cattle were 4.4 times more likely to be seropositive compared to males (95% CI: 2.69–7.19, p=0.000). Cross-breed cattle were more likely to be seropositive (OR=6.37, 95% CI: 5.04–15.14, p=006) compared to cattle of the local breed. Cattle on which ticks were detected during sampling, were more likely to be seropositive (OR=1.94, 95% CI: 1.20–3.14, p=0.006) compared to cattle with no ticks. Finally, cattle that were thin were more likely to be seropositive than others (OR=2.52, 95% CI: 1.09–5.82, p=0.03) (Table 2). When it comes to the multivariable regression, the results showed that cattle sex and breed had a significant influence on the cattle’s susceptibility to seropositivity. Females (OR=1.99, 95% CI: 1.1–3.6, p=0.023) were more susceptible to being seropositive than males. In addition, cross-breed cattle were more likely to be seropositive compared to the local breed (OR=6.42, 95% CI: 1.71–24.14, p=0.006) (Table 3). DiscussionIn this study, we investigated the seroprevalence of CCHF in cattle in two regions of Burkina Faso. To the best of our knowledge, no study was conducted on CCHF in animals in Burkina Faso. As the first epidemiological investigation in the country, cattle species were selected as previous studies reported frequent outbreaks in large mammals, which are the preferred hosts of adult ticks, Hyalomma species (Shepherd et al., 1989). The choice of the study areas was based on epidemiological considerations and the ease of access to cattle, regarding the lack of security in several parts of Burkina Faso during the study period. In the two provinces of the study, cattle’s breeding is widespread and cattle are bred mainly under the extensive breeding system with important animal movements. Several studies reported the presence of the CCHF virus in neighboring countries, such as Niger (Maïna et al., 2020) and Mali (Maiga et al., 2017). In the case of Mali, human cases of CCHF were also reported and the virus has been noted in tick (Zivcec et al., 2014; Baumann et al., 2019). In this study, we demonstrated that cattle in the Mouhoun and Kénédougou provinces were previously exposed to CCHF virus with an overall seroprevalence of CCHF of 72.2%. This high seroprevalence indicates that the CCHF virus had significantly circulated among cattle in the study area. Ticks from Hyalomma genus mainly Hyalomma marginatum and Hyalomma rufipes are known to be reservoirs and vectors of the virus (Hoogstraal, 1979). In Burkina Faso, studies have noted the presence of Hyalomma ticks on cattle (Biguezoton et al., 2016; Compaoré et al., 2022) and the presence of the virus in the country has been demonstrated (Saluzzo et al., 1984). Animals from all provinces and communes included in the study experienced contact with the virus, suggesting a large distribution of the disease in the country. Indeed, the local climate and vegetation are favorable to the development of ticks (Stachurski et al., 2004), which are vectors of the CCHF virus. It is known that the Sudan-Sahelian climate with high rainfall and abundant vegetation is well suited for the development of ticks. The association between climatic conditions and the incidence of CCHF has been demonstrated (Ansari et al., 2014). The seroprevalence observed was higher than data noted in Niger (Maïna et al., 2020), Ghana (Akuffo et al., 2016), Nigeria (Oluwayelu et al., 2020), Senegal (Mangombi et al., 2020) and Sudan (Ibrahim et al., 2015). Also, in Corsica, Grech-Angelini et al. (2020) reported a prevalence of 13%, and in India, Mourya et al. (2014) found a prevalence of 12.09%. This higher seroprevalence reported in the present study could be explained by a higher density of the ticks on animals despite the control of ticks by farmers. During discussions with farmers, they reported that they used to treat animals against ticks by spraying animals with acaricides. However, several studies reported high resistance of ticks against commonly used acaricide in Burkina Faso (Adakal et al., 2012; Ouedraogo et al., 2021)little information exists on tick-control practices used by livestock farmers. We interviewed 60 stockbreeders working in traditional farming systems to obtain the first data on tick-control practices. Sixteen farmers (27 %. However, in Nigeria, authors recently described a higher prevalence in Camels (Adamu et al., 2024) Kano State, Nigeria, to estimate the seropositivity of CCHFV in camels using a commercial multi-species competitive enzyme-linked immunosorbent assay (ELISA. Findings noted that risk factors were animal sex and breed. Female cattle were more likely to be seropositive compared to males. The same observations were made by Mangombi et al. (2020) in Senegal. Regarding the management of animals in herds in Burkina Faso, females are preferred by breeders for reproduction and therefore kept longer than males. As female cattle stay longer in herds, they are highly exposed to tick bites, increasing the risk of contamination. The breed of the animals was also found to be a risk factor; with cross-breed cattle being more susceptible to be seropositive. This could be explained by the higher susceptibility of cross-breed cattle to tick infestation (Yessinou et al., 2018). In contrast, the indigenous breeds are relatively resistant to tick infestation hence, they were at lower risk for the disease compared to cross breeds. The main limitation of this work is its geographical extent. This study was only conducted in two provinces in Burkina Faso. Because of security issues, some areas at the Mali borders have been excluded from sampling. Moreover, data provided by farmers on treatment against ticks were neither accurate nor reliable, and then have been removed for analysis. ConclusionThe CCHF is a zoonotic disease that is transmitted by ticks and affects different domestic animals and humans. This study provided evidence of animal exposure to CCHF in Burkina Faso, with a high seroprevalence observed in the study areas. According to findings, females and the cross-bred cattle were more likely to be seropositive than other animals. Finally, it would be interesting to extend this study to other areas of the country to better capture the epidemiological status and risk factors of the disease. As it is a major zoonotic disease mass awareness-raising activities should be promoted to reduce the transmission risk to human populations. AcknowledgmentsThe authors are grateful to the Royal Society for Tropical Medicine and Hygiene (RSTMH) for funding this study and to the National Livestock Laboratory [Laboratoire National d’Elevage (LNE) de la Direction Générale des Services Vétérinaires (DGSV), Burkina Faso] for the technical support during the serological analyses. The authors also thank the Provincial Directorates of Mouhoun and Kenedougou as well as the communal officers for their valuable assistance during field investigations. Conflict of interestThe authors declare that there are no conflicts of interest. FundingThis study was funded by the Royal Society for Tropical Medicine and Hygiene through its small grant program. Authors’ contributionsDAHOUROU Laibané Dieudonné: Conceptualization; Methodology; Formal analysis; Funding acquisition; Software; Writing—original draft; DERA Mikhailou, ABGA Lamoussa Roland, AKIO Salimata, YOUGBARE Bernadette, OUOBA Lalidia Bruno, TAPSOBA Arnaud Stéphane Rayangnéwêndé, SAVADOGO Madi, ZERBO Lamouni Habibata: Investigation; Writing—review and editing; TRAORE Amadou: Visualization; Writing—review and editing; BADA ALAMBEDJI Rianatou: Validation; Supervision; Writing—review and editing. All authors reviewed and validated the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAdakal, H., Stachurski, F. and Chevillon, C. 2012. Tick control practices in Burkina Faso and acaricide resistance survey in Rhipicephalus (Boophilus) geigyi (Acari: Ixodidae). Exp. Appl. Acarol. 59, 483–491. Adamu, A.M., Onoja, A.B., Ugbodu, V.E., Bala, R.S., Maina, M., Salisu, U.S., Pewan, S.B., David, E., Malgwi, A., Adamu, C., Adeiza, A., Herbert, M., Horwood, P. and Adegboye, O. 2024. Investigating Crimean–Congo haemorrhagic fever virus seropositivity in camels and human behavioural risks in an abattoir in Nigeria. Epidemiol. Infect. 152, e29. Akuffo, R., Brandful, J.A.M., Zayed, A., Adjei, A., Watany, N., Fahmy, N.T., Hughes, R., Doman, B., Voegborlo, S.V., Aziati, D., Pratt, D., Awuni, J.A., Adams, N. and Dueger, E. 2016. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect. Dis. 16, 324. Ansari, H., Shahbaz, B., Izadi, S., Zeinali, M., Tabatabaee, S.M., Mahmoodi, M., Holakouie Naieni, K. and Mansournia, M.A. 2014. Crimean-Congo hemorrhagic fever and its relationship with climate factors in southeast Iran: a 13-year experience. J. Infect. Dev. Ctries. 8, 749–757. Baumann, J., Knüpfer, M., Ouedraogo, J., Traoré, B.Y., Heitzer, A., Kané, B., Maiga, B., Sylla, M., Kouriba, B. and Wölfel, R. 2019. Lassa and Crimean-Congo hemorrhagic fever viruses, Mali. Emerg. Infect. Dis. 25, 999–1002. Biguezoton, A., Adehan, S., Adakal, H., Zoungrana, S., Farougou, S. and Chevillon, C. 2016. Community structure, seasonal variations and interactions between native and invasive cattle tick species in Benin and Burkina Faso. Parasit. Vectors 9, 43. Compaoré, S., Boungou, M., Biguezoton, A.S., Thiombiano, N.G., Zannou, O.M., Ouedraogo, A.S. and Kabré, G.B. 2022. Tick species infesting cattle in the central region of Burkina Faso: presence of Rhipicephalus microplus less than ten years after its first identification in the Southwest part of the country. Ticks Tick Borne Dis. 13, 101983. FAO. 2019. Le devenir de l’élevage au Burkina Faso: opportunités et défis face aux incertitudes. Rome, Italy: FAO. Grech-Angelini, S., Lancelot, R., Ferraris, O., Peyrefitte, C.N., Vachiery, N., Pédarrieu, A., Peyraud, A., Rodrigues, V., Bastron, D., Libeau, G., Fernandez, B., Holzmuller, P., Servan de Almeida, R., Michaud, V., Tordo, N., Comtet, L., Métras, R., Casabianca, F. and Vial, L. 2020. Crimean-Congo hemorrhagic fever virus antibodies among livestock on Corsica, France, 2014–2016. Emerg. Infect. Dis. 26, 1041–1044. Hoogstraal, H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15, 307–417. Ibrahim, A.M., Adam, I.A., Osman, B.T. and Aradaib, I.E. 2015. Epidemiological survey of Crimean Congo hemorrhagic fever virus in cattle in East Darfur State, Sudan. Ticks Tick Borne Dis. 6, 439–444. INSD. 2020. Annuaire statistiques du Burkina Faso, 2019, Institut National de la Statistiques et de la Démographie (INSD). Ouagadougou, Burkina Faso: INSD. INSD. 2021. Annuaire statistique du Burkina Faso, 2020, Institut National de la Statistiques et de la Démographie (INSD). Ouagadougou, Burkina Faso: INSD. Maiga, O., Sas, M.A., Rosenke, K., Kamissoko, B., Mertens, M., Sogoba, N., Traore, A., Sangare, M., Niang, M., Schwan, T.G., Maiga, H.M., Traore, S.F., Feldmann, H., Safronetz, D. and Groschup, M.H. 2017. Serosurvey of Crimean-Congo hemorrhagic fever virus in Cattle, Mali, West Africa. Am. J. Trop. Med. Hyg. 96, 1341–1345. Maïna, A., Ibrahim, A.I., Alassane, A. and Adakal, H. 2020. Epidémiologie de la fièvre Hémorragique de Crimée-Congo (FHCC) chez les bovins dans le département de Boboye au Niger. Int. J. Bio. Chem. Sci. 14, 698–705. Mangombi, J.B., Roqueplo, C., Sambou, M., Dahmani, M., Mediannikov, O., Comtet, L. and Davoust, B. 2020. Seroprevalence of Crimean-Congo hemorrhagic fever in domesticated animals in Northwestern Senegal. Vector Borne Zoonotic Dis. 20, 797–799. Mourya, D.T., Yadav, P.D., Shete, A., Majumdar, T.D., Kanani, A., Kapadia, D., Chandra, V., Kachhiapatel, A.J., Joshi, P.T., Upadhyay, K.J., Dave, P. and Raval, D. 2014. Serosurvey of Crimean-Congo hemorrhagic fever virus in domestic animals, Gujarat, India, 2013. Vector Borne Zoonotic Dis. 14, 690–692. Oluwayelu, D., Afrough, B., Adebiyi, A., Varghese, A., Eun-Sil, P., Fukushi, S., Yoshikawa, T., Saijo, M., Neumann, E., Morikawa, S., Hewson, R. and Tomori, O. 2020. Prevalence of antibodies to Crimean-Congo hemorrhagic fever virus in Ruminants, Nigeria, 2015. Emerg. Infect. Dis. 26, 744–747. Ouedraogo, A.S., Zannou, O.M., Biguezoton, A.S., Patrick, K.Y., Belem, A.M.G., Farougou, S., Oosthuizen, M., Saegerman, C. and Lempereur, L. 2021. Efficacy of two commercial synthetic pyrethroids (cypermethrin and deltamethrin) on Amblyomma variegatum and Rhipicephalus microplus strains of the south-western region of Burkina Faso. Trop. Anim. Health Prod. 53, 402. Saluzzo, J.F., Digoutte, J.P., Cornet, M., Baudon, D., Roux, J. and Robert, V. 1984. Isolation of Crimean-Congo haemorrhagic fever and Rift Valley fever viruses in Upper Volta. Lancet 1, 1179. Sas, M.A., Comtet, L., Donnet, F., Mertens, M., Vatansever, Z., Tordo, N., Pourquier, P. and Groschup, M.H. 2018. A novel double-antigen sandwich ELISA for the species-independent detection of Crimean-Congo hemorrhagic fever virus-specific antibodies. Antiviral Res. 151, 24–26. Shepherd, A.J., Swanepoel, R. and Leman, P.A. 1989. Antibody response in Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11(Suppl. 4), S801–S806. Stachurski, F., Adakal, H. and Desquesnes, M. 2004. La cowdriose : épidémiologie et contrôle. Bobo Dioulasso, Burkina Faso: CIRDES. Temur, A.I., Kuhn, J.H., Pecor, D.B., Apanaskevich, D.A. and Keshtkar-Jahromi, M. 2021. Epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Africa—underestimated for decades. Am. J. Trop. Med. Hyg. 104, 1978–1990. Yessinou, R.E., Adoligbe, C., Akpo, Y., Adinci, J., Youssao Abdou Karim, I. and Farougou, S. 2018. Sensitivity of different cattle breeds to the infestation of cattle ticks Amblyomma variegatum, Rhipicephalus microplus, and Hyalomma spp. on the Natural Pastures of Opkara Farm, Benin. J. Parasitol. Res. 2018, 2570940. Zivcec, M., Maïga, O., Kelly, A., Feldmann, F., Sogoba, N., Schwan, T.G., Feldmann, H. and Safronetz, D., 2014. Unique strain of Crimean–Congo hemorrhagic fever virus, Mali. Emerg. Infect. Dis. 20, 911–913. | ||
| How to Cite this Article |
| Pubmed Style Dahourou LD, Savadogo M, Dera MK, Abga LR, Ouoba BL, Tapsoba RSA, Yougbare B, Akio S, Zerbo LH, Traore A, Alambedji RB. Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Vet. J.. 2024; 14(8): 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 Web Style Dahourou LD, Savadogo M, Dera MK, Abga LR, Ouoba BL, Tapsoba RSA, Yougbare B, Akio S, Zerbo LH, Traore A, Alambedji RB. Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. https://www.openveterinaryjournal.com/?mno=199973 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.19 AMA (American Medical Association) Style Dahourou LD, Savadogo M, Dera MK, Abga LR, Ouoba BL, Tapsoba RSA, Yougbare B, Akio S, Zerbo LH, Traore A, Alambedji RB. Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Vet. J.. 2024; 14(8): 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 Vancouver/ICMJE Style Dahourou LD, Savadogo M, Dera MK, Abga LR, Ouoba BL, Tapsoba RSA, Yougbare B, Akio S, Zerbo LH, Traore A, Alambedji RB. Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 Harvard Style Dahourou, L. D., Savadogo, . M., Dera, . M. K., Abga, . L. R., Ouoba, . B. L., Tapsoba, . R. S. A., Yougbare, . B., Akio, . S., Zerbo, . L. H., Traore, . A. & Alambedji, . R. B. (2024) Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Vet. J., 14 (8), 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 Turabian Style Dahourou, Laibané Dieudonné, Madi Savadogo, Mikhailou Kiswend-sida Dera, Lamoussa Roland Abga, Bruno Lalidia Ouoba, Rayangnéwêndé Stéphane Arnaud Tapsoba, Bernadette Yougbare, Salimata Akio, Lamouni Habibata Zerbo, Amadou Traore, and Rianatou Bada Alambedji. 2024. Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Veterinary Journal, 14 (8), 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 Chicago Style Dahourou, Laibané Dieudonné, Madi Savadogo, Mikhailou Kiswend-sida Dera, Lamoussa Roland Abga, Bruno Lalidia Ouoba, Rayangnéwêndé Stéphane Arnaud Tapsoba, Bernadette Yougbare, Salimata Akio, Lamouni Habibata Zerbo, Amadou Traore, and Rianatou Bada Alambedji. "Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso." Open Veterinary Journal 14 (2024), 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 MLA (The Modern Language Association) Style Dahourou, Laibané Dieudonné, Madi Savadogo, Mikhailou Kiswend-sida Dera, Lamoussa Roland Abga, Bruno Lalidia Ouoba, Rayangnéwêndé Stéphane Arnaud Tapsoba, Bernadette Yougbare, Salimata Akio, Lamouni Habibata Zerbo, Amadou Traore, and Rianatou Bada Alambedji. "Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso." Open Veterinary Journal 14.8 (2024), 1912-1920. Print. doi:10.5455/OVJ.2024.v14.i8.19 APA (American Psychological Association) Style Dahourou, L. D., Savadogo, . M., Dera, . M. K., Abga, . L. R., Ouoba, . B. L., Tapsoba, . R. S. A., Yougbare, . B., Akio, . S., Zerbo, . L. H., Traore, . A. & Alambedji, . R. B. (2024) Detection of Crimean-Congo Haemorrhagic Fever virus antibodies in cattle in Kenedougou and Mouhoun provinces in Burkina Faso. Open Veterinary Journal, 14 (8), 1912-1920. doi:10.5455/OVJ.2024.v14.i8.19 |